Abstract
Tapered titanium porous plasma-sprayed components have performed well in primary THA. To confirm the literature at longer followup we retrospectively reviewed all 1639 patients who underwent 2000 THAs in which a specific porous femoral component was used. One hundred fourteen patients (134 hips) were lost to followup leaving a cohort of 1525 patients (1866 THAs). The component is a tapered titanium plasma spray-coated design that remained relatively unchanged since its first implantation except for circumferential proximal porous coating added in 1986 and an offset option added in 1999. Minimum followup was 24 months (average, 119 months; range, 24 to 275 months). To date there have been 39 femoral revisions for an implant survival of 98%. Using the Kaplan-Meier method, cumulative survival with any stem revision as the end point was 98.6% at 5 years, 98.4% at 10 years, 97.1% at 15 years, and 95.5% at 20 years. Using aseptic revision for failure of ingrowth as the endpoint, stem survival was 99.1%. Kaplan-Meier cumulative survival with aseptic revision for failure of ingrowth as the endpoint was 99.4% at 5 years, 99.3% at 10, 15 and 20 years. Harris hip pain and total scores improved. This titanium, porous plasma spray-coated femoral component continues to demonstrate high long-term survival with a low rate of component revision for any reason or aseptic failure of ingrowth.
Level of Evidence: Level IV, therapeutic study (case series). See the Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the United States, the pendulum of fixation of the femoral component in primary total hip arthroplasty has swung from cemented fixation to cementless fixation, with cementless femoral components used in an estimated 56% of total hip arthroplasties in 2006 [1, 8, 52, 54]. The rationale for this enthusiasm rests in favorable rates of survival observed in multiple mid- and long-term followups of cementless primary hip arthroplasty [2–4, 7, 13–19, 22, 23, 26, 27, 30–35, 38–40, 44–51, 53, 55–58, 60–62, 69]. However, this trend is not the case worldwide. Cemented fixation is favored in some European countries such as England and Sweden [1, 42, 43, 54, 68], although there has been steady increase in the number of cementless femoral components implanted [20, 25, 68].
Modular devices have demonstrated promising results, with survival ranging from 98% at 8.4 years to 99.6% at 11 years [9, 10, 65]. However, concerns exist with respect to corrosion and modular junctions, and potential for increased risk of fretting, osteolysis, and fracture at modular junctions [6]. Cementless straight, cylindrical porous-coated devices have high survival rates ranging from 98% at 11.3 years to over 95% at 15 years [18, 35]. However, these devices may have issues related to thigh pain and perhaps more importantly proximal stress shielding [19]. Anatomic devices have had encouraging reports, with 100% survival at 8.4 years [23]. However, longer-term followup questions the durability of these designs, with failure rates of 11% at 11.2 years and 6% at 15 years [34, 38]. Tapered stems of various designs have enjoyed a high degree of success (Tables 1, 2) [44, 46, 47]. They are appropriate in both Dorr Type A [12] bone typical of young patients [13, 14, 20] and Dorr Type C [12] bone typically seen in older patients [3, 31, 60]. They do not fail from varus malalignment (96% survival at 10 years) [4, 32]. While somewhat prone to proximal fracture at the time of insertion, they have demonstrated high survivorship (100% at 7.5 years) if treated with a proximal cerclage cable or wire [2]. These tapered titanium stems also seem to respect the proximal femur with minimal proximal stress shielding [16, 39, 40, 44–47].
To confirm the literature we first analyzed the survivorship of the first 2000 tapered titanium porous plasma-sprayed femoral components used in primary THA at a single institution. All failures were identified. Second, we sought to define and determine the etiology for failures. Third, we compared pre- and postoperative clinical pain and function scores.
Materials and Methods
We retrospectively reviewed all 1639 patients who underwent 2000 primary or conversion THAs with a porous femoral component performed by four surgeons (THM, AVL, BKV, RAF) from August 1984 through July 2001. Initially (i.e., from 1984 to 2000) younger patients with Dorr type A or B [12] bone quality were selected for cementless devices. As confidence with the device grew, selection criteria were expanded to include patients with Dorr type C [12] bone and more extensive femoral deformity (from 2000 to 2001). There were 882 (54%) men and 759 (46%) women. The average age of the patients at the time of surgery was 53 years (range, 19–92 years) and the average body mass index was 30.3 kg/m2 (range, 15.3–64.0 kg/m2). The underlying diagnosis was osteoarthritis in 1358 (68%) hips, avascular necrosis in 229 (12%), developmental dysplasia in 138 (7%), posttraumatic arthritis in 109 (6%), rheumatoid arthritis in 61 (3%), Legg-Calvé-Perthes in 47 (2%), slipped capital femoral epiphysis in 36 (2%), ankylosing spondylitis in 13 (1%), acute femoral fracture in four, and failed resurfacing arthroplasty in five. Charnley classification [11] was A (unilateral hip disease) for 793 (40%) hips, B (bilateral disease) for 906 (45%), and C (systemic illness or other joint involvement) for 301 (15%). One hundred fourteen patients (134 hips) were lost to followup before 24 months, despite extensive efforts to locate them, leaving a cohort of 1525 patients (1866 THA). During the followup period, 229 patients (278 hips) died at an average of 8.2 years postoperatively; the status of their hip arthroplasty was known in each patient. There was one perioperative death of a woman who suffered an immediate myocardial infarction. Another woman suffered a fatal pulmonary embolism at 3 weeks postoperatively despite an uneventful course. Minimum followup for surviving patients was 24 months and followup averaged 10.0 years (range, 2–23 years).
The Mallory-Head porous femoral component (Biomet, Inc., Warsaw, IN) was introduced in 1984. Its key characteristics are a proximal-to-distal taper in both the coronal and sagittal planes. Proximal anterior, posterior, and lateral fins engage the cortical-cancellous junction. Finite element analysis demonstrates these fins resist rotational moments about the implant in the proximal femur [45]. The implant is divided into three equal segments of different surface preparations along its length. The proximal third is plasma-sprayed titanium alloy with an average porosity of 43.6% and a coating thickness between 635 and 889 μm. The midsection is grit-blasted with an average surface finish of 30 mesh, and the distal surface is a smooth satin finish. The components range in sizes from 6 mm to 19 mm in 1-mm increments. Initially, the device lacked circumferential plasma spray coating. However, with an enhanced understanding of the need for circumferential endosteal coating, as noted in several studies, circumferential plasma spray was added in 1986 [5, 17, 66, 67]. The component was offered in a standard offset through 1999, when a high offset, lateralized option was added to the product line. The component was designed without a collar to ensure intimate contact of a taper within a taper in the proximal femur. A reduced profile 7° included angle taper neck was incorporated into the prosthesis with anterior and posterior flats to enhance range of motion prior to impingement. Initially, the modular femoral heads were made of titanium alloy, which proved an inferior bearing material and in 1989 chrome-cobalt alloy became the bearing material of choice [41]. Later, options for ceramics were added.
One hundred fifty cases were performed before the introduction of circumferential porous coating. Stems featuring the lateralized offset option were implanted in 130 (7%) hips. The material of the modular femoral head was titanium in 218 (11%) hips, ion-bombarded titanium in 60 (3%), chrome cobalt in 1500 (75%), and ceramic in 222 (11%). Over this period of nearly two decades, a wide variety of acetabular components were utilized, beginning with predominately S-ROM Poly-Dial-type cups (DePuy; 251 hips, 13% overall) and Mallory-Head HexLoc devices (Biomet; 187 hips, 9% overall) and evolving to Mallory-Head RingLoc devices (Biomet; 1307 hips, 65% overall) and M2a-Taper metal-on-metal devices (Biomet; 201 hips, 10% overall).
The surgical technique has remained constant. All operative procedures were performed in a clean-air laminar flow room with all members of the surgical team in personal isolator suits. The operative approach was the direct lateral approach to the hip as described by Frndak et al. [21]. Preoperative templating assisted in the determination of the level of neck resection. The femoral canal was opened with a standard starting femoral reamer. Conical reaming of the proximal femur then followed in a sequential matter. The proximal femur was then broached with incremental sizes of rasps. Phantoms of the true prosthesis without the plasma spray were then used to determine appropriate size and to trial for appropriate leg length. If a proximal fracture occurred during preparation or implantation of the final component, a cerclage cable or wire was placed. Patients were allowed immediate full weight bearing as tolerated with crutches or a walker for a period of 4 to 6 weeks. Patients were advised to progress without ambulatory aids when they were pain-free and without limp.
Patients were followed in the immediate postoperative period at approximately six weeks and encouraged to return yearly thereafter. We (AVL, KRB, THM, BKV, RAF) assessed patients at each followup time using the Harris hip score [24] and beginning in October 2006 using the Lower Extremity Activity Scale [63].
Survival estimates and cumulative survivorship were determined using the Kaplan-Meier method [29], examining both septic and aseptic revisions. Aseptic revisions were further stratified by failure of ingrowth, or revision of a well-fixed stem. All patients enrolled were included in Kaplan-Meier analyses. Patients were censored by date of last followup including those without minimum 2-year. Differences between preoperative and postoperative Harris hip total score and pain component were compared with unpaired Student’s t-test. Power analysis was performed using 80%, and confidence intervals were calculated at 95%. All analyses were performed using StatsDirect (StatsDirect Ltd., Altrincham, Cheshire, UK).
Results
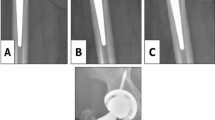
We revised 39 (2%) femoral components, with 12 (1%) revisions attributed to failure of ingrowth (Fig. 1A–D) (Table 3). Revisions for failure of ingrowth were performed at an average of 21 months postoperatively (range, 3–69 months). Eight stems were revised as part of two-stage treatment of sepsis at an average of 83 months postoperatively (range, 2–211 months). Six stems were revised secondary to periprosthetic femoral fracture at an average of 70 months postoperatively (range, 0.4–211 months). Twelve well-fixed stems were revised for pain secondary to malalignment in one hip at 29 months postoperatively, secondary to severe osteolysis in 7 hips at an average of 117 months postoperatively (range, 37–242 months), and for reasons unknown in four hips (range, 4–206 months). One well-fixed stem underwent revision at 184 months postoperatively because of component breakage at the neck.
A case is presented in which the femoral component was revised for aseptic loosening. (A) The preoperative radiograph of the right hip of 43-year-old man who presented with pain and discomfort reveals the presence of avascular necrosis. (B) Immediate postoperative radiograph reveals treatment with primary cementless total hip arthroplasty. (C) Radiographs at 20 months postoperative reveal radiolucency in zone 3 of the acetabular component and zones 2, 3, 5, and 6 of the femoral component, with pedestal formation noted and evidence of subsidence. (D) Radiograph reveals treatment with revision total hip arthroplasty including the acetabular liner, the femoral head and the femoral component.
With any revision of the femoral component as the endpoint (Fig. 2), Kaplan-Meier estimated survival proportion was 98.6% at 5 years (1589 at risk, standard error 0.003), 98.4% at 10 years (871 at risk, standard error 0.003), 97.1% at 15 years (380 at risk, standard error 0.006), and 95.5% at 20 years (196 at risk, standard error 0.009).Using aseptic revision of the femoral component for failure of ingrowth as the endpoint (Fig. 3), Kaplan-Meier estimated survival proportion was 99.4% at 5 years (1508 at risk, standard error 0.002), 99.3% at 10 years (795 at risk, standard error 0.002), 99.3% at 15 years (305 at risk, standard error 0.002), and 99.3% at 20 years (121 at risk, standard error 0.002). Using aseptic revision of a well-fixed femoral component as the endpoint (Fig. 4), Kaplan-Meier estimated survival proportion was 99.5% at 5 years (1508 at risk, standard error 0.002), 99.4% at 10 years (795 at risk, standard error 0.002), 98.2% at 15 years (305 at risk, standard error 0.006), and 96.6% at 20 years (121 at risk, standard error 0.01).
Kaplan-Meier survivorship analysis using revision of the femoral component for any reason as the endpoint reveals estimated survival proportion of 98.6% at 5 years (1589 at risk, standard error 0.003), 98.4% at 10 years (871 at risk, standard error 0.003), 97.1% at 15 years (380 at risk, standard error 0.006), and 95.5% at 20 years (196 at risk, standard error 0.009).
Kaplan-Meier survivorship analysis using aseptic revision of the femoral component secondary to failure of ingrowth as the endpoint reveals survival proportion of 99.4% at 5 years (1508 at risk, standard error 0.002), 99.3% at 10 years (795 at risk, standard error 0.002), 99.3% at 15 years (305 at risk, standard error 0.002), and 99.3% at 20 years (121 at risk, standard error 0.002).
Kaplan-Meier survivorship analysis using aseptic revision of a well-fixed femoral component secondary to severe osteolysis as the endpoint reveals estimated survival proportion of 99.5% at 5 years (1508 at risk, standard error 0.002), 99.4% at 10 years (795 at risk, standard error 0.002), 98.2% at 15 years (305 at risk, standard error 0.006), and 96.6% at 20 years (121 at risk, standard error 0.01).
The Harris hip pain score [24] increased (p < 0.00001) from a preoperative average of 13 (0–44 possible; range, 0–44) to an average of 38 at most recent evaluation (range, 10–44). The Harris hip total score [24] improved (p < 0.00001) from a preoperative average of 46 (0–100 possible; range, 5–46) to an average of 83 at most recent evaluation (range, 18–100). The lower extremity activity scale [63] averaged 10 (1–18 possible; range, 3–17) at most recent evaluation, with 10 corresponding to the ability to be up and about at will in the house and work outside the house in a minimally active job.
Discussion
Tapered titanium porous plasma-sprayed components have performed well in primary THA [2–4, 7, 13–17, 22, 26, 27, 30–32, 39, 40, 44–51, 53, 55–58, 60–62]. To confirm the literature at longer followup we analyzed the survivorship of the first 2000 tapered titanium porous plasma-sprayed femoral components used in primary THA at a single institution. We then sought to define and determine the etiology for failures. Finally, we compared pre- and postoperative clinical pain and function scores.
The current series and research project contain several limitations. The project, while encompassing a large volume of patients across a very large demographic range and across more than two decades of practice, has 7% lost to followup. However, based on studies by King et al. [36] and Joshi et al. [28], these patients lost to followup should not substantially detract from our positive overall results. Patients’ demographics and factors that could act as independent variables for survivorship analysis were not independently investigated in this study, and this may affect survivorship in specific populations and disease etiologies. Finally, we acknowledge surgeon bias in selection of patients to undergo cementless THA using the stem design. As mentioned previously, early in the experience young patients with good bone quality and normal shape of the femora were selected to receive the cementless device. As our experience increased, confidence with the design grew and the inclusion criteria expanded to encompass elderly patients, those with mild to moderate deformity, and those patients with poor bone quality such as Dorr type C [12].
The overall stem survivorship was 98% with respect to stem revision for any reason. At over 20 years, the survivorship with failure of ingrowth as the etiology for aseptic revision was 99%. During its 24-year life, this stem has gone through only two minor revisions. In 1986, 2 years after its introduction, circumferential porous coating was added. The benefit of this addition was documented by Emerson et al. [17] who reported on 126 Mallory-Head porous components with noncircumferential coating followed an average of 7.8 years. These cases demonstrated remote osteolysis of 11% whereas 90 THAs followed for an average of 7.5 years with circumferential porous coating demonstrated no remote osteolysis. Seven stems in our series (0.4%) underwent revision for osteolysis despite being well fixed. This occurred only in stems without circumferential porous coating. No stem with radiographic signs of osteointegration [44–47] failed from loosening at any point in the 24-year period of study. The only other modification of this implant was the addition of a high offset lateralized component option in 1999. The current authors evaluated 49 cases performed before the availability of the offset stem to 49 cases performed after the availability of the offset stem [53]. Not only did the offset stem assist in a higher percentage of patients obtaining appropriate postoperative offset when compared to preoperative, but also it was associated with a decreased incidence of dislocation and an increased ability to obtain appropriate leg length.
This device has demonstrated applicability in all patient age groups. In this series spanning patients aged 19 to 93 years, we noted substantial and sustained improvement in Harris hip pain and total scores, as well as high lower extremity activity scales at most recent encounter. The current authors have specifically investigated the results of this device in this large patient age span. We reviewed our experience with 249 total hip arthroplasties in 201 patients, age 40 years or younger [14]. Sixty-five percent of these patients were men. Minimum followup for this group of patients was 5 years. In this series, four stems were revised: one for pain of an unknown etiology, another for sepsis, and two for aseptic loosening. One of the two for aseptic loosening was a conversion of a cephalomedullary nail to a THA for failed open reduction internal fixation of a femoral neck fracture. Similar results with this device have been reported in patients age 50 or younger by Dowdy et al. [13]. They reported 100% survivorship at 5.3 years. We also reported 39 total hip arthroplasties in 47 patients, age 74 years or older [3]. Twenty percent of the patients in this study demonstrated Dorr Type C bone [12]. Using aseptic loosening as the end point, 100% survivorship was noted. Reitman et al. [60] reported 72 cementless Mallory-Head Porous implants in 62 patients age 65 and older. Nearly 50% were Dorr Type C bone [12]. Followup averaged 13.2 years and no femoral component revisions for aseptic loosening were performed. Concern may remain that implantation of tapered stems may be complicated by varus malposition or proximal calcar fractures. This porous plasma-sprayed titanium tapered cementless stem was associated with high survival and good results with an average postoperative Harris hip score of 88 despite varus malalignment. We reported a review of 1030 primary Mallory-Head Porous femoral components inserted from 1986 to 1997 [4]. Twenty-six hips (2.4%) in 25 patients were placed in 5° or more varus malalignment. With a mean followup of 10 years and minimum 5 years, one well-fixed stem was revised for unexplained pain for an overall survivorship of 96%. With aseptic loosening as the end point there was 100% survivorship. Similar success with varus angulation of a grit-blasted tapered prosthesis has been reported [32]. In a review of 1320 primary Mallory-Head Porous femoral components, 4% suffered an intraoperative proximal fracture [2]. All patients were treated with either a cerclage wire or cable and immediate full weight bearing. At a minimum followup of 2 years, (mean, 7.5 years; range, 2–16 years), no component suffered substantial subsidence. The key to success with these nondisplaced or minimally displaced proximal femoral fractures is the attainment of fracture and prosthetic stability with cerclage wires or cables [37, 59, 64].
Our data confirm high mid- to long-term survivorship with porous plasma-sprayed titanium tapered cementless stems (Fig. 5A–C). Numerous studies document mid- to long-term followup survivorship of porous plasma-sprayed titanium tapered cementless prosthesis in 97% to 100% of patients. We found the porous plasma-sprayed titanium tapered cementless femoral components versatile. They were appropriate for use in all ages and types of femora. Varus malalignment or proximal femoral fracture did not seem to influence survival. From these data and others, cementless femoral fixation with this device provides ongrowth and is durable.
A case is presented with a good long-term result. (A) A radiograph of the left hip of a 41-year-old woman who presented with pain and discomfort reveals the presence of developmental dysplasia. (B) Immediate postoperative radiograph reveals treatment in the form of primary cementless total hip arthroplasty. (C) Radiograph at 20 years postoperative reveals components in satisfactory position and alignment with firm fixation.
References
American Academy of Orthopaedic Surgeons. Osteoarthritis of the hip: Hip joint replacement. Available at: http://www.aaos.org/Research/documents/OAinfo_hip_jointrepl.pdf. Accessed June 11, 2008.
Berend KR, Lombardi AV Jr, Mallory TH, Chonko DJ, Dodds KL, Adams JB. Cerclage wires or cables for the management of intraoperative fracture associated with a cementless, tapered femoral prosthesis: results at 2 to 16 years. J Arthroplasty. 2004; 19(7 Suppl 2):17–21.
Berend KR, Lombardi AV, Mallory TH, Dodds KL, Adams JB. Cementless double-tapered total hip arthroplasty in patients 75 years of age and older. J Arthroplasty. 2004;19:288–295.
Berend KR, Mallory TH, Lombardi AV Jr, Dodds KL, Adams JB. Tapered cementless femoral stem: difficult to place in varus but performs well in those rare cases. Orthopedics. 2007;30:295–297.
Bobyn JD, Jacobs JJ, Tanzer M, Urban RM, Aribindi R, Sumner DR, Turner TM, Brooks CE. The susceptibility of smooth implant surfaces to periimplant fibrosis and migration of polyethylene wear debris. Clin Orthop Relat Res. 1995;311:21–39.
Bobyn JD, Tanzer M, Krygier JJ, Dujovne AR, Brooks CE. Concerns with modularity in total hip arthroplasty. Clin Orthop Relat Res. 1994;298:27–36.
Bourne RB, Rorabeck CH, Patterson JJ, Guerin J. The proximal porous coating alternative for primary total hip arthroplasty. Clin Orthop Relat Res. 2001;393:112–120.
Callaghan JJ, Mallory TH. Optimal fixation for femoral components: cemented or cementless. J Arthroplasty. 1995;10:401–404.
Cameron HU, Keppler L, McTighe T. The role of modularity in primary total hip arthroplasty. J Arthroplasty. 2006;21(4 Suppl 1):89–92.
Christie MJ, DeBoer DK, Trick LW, Brothers JC, Jones RE, Vise GT, Gruen TA. Primary total hip arthroplasty with use of the modular S-ROM prosthesis. Four to seven-year clinical and radiographic results. J Bone Joint Surg Am. 1999;81:1707–716.
DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32.
Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14:231–242.
Dowdy PA, Rorabeck CH, Bourne RB. Uncemented total hip arthroplasty in patients 50 years of age or younger. J Arthroplasty. 1997;12:853–862.
Ellison B, Berend KR, Lombardi AV Jr, Mallory TH. Tapered titanium porous plasma-sprayed femoral component in patients aged 40 years and younger. J Arthroplasty. 2006;21(6 Suppl 2):32–37.
Emerson RH Jr, Head WC, Emerson CB, Rosenfeldt W, Higgins LL. A comparison of cemented and cementless titanium femoral components used for primary total hip arthroplasty: a radiographic and survivorship study. J Arthroplasty. 2002;17:584–591.
Emerson RH Jr, Head WC, Higgins LL. Clinical and radiographic analysis of the Mallory-Head femoral component in revision total hip arthroplasty. A minimum 8.8-year and average eleven-year follow-up study. J Bone Joint Surg Am. 2003;85:1921–1926.
Emerson RH Jr, Sanders SB, Head WC, Higgins L. Effect of circumferential plasma-spray porous coating on the rate of femoral osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 1999;81:1291–1298.
Engh CA, Hopper RH Jr. The odyssey of porous-coated fixation. J Arthroplasty. 2002;17(4 Suppl 1):102–107.
Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;393:137–146.
Eskelinen A, Remes V, Helenius I, Pulkkinen P, Nevalainen J, Paavolainen P. Uncemented total hip arthroplasty for primary osteoarthritis in young patients: a mid-to long-term follow-up study from the Finnish Arthroplasty Register. Acta Orthop. 2006;77:57–70.
Frndak PA, Mallory TH, Lombardi AV Jr: Translateral surgical approach to the hip: The abductor muscle “split”. Clin Orthop Relat Res. 1993;295:135–141.
Gosens T, van Langelaan EJ, Tonino AJ. Cementless mallory-head HA-coated hip arthroplasty for osteoarthritis in hip dysplasia. J Arthroplasty. 2003;18:401–410.
Harris M, Dorr LD, Wan Z, Sirianni L, Boutary M. Total hip arthroplasty with the APR stem and cup. Follow-up of a previous report. J Arthroplasty. 2005;20:828–831.
Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755.
Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian arthroplasty register. 11 years and 73,000 arthroplasties. Acta Orthop Scand. 2000;71:337–353.
Head WC, Mallory TH, Emerson RH Jr. The proximal porous coating alternative for primary total hip arthroplasty. Orthopedics. 1999;22:813–815.
Hozack WJ, Rothman RH, Eng K, Mesa J. Primary cementless hip arthroplasty with a titanium plasma sprayed prosthesis. Clin Orthop Relat Res. 1996;333:217–225.
Joshi AB, Gill GS, Smith PL. Outcome in patients lost to follow-up. J Arthroplasty. 2003;18:149–153.
Kaplan EL, Meier P. Nonparametric estimation from incomplete data. J Am Statistical Assoc 1958;53:457–481.
Keisu KS, Orozco F, McCallum JD 3rd, Bissett G, Hozack WJ, Sharkey PF, Rothman RH. Cementless femoral fixation in the rheumatoid patient undergoing total hip arthroplasty: minimum 5-year results. J Arthroplasty. 2001;16:415–421.
Keisu KS, Orozco F, Sharkey PF, Hozack WJ, Rothman RH, McGuigan FX. Primary cementless total hip arthroplasty in octogenarians. Two to eleven-year follow-up. J Bone Joint Surg Am. 2001;83:359–363.
Khalily C, Lester DK. Results of a tapered cementless femoral stem implanted in varus. J Arthroplasty. 2002;17:463–466.
Kim YH. Long-term results of the cementless porous-coated anatomic total hip prosthesis. J Bone Joint Surg Br. 2005;87:623–627.
Kim YH, Kim JS, Cho SH. Primary total hip arthroplasty with a cementless porous-coated anatomic total hip prosthesis: 10- to 12-year results of prospective and consecutive series. J Arthroplasty. 1999;14:538–548.
Kim YH, Kim JS, Cho SH. Primary total hip arthroplasty with the AML total hip prosthesis. Clin Orthop Relat Res. 1999;360:147–158.
King PJ, Malin AS, Scott RD, Thornhill TS. The fate of patients not returning for follow-up five years after total knee arthroplasty. J Bone Joint Surg Am. 2004;86:897–901.
Kyle RF, Crickard GE. Hip arthroplasty: management problems; periprosthetic fractures associated with total hip arthroplasty. Orthopedics. 1998;21:982–984.
Little BS, Wixson RL, Stulberg SD. Total hip arthroplasty with the porous-coated anatomic hip prosthesis: results at 11 to 18 years. J Arthoplasty. 2006;21:338–343.
Lombardi AV Jr, Berend KR, Mallory TH. Hydroxyapatite-coated titanium porous plasma spray tapered stem: Experience at 15–18 years. Clin Orthop Relat Res. 2006;453:81–85.
Lombardi AV Jr, Mallory TH, Fada RA, Adams JB. Stem modularity: rarely necessary in primary total hip arthroplasty. Orthopedics. 2002;25:1385–1387.
Lombardi AV Jr, Mallory TH, Vaughn BK, Drouillard P: Aseptic loosening in total hip arthroplasty secondary to osteolysis induced by wear debris from titanium alloy modular femoral heads. J Bone Joint Surg Am. 1989;71:1337–1342.
Malchau H, Herberts P, Eisler T, Garellick G, Söderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84(Suppl 2):2–20.
Malik MHA, Gambhir AK, Bale L, Pradhan N, Porter ML. Primary total hip replacement: a comparison of a nationally agreed to best practice and current surgical technique as determined by the North West Regional Arthroplasty Register. Ann R Coll Surg Engl. 2004;86:113–118.
Mallory TH, Head WC, Lombardi AV Jr. Tapered design for the cementless total hip arthroplasty femoral component. Clin Orthop Relat Res. 1997;344:172–178.
Mallory TH, Head WC, Lombardi AV Jr, Emerson RH Jr, Eberle RW, Mitchell MB. Clinical and radiographic outcome of a cementless, titanium, plasma spray-coated total hip arthroplasty femoral component. Justification for continuance of use. J Arthroplasty. 1996;11:653–660.
Mallory TH, Lombardi AV Jr, Leith JR, Fujita H, Hartman JF, Capps SG, Kefauver CA, Adams JB, Vorys GC. Minimal 10-year results of a tapered cementless femoral component in total hip arthroplasty. J Arthroplasty. 2001;16(8 Suppl 1):49–54.
Mallory TH, Lombardi AV Jr, Leith JR, Fujita H, Hartman JF, Capps SG, Kefauver CA, Adams JB, Vorys GC. Why a taper? J Bone Joint Surg Am. 2002;84(Suppl 2):81–89.
McLaughlin JR, Lee KR. Total hip arthroplasty with an uncemented femoral component. Excellent results at ten-year follow-up. J Bone Joint Surg Br. 1997;79:900–907.
McLaughlin JR, Lee KR. Total hip arthroplasty in young patients. 8- to 13-year results using an uncemented stem. Clin Orthop Relat Res. 2000;373:153–163.
McLaughlin JR, Lee KR. The outcome of total hip replacement in obese and non-obese patients at 10- to 18-years. J Bone Joint Surg Br. 2006;88:1286–1292.
McLaughlin JR, Lee KR. Total hip arthroplasty with an uncemented tapered femoral component. J Bone Joint Surg Am. 2008;90:1290–1296.
Mendenhall S. Hospital resources and implant cost management—a 2006 update. Orthopedic Network News. 2007;18:13–19.
Mineo R, Berend KR, Mallory TH, Lombardi AV Jr. A lateralized tapered titanium cementless femoral component does not increase thigh or trochanteric pain. Surg Tech Int. 2007;16:210–214.
Morshed S, Bozic KJ, Ries MD, Malchau H, Colford JM Jr. Comparison of cemented and uncemented fixation in total hip replacement. A meta-analysis. Acta Orthop. 2007;78:315–326.
Park MS, Choi BW, Kim SJ, Park JH. Plasma spray-coated Ti femoral component for cementless total hip arthroplasty. J Arthroplasty. 2003;18:626–630.
Parvizi J, Keisu KS, Hozack WJ, Sharkey PF, Rothman RH. Primary total hip arthroplasty with an uncemented femoral component: a long-term study of the Taperloc stem. J Arthroplasty. 2004;19:151–156.
Purtill JJ, Rothman RH, Hozack WJ, Sharkey PF. Total hip arthroplasty using two different cementless tapered stems. Clin Orthop Relat Res. 2001;393:121–127.
Rao RR, Sharkey PF, Hozack WJ, Eng K, Rothman RH. Immediate weightbearing after uncemented total hip arthroplasty. Clin Orthop Relat Res. 1998;349:156–162.
Reis MD. Hip arthroplasty: management problems; periprosthetic fractures: early and late. Orthopedics. 1997;20:789–800.
Reitman RD, Emerson R, Higgins L, Head W. Thirteen year results of total hip arthroplasty using a tapered titanium femoral component inserted without cement in patients with type C bone. J Arthroplasty. 2003;18(7 Suppl 1):116–121.
Rorabeck CH, Bourne RB, Mulliken BD, Nayak N, Laupacis A, Tugwell P, Feeney D. Comparative results of cemented and cementless total hip arthroplasty. Clin Orthop Relat Res. 1996;325:330–344.
Sakalkale DP, Eng K, Hozack WJ, Rothman RH. Minimum 10-year results of a tapered cementless hip replacement. Clin Orthop Relat Res. 1999;362:138–144.
Saleh KJ, Mulhall KJ, Bershadsky B, Ghomrawi HM, White LE, Buyea CM, Krackow KA. Development and validation of a lower-extremity activity scale. Use for patients treated with revision total knee arthroplasty. J Bone Joint Surg Am. 2005;87:1985–1994.
Schmidt AH, Kyle RF. Periprosthetic fractures of the femur. Orthop Clin North Am. 2002;33:143–152.
Tanzer M, Chan S, Brooks CE, Bobyn JD. Primary cementless total hip arthroplasty using a modular femoral component: a minimum 6-year follow-up. J Arthroplasty. 2001;16(8 Suppl 1):64–70.
Tanzer M, Maloney WJ, Jasty M, Harris WH. The progression of femoral cortical osteolysis in association with total hip arthroplasty without cement. J Bone Joint Surg Am. 1992;74:404–410.
Urban RM, Jacobs JJ, Sumner DR, Peters CL, Voss FR, Galante JO. The bone-implant interface of femoral stems with non-circumferential porous coating. J Bone Joint Surg Am. 1996;78:1068–1081.
Wirz D, Daniels AU, Göpfert B, Morscher EW. Clinical development and current status: Europe. Orthop Clin N Am. 2005;36:63–73.
Xenos JS, Callaghan JJ, Heekin RD, Hopkinson WJ, Savory CG, Moore MS. The porous-coated anatomic total hip prosthesis, inserted without cement. A prospective study with a minimum of ten years of follow-up. J Bone Joint Surg Am. 1999;81:74–82.
Acknowledgments
We thank J. Brooke Harris and Tawnya L. Tucker, M.T., for their assistance in preparation of this manuscript. Cases included in this review include surgeries performed by Bradley K. Vaughn, MD, and Robert A. Fada, MD, during their associations with Joint Implant Surgeons, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the authors (AVL, KRB, THM) receive royalties and have consulting agreements with Biomet, Inc. (Warsaw, IN). Institutional financial support is received from Biomet, Inc. Foundation support has been received from Allergan, Biomet, Inc.; GlaxoSmithKline; Medtronics; Merck; Mount Carmel New Albany Surgical Hospital; Pivotal Research Solutions, Inc., Pozen and Tornier.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Lombardi, A.V., Berend, K.R., Mallory, T.H. et al. Survivorship of 2000 Tapered Titanium Porous Plasma-sprayed Femoral Components. Clin Orthop Relat Res 467, 146–154 (2009). https://doi.org/10.1007/s11999-008-0568-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-008-0568-x