Abstract
Clinical findings and blood parameters often are inconclusive in patients with periprosthetic joint infections. Among the accepted criteria for diagnosis, histologic analysis of debrided tissue can detect infection in most cases but does not allow intraoperative decision making. We evaluated the validity of intraoperative frozen sections for detection of prosthetic infections. The results from frozen and permanent sections of periprosthetic membranes of 64 consecutive patients who underwent exchange procedures after hip arthroplasty were compared using the histopathologic consensus classification of Morawietz et al. Blood parameters (erythrocyte sedimentation rate, leukocyte count, C-reactive protein) and culture results of preoperatively aspirated joint fluid and intraoperative tissue samples were correlated with the histologic results. In 50 patients (78.1%), agreement was found between the frozen and permanent sections. Two patients (3.1%) revealed a discrepancy between the two histologic methods. In 12 patients (18.8%), a diagnosis was not possible based on the frozen sections because the tissue samples were not representative enough for definite classification. For the analyzable cases (n = 52), the sensitivity of frozen-section histologic analysis was 86.6%, specificity 100%, and accuracy 96.2%. Our data support a recommendation for use of intraoperative frozen sections for diagnosis of septic versus aseptic loosening in revision hip surgery.
Level of Evidence: Level II, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The decision to remove an endoprosthetic implant attributable to infection results in severe hardship for the patients. The present preoperative diagnostic algorithms including the analysis of blood parameters and microbiologic evaluation of joint aspirates often do not confirm the presence of infection owing to low sensitivity and/or specificity. As a result, preoperative confirmation of periprosthetic infection, especially low-grade infection, remains a diagnostic challenge for the clinician.
Often, preoperative laboratory analysis is inconclusive and surgeons must proceed with an explanation based solely on the presence of pain and impaired joint function [7]. Preoperative laboratory analysis usually includes evaluation of the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, and culture results of joint fluid aspiration [3, 9, 19]. Joint fluid analysis that is suggestive of a periprosthetic infection, despite negative culture includes a total leukocyte count greater than 1700 to 3000 leukocytes (WBCs) per mL with greater than 60% neutrophils (PMNs) in the synovial fluid [9, 15, 20]. In joint aspiration, correct needle placement must be confirmed, especially in patients who are obese or patients with acetabular protrusions or periarticular ossifications. Dilution of the aspirate by saline irrigation into the joint compromises the cell quantification and the microbiologic analysis. It has been shown that microbiologic examination of joint aspirations and tissue samples obtained surgically without removal of the prosthesis can yield false-negative results in 28% and 14%, respectively [17]. The pseudomembrane of the bone-implant interface represents a tissue of high diagnostic value but is not accessible without exchange of the prosthesis [8, 13, 21].
Histopathologic analysis of periprosthetic tissue differentiates more precisely between aseptic and septic loosening. The number of PMNs at ×400 magnification (high-power field [HPF]) is the decisive parameter in histopathologic examination [6]. Some working groups have evaluated the usability of intraoperative frozen sections, using different thresholds for the PMN numbers with an acceptable range of sensitivity and specificity [1, 10]. However, an independent study came to a sensitivity of 28.5% and specificity of 100% for the criterion of Feldman et al. [10] (at least five PMNs in five HPFs) and a sensitivity of 71.4% and specificity of 64.2% for the criterion of Athanasou et al. [1] (at least one PMN per HPF in 10 HPFs) [6].
Our study is the first investigating the validity and reliability of intraoperative frozen sections using the consensus classification of Morawietz et al. [16]. The classification is based on neutrophil granulocytes number (at least two PMNs in 10 HPFs) and on additional histologic criteria, such as lymphocytes, plasma cells, multinucleated cells, and wear particles, allowing the differentiation between four types of loosening membranes (Table 1).
The aim of our study was to compare the results of intraoperative frozen sections with the final analysis of permanent histology using the above-stated classification system [16] in correlation with other diagnostic parameters of periprosthetic infection. Our hypothesis was that fresh-frozen-section histologic analysis of the periprosthetic membrane would substantially enhance the diagnostic outcome regarding detection of periprosthetic infections and therefore could facilitate the decision between one-stage or two-stage revision surgery. The primary questions were: (1) Would a representative analysis of frozen sections during the revision procedure be possible in each patient? (2) Would there be concordance between the intraoperative statement of the pathologist and the definitive result of the permanent histology, allowing intraoperative diagnosis of a periprosthetic infection. The secondary questions were: (1) How would the histologic results correlate with other diagnostic methods, such as blood parameters of infection and the microbiologic analysis of joint fluid aspiration and intraoperative samples? (2) Would the exclusive use of the intraoperative frozen section be sufficient for evaluation of a periprosthetic infection?
Materials and Methods
In this prospective study, 64 consecutive patients were included who underwent one- or two-stage exchange procedures attributable to component loosening after hip arthroplasty from January 2007 to June 2008. Patients preoperatively diagnosed as having septic and aseptic loosening were included. Surgery was performed by two surgeons (SWT, CP). The average age of the 37 female and 26 male patients was 66.7 years (range, 24–89 years) (Table 2). All patients gave informed consent before participating in the study.
A periprosthetic infection was diagnosed according to the following established criteria [2]: when the same bacterial organism was identified in at least two tissue samples, when purulence was detected in the joint, when the permanent sections showed evidence of infection [16], or when a hip-associated sinus tract was present. If none of these criteria was found, the loosening was defined as aseptic [21]. Nineteen of the 64 patients were diagnosed with a periprosthetic infection according to the above-stated criteria [2] (same bacterial organism in two tissue samples, purulence, positive permanent section [16], or hip-associated sinus tract).
Preoperative blood tests included ESR, peripheral WBC, and CRP. Elevation of the respective values greater than 30 mm/hour (ESR), 10.0 × 109/L (WBC), and 10 mg/L (CRP) were rated pathologic [3, 19]. Aspirates of joint fluid were obtained preoperatively from the affected hips of all patients. Care was taken that antibiotics had not been used by the patients for at least 14 days before revision surgery. In the cases where the clinical presentation suggested infection, but the result of the aspiration was not suggestive of infection, the latter was repeated once. Material from each aspiration was cultured for 14 days unless bacterial organisms were characterized sooner [12, 22]. During surgery, three representative samples from the synovial tissue and two samples from the bone-implant interface were harvested and cultured for the microbiologic analysis, which included at least 10 days of incubation [17].
Periprosthetic membranes were excised from the acetabulum and the femur in the case of a total exchange operation and from one site if only one component was exchanged. Tissue samples from the same region were either fresh frozen or fixed in formalin. The 5-μm sections were stained with hematoxylin and eosin. Fresh-frozen sections and permanent sections were classified according to the score of a consensus classification [16] by a pathologist (LM) experienced in the use of this classification in a blinded manner (Table 1). The interobserver reproducibility for the classification is 86%, which has been tested in a large patient cohort with five different investigators [16].
Student’s t test was used for analysis of normally distributed continuous data for comparison of aseptic and septic loosening. Categorical variables (positive/negative) were compared using the McNemar test. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for the diagnostic parameters used were calculated. A Pearson correlation analysis was performed between all parameters. For the latter, the microbiologic results of preoperative aspirations and intraoperative tissue samples were pooled.
Results
The comparison of the histopathologic methods (fresh-frozen versus permanent sections) yielded corresponding results in 50 of 64 patients (78.1%). In 12 patients (18.8%), the diagnosis could not be established definitely on the basis of the frozen sections as the tissue samples were not representative enough for cryohistology. In these patients, the tissue finally was classified in the permanent sections (Table 3). In two patients (3.2%), the diagnosis of the intraoperative frozen section was not confirmed by the permanent sections (Table 3). In one of these patients, evidence for a periprosthetic infection was present preoperatively (CRP, 15.4 mg/L; ESR, 78 mm/hour; Staphylococcus epidermidis in the joint aspiration) and was confirmed in the permanent sections (Type II) but was not detected in the frozen sections (Type I). Owing to the preoperative data, a two-stage exchange procedure was performed. The second patient had a loosened cemented stem and was diagnosed with an aseptic membrane type in the frozen sections (Type I), whereas the evaluation of the paraffin histology described a membrane of the combined type (infection and wear induced). This led to the final diagnosis of a low-grade infection with consecutive loosening as only the ESR (52 mm/hour) had met the criteria of a periprosthetic infection preoperatively. The patient underwent a complete one-stage-exchange again with cemented components and has not had any additional revision surgeries.
In eight patients whose diagnoses could not be established on the basis of the frozen sections, the final histologic results indicated aseptic loosenings. All parameters except for CRP elevation of 15.2 mg/dL in one patient and positive microbiology (gram-positive cocci) in one tissue sample of another patient (rated contaminated) confirmed these results. In all four patients diagnosed with septic loosenings in the permanent sections, the ESR had been elevated and two of them had the same organism in two or more intraoperative tissue samples. Preoperative joint aspirations were negative in all 12 of these patients and in the patients with histologically confirmed infections. The only diagnostic finding completely concordant with the permanent histology was the ESR (Table 3).
We found a difference between the two diagnostic groups (septic versus aseptic) only for the parameters ESR and frozen section. Peripheral WBC, CRP, and the microbiologic results did not show any differences (Table 2).
A Pearson analysis showed positive correlations between the histology (frozen and permanent sections) and all other diagnostic parameters apart from the peripheral WBC (Table 4).
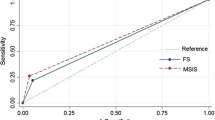
Sensitivity and specificity of the diagnostic tools have been referenced to the above-stated standard criteria for the diagnosis of infection. The sensitivity of the frozen sections (86.6%) was greater than that of peripheral WBC (21.7%; p < 0.002), microbiologic analysis (36.8%; p < 0.03), and CRP (57.9%; p < 0.05), but did not differ from that of the ESR (73.7%). Most interestingly, the specificity of the intraoperative frozen sections was 100% compared with the lower ratios of the other parameters (Table 5).
Discussion
Preoperative diagnostic parameters often are not conclusive enough to differentiate between aseptic and septic loosening. Frozen sections seem to be the only reliable tool for an intraoperative diagnosis. In this study we attempted to verify whether the analysis of frozen sections would allow a reliable decision regarding the infectious status of a loosened hip prosthesis. To answer this question, we compared the results of frozen sections of intraoperative samples with those of established diagnostic standards for infection including permanent histology and clear clinical signs of infection in 64 patients.
The use of only one pathologist analyzing the histologic sections is a potential limitation of the study. However, data for interobserver agreement when using the histopathologic consensus classification of Morawietz et al. [16], showed the agreement to be 85% to 87% (two to five pathologists involved in the study).
The major finding of our study was that frozen-section histologic analysis surpassed microbiologic methods and blood parameters concerning sensitivity and specificity for detection of periprosthetic infections. This was shown for the first time using the consensus classification of Morawietz et al. [16]. Nevertheless, a relevant percentage of unclear results (19%) obtained from the frozen sections pose a limitation to the method. Other studies investigating frozen-section histologic analysis as a diagnostic method for periprosthetic infections on the basis of the classification systems of Feldman et al. [10] or Athanasou et al. [1] do not report unclear results, but do report a lower sensitivity based on a high number of false-negative results [6, 11]. The unclear diagnoses in our study were caused by the inferior quality of fresh-frozen sections in comparison to the paraffin-embedded permanent sections. As the technical assistants who performed the cryohistology and the pathologist were highly experienced, an additional optimization concerning this problem is not to be expected.
If an unclear result was obtained from histologic analysis of the intraoperative specimen, the method was excluded from the diagnostic algorithm leading to a treatment decision, and only the synopsis of the other available parameters was used, causing no disadvantage for the patients. However, if a diagnostic decision could be made on the basis of the frozen sections, a comparison with the results of the permanent histology showed a high correlation between the two methods with a Pearson coefficient of 0.953. We observed only two false-negative results in all 64 patients, which, in our opinion, supports the use of the classification system for diagnosis of intraoperative infection. Nevertheless, false-negative results can occur and the intraoperative decision regarding the correct exchange procedure always should be made based on all available data.
Significant positive correlations of frozen-section histologic analysis, although to a lower extent, were found with all investigated parameters except for the peripheral WBC, supporting the diagnostic value of the method. The results of the statistical evaluation of standard blood parameters indicating infection were found to be in accordance with published results [19]. Sensitive parameters such as the ESR and CRP are not specific. Often they are depressed by antibiotic therapy [4, 14]. Furthermore, sensitivity and specificity are influenced and reduced by several factors, including accompanying illnesses. Chronic inflammatory disorders of the digestive tract and rheumatic diseases can lead to false-positive and false-negative results. The exclusive use of ESR and CRP for diagnosis of infection should not be relied on [3, 19]. Our results confirm the peripheral WBC should not be used for diagnosis of periprosthetic infections owing to unacceptable sensitivity and accuracy (Table 5).
The preoperative aspiration of joint fluid and microbiologic culture of intraoperative tissue samples yielded a high rate of false-negative results (Table 5), and therefore cannot be accepted as a gold standard for diagnosis of periprosthetic infection. However, when positive results are obtained, the specificities are 97% and 91%, respectively. Therefore a positive culture renders both methods valuable which also is supported by another study [5]. An underestimated and contributing problem is that aspiration of an adequate sample of fluid from the hip after arthroplasty usually is more difficult to achieve than from a surgically treated knee [18, 19].
Our results confirm the hypothesis that intraoperative frozen-section histologic analysis can reliably detect periprosthetic infections in most cases and consequently can be used as a tool for decision making during exchange procedures. In the case of an unclear diagnosis in the frozen sections, one still has to resort to other, above-discussed parameters of the diagnostic pool, which have less sensitivity or specificity but can provide crucial information in the synopsis. When early periprosthetic infections, or well-fixed, uncemented, or stable cemented implants are present, the sampling of material from the periprosthetic interface can be difficult and the harvested material may not be representative enough for frozen-section histology. Established methods such as permanent histology or innovative new tools such as culturing of biofilms harvested from implants with sonication [21] provide additional data to reach a diagnosis. Nevertheless, these can be used only for two-stage exchange procedures, such as when a definite diagnosis cannot be established on the basis of frozen-section histology and other parameters trigger suspicion for a periprosthetic infection.
Frozen-section histology provides a reliable method to detect a periprosthetic infection intraoperatively and has a high correlation to results obtained from final permanent sections, when using the consensus classification of Morawietz et al. [16]. We therefore recommend routine use of intraoperative frozen sections for differentiation of aseptic and infection-associated implant loosening, at least in cases with inconclusive preoperative findings.
References
Athanasou NA, Pandey R, de Steiger R, McLardy Smith P. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1997;79:1433–1434.
Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254.
Bernard L, Lubbeke A, Stern R, Bru JP, Feron JM, Peyramond D, Denormandie P, Arvieux C, Chirouze C, Perronne C, Hoffmeyer P (2004) Groupe D’Etude Sur L’Osteite. Value of preoperative investigations in diagnosing prosthetic joint infection: retrospective cohort study and literature review. Scand J Infect Dis 36:410–416.
Bernig T, Weigel S, Mukodzi S, Reddemann H. Antibiotic sequential therapy for febrile neutropenia in pediatric patients with malignancy. Pediatr Hematol Oncol. 2000;17:93–98.
Bori G, Soriano A, García S, Gallart X, Casanova L, Mallofre C, Almela M, Martínez JA, Riba J, Mensa J. Low sensitivity of histology to predict the presence of microorganisms in suspected aseptic loosening of a joint prosthesis. Mod Pathol. 2006;19:874–877.
Bori G, Soriano A, García S, Mallofre C, Riba J, Mensa J. Usefulness of histological analysis for predicting the presence of microorganisms at the time of reimplantation after hip resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 2007;89:1232–1237.
Bozic KJ, Rubash HE. The painful total hip replacement. Clin Orthop Relat Res. 2004;420:18–25.
Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322.
Della Valle CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):90–93.
Feldman DS, Lonner JH, Desai P, Zuckerman JD. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–1813.
Gallo J, Kolar M, Dendis M, Loveckova Y, Sauer P, Zapletalova J, Koukalova D. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31:97–104.
Gunthard H, Hany A, Turina M, Wust J. Propionibacterium acnes as a cause of aggressive aortic valve endocarditis and importance of tissue grinding: case report and review. J Clin Microbiol. 1994;32:3043–3045.
Ince A, Rupp J, Frommelt L, Katzer A, Gille J, Lohr JF. Is “aseptic” loosening of the prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in patients with low-grade infection? Clin Infect Dis. 2004;39:1599–1603.
Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6:311–315.
Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplasty. 2003;18:1038–1043.
Morawietz L, Classen RA, Schröder JH, Dynybil C, Perka C, Skwara A, Neidel J, Gehrke T, Frommelt L, Hansen T, Otto M, Barden B, Aigner T, Stiehl P, Schubert T, Meyer-Scholten C, König A, Ströbel P, Rader CP, Kirschner S, Lintner F, Rüther W, Bos I, Hendrich C, Kriegsmann J, Krenn V. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59:591–597.
Müller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. Diagnosis of periprosthetic infection following total hip arthroplasty: evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J Orthop Surg. 2008;3:31.
Somme D, Ziza JM, Desplaces N, Chicheportiche V, Chazerain P, Leonard P, Lhotellier L, Jacquenod P, Mamoudy P. Contribution of routine joint aspiration to the diagnosis of infection before hip revision surgery. Joint Bone Spine. 2003;70:489–495.
Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683.
Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562.
Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663.
Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654.
Acknowledgments
We thank Ines Poley for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Tohtz, S.W., Müller, M., Morawietz, L. et al. Validity of Frozen Sections for Analysis of Periprosthetic Loosening Membranes. Clin Orthop Relat Res 468, 762–768 (2010). https://doi.org/10.1007/s11999-009-1102-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-1102-5