Abstract
Prosthetic joint infections (PJI) caused by methicillin-resistant staphylococci represent a major therapeutic challenge. We examined the effectiveness of surgical treatment in treating infection of total hip or knee arthroplasty caused by methicillin-resistant staphylococcal strains and the variables influencing treatment success. One hundred and twenty-seven patients were treated at our institution between 1999 and 2006. There were 58 men and 69 women, with an average age of 66 years. Patients were followed for a minimum of 2 years or until recurrence of infection. Débridement and retention of the prosthesis was performed in 35 patients and resection arthroplasty in 92. Débridement controlled the infection in only 37% of cases whereas two-stage exchange arthroplasty controlled the infection in 75% of hips and 60% of knees. Preexisting cardiac disease was associated with a higher likelihood of failure to control infection in all treatment groups. Antibiotic-resistant Staphylococci continue to compromise treatment outcome of prosthetic joint infections, especially in patients with medical comorbidities. New preventive and therapeutic strategies are needed.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosthetic joint infection (PJI) is a serious complication that can develop after total joint arthroplasty (TJA) and affect the surgical outcome as well as the quality of life of the patient. The liberal use of antibiotics during the past decade has raised concern regarding the emergence of resistant organisms and their detrimental effect on joint arthroplasty [5, 8]. According to the latest National Nosocomial Infection Surveillance System (NNIS) report [11], there has been a substantial rise in incidence of antibiotic resistance among organisms isolated from intensive care units compared to previous years. It is still undetermined, however, if this has been associated with a rising incidence of these organisms in periprosthetic infections on a national level.
Several investigators [2, 9, 10, 13, 15] reported on the outcome of treatment of infections caused by antibiotic-resistant organisms, especially methicillin-resistant staphylococci. One study reported that among 19 patients with infected total hip arthroplasty, there were no differences in treatment failure rates between resistant and sensitive bacteria. Nonetheless, when this cohort was combined with 16 patients with infected total knee arthroplasty, a higher failure rate of treatment was observed among resistant bacterial infections [9]. Another study involving nine patients with infected total hip or knee arthroplasty due to resistant organisms found no differences in failure rates between infections with resistant bacteria and infections with sensitive ones [15]. Conversely, a higher failure rate of treatment was observed among seven patients with methicillin-resistant Staphylococcus aureus (MRSA) infections when compared with infections involving sensitive strains [2]. Similarly, a 50% failure rate of treatment was reported among 12 patients with infection after total hip and knee arthroplasty caused by MRSA [13]. The largest reported cohort of MRSA or methicillin-resistant Staphylococcus epidermidis (MRSE) infections consisted of 37 total knee arthroplasty patients from two centers treated between 1987 and 2003 with a 24% failure rate observed [10]. Higher failure rate of débridement with prosthesis retention in the treatment of PJI has been associated with postoperative drainage for more than 2 weeks, sinus tracts, a hinged prosthesis, and immunocompromised patients (diabetes mellitus and rheumatoid arthritis) [14]. Rheumatoid arthritis and multiple prior knee surgeries have also been identified as risk factors for failure of two-stage exchange arthroplasty in the treatment of PJI [7]. The rationale of this study, based partly on the results of these previous reports, was that surgery carries a high failure rate in the treatment of PJI caused by antibiotic-resistant staphylococci.
To confirm those results, we first asked whether débridement with prosthesis retention and two-stage exchange arthroplasty could control prosthetic joint infection caused by methicillin-resistant staphylococcal strains; we then sought to identify risk factors (eg, comorbidities of the host, prior revision surgeries, extent of bone loss, and soft tissue damage) associated with treatment failure.
Materials and Methods
Between 1999 and 2006, we treated 127 patients for prosthetic joint infection (PJI) caused by either methicillin-resistant Staphylococcus aureus (MRSA) or methicillin-resistant Staphylococcus epidermidis (MRSE). These included 66 patients who developed infection after total hip arthroplasty and 61 patients with infection following total knee arthroplasty. Diagnosis was confirmed by isolation of MRSA or MRSE in at least two intraoperative cultures. A subgroup of patients was treated with irrigation, débridement, and retention of the prosthesis, whereas another subgroup was treated with two-stage exchange arthroplasty. Patients were followed for a minimum of 2 years or until recurrence of infection. Two patients died before the minimum followup and two were lost to followup. The mean followup time for patients who were successfully treated was 4.8 years (range, 2.1–9 years).
Thirty-five patients (24 hips and 11 knees) presenting with PJI were initially treated with irrigation and débridement and exchange of the modular parts of the implants. Short duration of symptoms (< 4 weeks) and no radiographic signs of loosening were the two main indications for prosthesis retention. There were 13 men and 22 women, with an average age of 65 years. PJI developed after primary arthroplasty in 10 patients, following a prior two-stage exchange arthroplasty in two patients and following revision arthroplasty due to infection-unrelated reasons in the remaining 23 patients. Only one of the 35 patients was referred from an outside institution. Surgery entailed thorough débridement and extensive synovectomy and irrigation with 9 Liters of antibiotic-laden saline solution. Prostheses were stable in all cases. Postoperative antimicrobial therapy was started, first empirically, and then based on the results of intraoperative cultures was continued for 6 weeks after which the patient returned for followup. Intraoperative cultures revealed polymicrobial growth in three patients (Pseudomonas aeruginosa + MRSA in one patient, Proteus mirabilis + MRSE in one patient, and Enterobacter faecium group D + MRSE in the third patient). In vitro testing showed susceptibility of the isolated organisms to vancomycin and linezolid in all patients, and to rifampin in all but one patient. Patients were therefore placed on IV vancomycin for 6 weeks. Trough and peak vancomycin levels were carefully monitored during the treatment period and all patients were followed by an infectious disease consult. Suppressive therapy was not used in this cohort. There were no toxicities related to therapy.
Another group of 42 patients (19 men and 23 women; average age, 66 years) who developed periprosthetic infection following total hip arthroplasty were initially treated with resection arthroplasty. Infection occurred after primary arthroplasty in 20 patients and after revision arthroplasty due to aseptic causes in the other 22 patients. Sixteen of the 42 patients had undergone their initial arthroplasty at our institution while the remaining 26 patients were referred from outside institutions. Removal of total hip implants and cement in cemented arthroplasties was followed by thorough débridement of devitalized tissues and insertion of an antibiotic-laden cement spacer block. Four grams of vancomycin and 3.6 g of tobramycin were added to 40 g of cement in each patient. Cultures taken intraoperatively identified polymicrobial infection in two patients. Proteus mirabilis was isolated in one patient in addition to MRSA. Vancomycin-resistant enterococcus (VRE) was isolated in the other patient in association with MRSA. The patients were then treated with 6 weeks of intravenous antibiotics based on the results of the sensitivities of the organism cultured. The second-stage procedure consisted of delayed reimplantation with primarily cementless revision components at an average of 4 months (range, 1.6–20 months) after resection arthroplasty. Due to the extensive bone loss on the acetabular side, a cemented cup-cage construct was used in five patients. Thirty-two of the 42 patients (76%) underwent delayed reimplantation, while the remaining 10 patients were not reimplanted due to various reasons. Multiple comorbidities (including advanced congestive heart failure, metastatic tumors, end-stage liver and kidney disease) precluded reimplantation in three patients. Three more patients died postoperatively following the first-stage resection arthroplasty secondary to the lethal combination of methicillin-resistant Staphylococcal septicemia and end-stage organ failure. All three patients had preexisting liver and/or renal dysfunction. Two patients were lost to followup shortly after resection arthroplasty. Other causes that precluded reimplantation were the presence of a long history of infection combined with extensive bone loss in one patient and the presence of an ongoing septic focus (retroperitoneal sepsis) in another. Reimplantation was performed when clinical and serological confirmation of control of infection was available. One of five intraoperative culture samples taken at the time of reimplantation was positive for MRSA in four patients. They were treated with 6 weeks of IV vancomycin followed by 3 months of oral trimethoprim/sulfamethoxazole in two patients and doxycycline in two patients. Reimplantation was deferred and further débridement and insertion of a new spacer block were performed in eight patients (25%) due to the suspicion of persistent infection based on clinical (persistent pain, continuous drainage) and serological (elevated erythrocyte sedimentation rate and C-reactive protein values) findings. Cultures were positive for MRSA at the time of repeat débridement and spacer exchange in three patients.
A third group of 50 patients (26 men and 24 women; average age, 68 years) presented with infection after primary total knee replacement (35 patients) and after revision total knee replacement for aseptic reasons (15 patients). They were treated with a first-stage resection arthroplasty with removal of total knee implants, proper débridement of devitalized tissues, and insertion of an antibiotic-laden cement spacer. Articulating spacers were used in five patients due to surgeon’s preference. Four grams of vancomycin and 3.6 g of tobramycin were added to 40 g of cement in each patient. The patients were then treated with intravenous antibiotics according to the results of the sensitivities of the organism cultured. The second-stage reimplantation was performed on 40 of these 50 patients (80%). Poor general health, compromised soft tissues especially in association with extensive bone loss, ongoing immunosuppression, and septic foci precluded reimplantation in the remaining 10 patients. The final outcome in those 10 patients was knee arthrodesis (five patients), knee pseudarthrosis (three patients), and above-the-knee amputation (one patient), whereas one patient was satisfied with a dynamic knee spacer and opted not to undergo reimplantation surgery. Delayed reimplantation with constrained revision components was performed at an average of 3 months (range, 1.5-9 months) after resection arthroplasty. All reconstructions were performed with stemmed varus-valgus constrained components secondary to the bone deficiency and soft tissue laxity encountered at the time of reimplantations. Antibiotic-impregnated cement containing 1.2 gm of tobramycin per pack of cement was used in all cases. Postoperatively, two patients were placed on IV vancomycin for 6 weeks followed by oral trimethoprim/sulfamethoxazole for 3 months secondary to positive intraoperative cultures during reimplantation. Reimplantation was deferred and further débridement and exchange of the spacer block were performed in five patients (13%) due to the suspicion of persistent infection. Cultures were positive for MRSA at the time of repeat débridement and spacer exchange in three patients.
Different demographic, clinical, and surgical variables were studied to identify the possible predictors of outcome. We defined treatment failure as the need for resection arthroplasty or recurrent microbiologically proven infection. Demographic variables included age, gender, and body mass index. Clinical risk factors included type of previous arthroplasty (primary versus revision), presence of a sinus tract, type of organism (MRSA versus MRSE), ASA (American Society of Anesthesiologists) score, comorbidities (diabetes, anemia, smoking, urinary tract infection at the time of surgery, malignancy, cardiovascular disease, kidney disease based on preoperative creatinine), and steroid or immunosuppressant use. Surgical risk factors were extent of bone loss, duration of surgery, and estimated blood loss. Doses and duration of antibiotics received after hospital discharge were not always accurately reported in the medical records.
All 127 patients were followed up prospectively with radiographs. Bone loss prior to total hip reimplantation was determined using the Paprosky classification scheme for the acetabulum and femur [12]. Bone loss prior to total knee reimplantation was determined using the Anderson Orthopedic Research Institute (AORI) for the tibia and femur [4]. Duration of symptoms (time to intervention) was studied in the patient group who were treated with débridement alone, whereas time period from resection to reimplantation was studied in patients treated with two-stage exchange arthroplasty.
To identify potential variables associated with treatment failure, we performed a univariate analysis with means and standard deviations for continuous variables and proportions for categorical variables (Table 1). The outcome measure was success or failure of each surgical procedure in eradicating infection. The means of continuous variables were compared using the parametric test (t test) if the values followed a normal distribution and the nonparametric (Wilcoxon) test if otherwise. Proportions of categorical variables were compared using the parametric (Fisher) test if any of the proportions were less than five and using the nonparametric (chi square) test if all the proportions were more than five. This univariate analysis assessed the differences in demographics, comorbidities, and intraoperative variables between patients who failed treatment and those who had a successful outcome (Table 1). Subsequently, multiple regression analysis was performed after adjusting for potential confounders to determine the risk factors for reinfection. All statistical analyses were performed using SAS version 9.1 software (SAS Institute Inc., Cary, NC).
Results
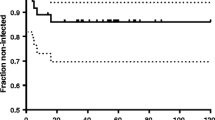
Débridement with retention of prosthesis was successful in 13 patients (37%) and failed in 22 patients (63%) (Table 2). Recurrent or persistent infection was diagnosed in eight patients (25%) at an average of 6 months (range, 0.8-15 months) after the second-stage reimplantation procedure of the hip (Table 2). Recurrence or persistence of infection was diagnosed based on recurrent purulent drainage, significant systemic symptoms like fever and/or persistent hip pain. Positive intraoperative culture on solid media was confirmed in seven of the eight cases. However, the organism(s) isolated from the site of recurrent infection was the same as that cultured from time of resection in only four cases, while a different organism(s) manifested in the remaining three cases. Irrigation and débridement was the initial treatment for five of those patients. Infection eradication with retention of components was achieved in two patients. They showed no signs of infection at their latest followup. The remaining six patients underwent resection arthroplasty for relapsing PJI. Two of the six patients underwent secondary reimplantation; whereas the remaining five patients had a resection arthroplasty. Due to poor general health, reimplantation was never performed on those patients. In patients in whom reimplantation surgery resulted in successful eradication of infection, a total of four patients were re-revised for aseptic failure at a mean of 10 months (range, 2–25 months) after reimplantation. Failure of the constrained liner occurred in two patients, whereas loosening of the acetabular component was the cause of reoperation in the other two patients. Multiple intraoperative specimens were obtained during revision surgery; all were reported negative after the initial 5 days of incubation. Therefore, the reinfection rate in this patient group was 25%, while the combined revision rate for both reinfection and mechanical failure after reimplantation reached 37.5% at the latest followup. Recurrent infection was diagnosed in 16 patients (40%) after the second-stage reimplantation procedure of the knee at an average of 18 months (range, 42 days to 7 years) (Table 2). An organism was identified in 15 of 16 patients. The same organism was isolated in 12 patients as that of the index resection while a different organism manifested in the remaining three cases. Eight cases were managed initially with irrigation and débridement procedures. Eradication of infection was attained in three patients. The remaining 13 patients underwent resection arthroplasty for relapsing PJI. Only 10 of the 13 patients underwent secondary reimplantation, whereas knee arthrodesis was performed in the remaining three patients due to uncontrolled infection and poor soft tissue condition. Of the patients who had successful infection control, one patient was re-revised for aseptic loosening 4 years after reimplantation. Intraoperative cultures were negative. Therefore, the reinfection rate in this group was 40%, while the combined revision rate for both reinfection and mechanical failure after reimplantation reached 42.5%.
For the irrigation and débridement group, higher body mass index (BMI) (p = 0.03), purulence around the prosthesis (p = 0.005), and postoperative drainage or hematoma after irrigation and débridement (p = 0.04) predicted treatment failure (Table 1). After adjusting for the effect of confounding factors, purulence deep to the fascia or capsule (p = 0.007, 95% confidence interval (CI) = 1.80–43.81) was associated with failure of irrigation and débridement with retention of hardware to control infection in this patient group. Preexisting cardiac disease was more commonly encountered in the patient group that developed reinfection after two-stage exchange arthroplasty (Tables 3, 4). No difference in infection control rates was noted between the five surgeons who contributed to this study.
Discussion
From 2002 to 2007, 34% of periprosthetic infections treated at our institution were due to either MRSA or MRSE. The purpose of our study was twofold. First, we asked whether débridement with prosthesis retention and two-stage exchange arthroplasty could control prosthetic joint infection caused by methicillin-resistant staphylococcal strains. We presumed, based partly on previous reports, that treating those infections with débridement and prosthesis retention or with two-stage exchange arthroplasty with delayed reimplantation results in a high rate of infection recurrence. Second, we sought to identify clinical, surgical, and patient-related characteristics associated with a higher incidence of recurrent infection.
The study does have some limitations. First, our retrospective study may introduce variability in data collection and recall bias: doses and duration of antibiotics received after hospital discharge were not always accurately reported in the medical records. Second, we have no comparative data on the outcome of the two surgical procedures for treating methicillin-sensitive staphylococcal infections. Third, although the sample size of our study group is relatively large, some of the important variables may not have been statistically identified owing to underpowering. However, we still believe that the results of the study provide important information.
Our data suggest that at a high-volume tertiary institution, joint irrigation and débridement for management of acute periprosthetic infection caused by antibiotic-resistant organisms carries a high failure rate (63%). Similar high failure rates were reported in previous studies [2, 9, 13]. Salgado et al. [13] reported an 83% failure rate of irrigation and débridement in five patients treated for prosthetic joint infection due to MRSA or MRSE. Evidence from recent studies suggests using combination oral antibiotic regimens that include rifampin improves outcome in methicillin-resistant staphylococcal PJI treated with débridement and prosthesis retention [1]. Several investigators [6, 7, 9, 10, 13] have reported a low success rate of two-stage exchange arthroplasty in treating infected total joint replacements involving resistant organisms. In total hip arthroplasty, Hanssen and Osmon [6] documented a reinfection rate of 22% in nine patients treated with two-stage reimplantation for methicillin-resistant staphylococcus infection. In total knee arthroplasty, Mittal et al. [10] demonstrated a 24% reinfection rate after two-stage exchange arthroplasty for periprosthetic knee infection. Although Volin et al. [15] demonstrated successful delayed reconstruction of six infected total hip replacements caused by methicillin-resistant organisms, one of three patients with infected total knee replacement caused by MRSE in their series developed recurrent infection following delayed reimplantation and subsequently underwent knee fusion. Another study (Salgado et al. [13]) reported a 17% failure rate for MRSA infections treated with delayed reimplantation, whereas no failures were noted among infections caused by MSSA.
We found higher body mass index (BMI) and heart disease were associated with a poorer outcome of surgical débridement. We also found purulence around the prosthesis encountered intraoperatively to be a major predictor of failure of prosthesis retention. Purulence is a marker of established infection, tissue devitalization, and a higher likelihood of extension into the prosthesis-bone interface. Our data suggested preexistent cardiac disease (ischemic heart disease or cardiac failure) correlated with the outcome of two-stage exchange arthroplasty in treating infection caused by methicillin-resistant staphylococci. Cardiac insufficiency has been recognized as an important systemic compromising factor in staging systems of periprosthetic infection [3, 6]. Retention of hardware, infections occurring around total knee arthroplasty (as opposed to total hip arthroplasty) and MRSA (as opposed to MRSE) are major risk factors of failure in treating PJI caused by resistant organisms [13].
Our data emphasize the high failure rate of irrigation and débridement with prosthesis retention in the management of periprosthetic infection involving resistant organisms. Despite being the most commonly agreed upon strategy for management of periprosthetic infections, two-stage exchange arthroplasty also carries a high failure rate in the management of infections due to resistant bacteria. As current strategies remain imperfect, new modalities and changes to the current protocols are desperately needed to avoid more serious problems in the near future.
References
Aboltins CA, Page MA, Buising KL, Jenney AW, Daffy JR, Choong PF, Stanley PA. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clin Microbiol Infect. 2007;13:586–591.
Barberan J, Aguilar L, Carroquino G, Gimenez MJ, Sanchez B, Martinez D, Prieto J. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am J Med. 2006;119:993–1000.
Cierny G, III, DiPasquale D. Periprosthetic total joint infections: staging, treatment, and outcomes. Clin Orthop Relat Res. 2002;403:23–28.
Engh GA: Bone defect classification. In: Engh GA, Rorabeck CH, eds. Revision Total Knee Arthroplasty. Baltimore, MD. Williams and Wilkins; 1997:63–120.
Fulkerson E, Valle CJ, Wise B, Walsh M, Preston C, Di Cesare PE. Antibiotic susceptibility of bacteria infecting total joint arthroplasty sites. J Bone Joint Surg Am. 2006;88:1231–1237.
Hanssen AD, Osmon DR. Evaluation of a staging system for infected hip arthroplasty. Clin Orthop Relat Res. 2002;403:16–22.
Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28.
Ip D, Yam SK, Chen CK. Implications of the changing pattern of bacterial infections following total joint replacements. J Orthop Surg (Hong Kong). 2005;13:125–130.
Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Relat Res. 2002;404:116–124.
Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum SM, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231.
National Nosocomial Infections Surveillance (NNIS) System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485.
Paprosky WG, Bradford MS, Younger TI. Classification of bone defects in failed prostheses. Chir Organi Mov. 1994;79:285–291.
Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53.
Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;404:125–131.
Volin SJ, Hinrichs SH, Garvin KL. Two-stage reimplantation of total joint infections: a comparison of resistant and non-resistant organisms. Clin Orthop Relat Res. 2004;427:94–100.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors did not receive any outside funding or grants in support of their research for or preparation of this work. One or more of the authors (JP, RHR) has received funding from Stryker Orthopaedics (Mahwah, NJ) and Smith & Nephew (Memphis, TN) in support of their research.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Parvizi, J., Azzam, K., Ghanem, E. et al. Periprosthetic Infection Due to Resistant Staphylococci: Serious Problems on the Horizon. Clin Orthop Relat Res 467, 1732–1739 (2009). https://doi.org/10.1007/s11999-009-0857-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-0857-z