Abstract
We retrospectively studied nine children and adolescents with congenital malformations, large reconstruction after tumor excision, fractures and osteotomies of the upper extremity, and hand trauma with bone and soft tissue defects treated by internal synthesis using a biocopolymer of L- and DL-stereoisomers of lactic acid polymers and trimethylenecarbonate. A total of 52 biodegradable implants were placed in bone. At a minimum followup of 7 months (mean, 17 months; range, 7–22 months), wound healing was uncomplicated; local or systemic inflammatory tissue reactions, foreign body reactions, and infections were not observed. Bone healing was complete. Six biodegradable screws broke during insertion because of inadequate drilling and tapping, and three biodegradable screws had to be replaced because of damage to the screw head during assembly with the screwdriver. Biodegradable copolymers of poly-L-lactic-poly-DL-lactic acid and trimethylenecarbonate can be used safely and effectively for reconstruction and fixation of bone in children and adolescents.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, metal has been the most popular material for fracture fixation; however, although it has excellent results, it is not without complications, including stress shielding [12], accumulation of metals in tissues [19, 20], hypersensitivity [27], growth restriction [40], pain [33], corrosion [1], implant migration [35], and imaging interference [36]. These complications may necessitate revision surgery or removal once the healing process is complete [14]. Because of these issues, a new class of internal fixation devices called bioabsorbable or biodegradable implants is being developed [28, 30]. The main attraction of biodegradable implants to surgeons and patients is they provide the correct amount of strength while the bone is healing and then harmlessly degrade with time [14, 24, 30], overcoming the inherent disadvantages of metal implants [17, 22, 26, 32, 37].
Numerous biodegradable polymers have been approved and have been used safely in surgical applications for the past 35 years, initially as suture materials such as Dexon® suture (polyglycolide [PGA]), Vicryl® suture (PGA/polylactide [PLA], 90:10), PDS® suture (polydioxanone [PDS]), and the Maxon® suture (PGA/tri-methylene-carbonate [TMC]). In addition, fixation devices such as the Endo Screw® and the Suretac® (polyglyconate/TMC) have been developed. During the last two decades, the use of biodegradable materials has expanded to include fixation applications with optimal biomechanical properties for specific clinical uses [9, 10, 14, 18, 23, 30, 38].
Some of the earlier biodegradable implants caused problems because they typically were created from one type of polymer. Some degraded too quickly causing tissue reactions or they took too long to degrade offering no real advantages over metal [3, 4, 39]. In addition, biodegradable implants induce a nonsymptomatic but histopathologically recognizable tissue response that seems to be a phenomenon inherent in the degradation and absorption processes [32]. This is expected and normal as long as it does not cause any clinical signs. However, the incidence of adverse tissue reactions to implants made of PGA related to its rapid degradation rate has been reported from 2.0% to 46.7% [2, 3, 10, 29].
For musculoskeletal applications, the ideal biodegradable material should have mechanical properties equal to those of standard stainless steel implants [15, 24, 30]. Moreover, the use of biodegradable implants for pediatric fracture fixation is particularly appealing because it obviates implant removal. However, little is known regarding the use and effectiveness of biodegradable implants in pediatric patients [9, 23, 24]. In the current study, we used a biodegradable copolymer made of a blend of safe and biocompatible monomers [10, 25, 32] of rigid and elastic L- and DL-stereoisomers of lactic acid polymers and trimethylenecarbonate (TMC-PLLA-PDLLA; Inion OTPS™, Tampere, Finland) for fixation of fractures and osteotomies in pediatric patients. The TMC-PLLA-PDLLA biodegradable copolymer is completely or substantially amorphous, which is ideal for biodegradable implants; TMC adds ductility and toughness to the biocopolymer. Amorphous polymers have a random structure and are completely and more easily degraded by hydrolysis. In contrast, crystalline polymers have a regular internal structure and, because of the orderly arrangement, are slow to degrade. The TMC-PLLA-PDLLA biocopolymer degrades by hydrolysis and is metabolized into carbon dioxide and water, which then is exhaled and excreted. As a guide, the TMC-PLLA-PDLLA biocopolymer loses most of its strength by 18 to 36 weeks. At 36 weeks, it will have lost 90% of its strength and the total resorption time is 2 to 4 years.
The rationale of our retrospective study was to evaluate the use of the TMC-PLLA-PDLLA biodegradable copolymer in pediatric patients and to discuss issues regarding complications and reactions to the breakdown of these materials and their ability to function as fixation devices to the completion of healing; the primary analysis was to evaluate the biologic effect and the secondary analysis was to evaluate the biomechanical performance of these implants in this age group. Evaluation was done using clinical and imaging analysis. The hypothesis was that the TMC-PLLA-PDLLA biodegradable implants could provide stability for bone fixation in children and adolescents with fractures and malformations without any adverse tissue reaction.
Materials and Methods
We retrospectively studied a series of nine pediatric patients in whom screws, plates, and pins made of the TMC-PLLA-PDLLA biocopolymer (Inion OTPS™) were used for management of congenital malformations, fracture fixation, large reconstruction after tumor excision, and hand trauma with bone and soft tissue defects. There were six boys and three girls, aged 2.5 to 15 years (Table 1), admitted and treated at the authors’ institution from July 2006 through November 2007. None of the patients was skeletally mature at the time of surgery. The minimum followup of the patients involved in this study was 7 months (mean, 17 months; range, 7–22 months). All patients were included in the postoperative clinical and imaging evaluations. All patients and their families gave written informed consent to be included in this study. The study was approved by the ethical committee of our hospital.
The indications for biodegradable implants used here were the advantages of no removal operation and postoperative imaging interference given the young ages and the diagnoses of the patients. We intend to use biodegradable implants for internal fixation of similar fractures and osteotomies in all our patients in this age group because of the advantages of these implants. Exclusion criteria were active infection and insufficient quantity or quality of bone for screw anchoring and stable fixation. The operations were performed with the patients under axillary nerve block or general anesthesia. Surgical procedures depended on the diagnosis for each patient (Table 1). Under aseptic surgical technique, the implants were positioned in place after adequate preparation according to the manufacturer’s guidelines. Postoperative assessment included clinical evaluation for signs of foreign body reaction such as localized swelling, pain and sterile discharging sinus [10], imaging evaluation for fracture stability and union, and osteolytic areas next to the absorbable devices [10]. Clinical and imaging evaluations were done by the senior authors of this study (ADK, PJP, PNS).
Results
Adverse tissue reactions to the biodegradable implants were not observed in any of the patients; 52 biodegradable implants were placed in bone (Figs. 1–3). In all patients, wound healing was uneventful. At the latest examination, local or systemic inflammatory tissue reactions, foreign body reactions, infection, or complications related to the adipofascial flap (Patient 9), such as wound healing problems, infection and flap necrosis, or the bone graft (Patient 4), such as osteolysis and graft resorption, were not observed.
The radiographs for Patient 2, a 5-year-old girl with bilateral congenital ulna claw hand and aplasia of the left thumb are shown. (A) Initial treatment included osteotomy of the left ulna stabilized with a Steinmann pin. The Steinmann pin was removed and a corrective osteotomy was performed, which was stabilized with a biodegradable six-hole plate and screws. Postoperative radiographs obtained at (B) 3 and (C) 12 months show partial and complete bone healing of the osteotomy, respectively.
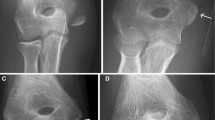
Patient 4 was a 13-year-old boy with a chondroblastoma of the right proximal tibia. His initial treatment consisted of surgical excision of the tumor and reconstruction with a tricortical autogenous bone graft stabilized with a metallic 6.5-mm cancellous bone screw as seen in these (A) lateral and (B) anteroposterior radiographs. The metallic screw was replaced, as is evident in these (C) anteroposterior and (D) lateral views, for imaging and for oncologic evaluation. (E) Frontal and (F) axial MR images obtained 6 months postoperatively show no evidence of tumor recurrence or inflammatory tissue reaction next to the biodegradable screw.
Patient 8 was a 14-year-old boy with distal radius and ulna fractures of the left forearm. His (A) anteroposterior and (B) lateral radiographs show the distal radius and ulna fractures of the left forearm. Open reduction and internal fixation of the ulna fracture were performed with a biodegradable six-hole plate and screws. Closed reduction and percutaneous fixation of the distal radius fracture were performed with two Kirschner wires, as seen on these (C) anteroposterior and (D) lateral radiographs. At the latest examination, 18 months postoperatively, complete bone healing of both fractures is evident on the (E) lateral and (F) anteroposterior radiographs.
Postoperative radiographs showed complete bone healing and union of the osteotomies and fractures; loss of reduction or malunion was not observed in any of the patients. Six biodegradable screws broke at the screw thread during insertion; this was the result of improper technique (inadequate drilling and tapping) before insertion of the screws. In all cases, removal of the broken screws was done intraoperatively by using a forceps, and new screws were positioned. Three biodegradable screws had to be replaced intraoperatively because of damage to the screw head during assembly with the screwdriver. Metallic implants were used in three patients (Patients 6, 7, 8) and were removed 4 weeks postoperatively.
Discussion
Biodegradable technology was first described in the mid1960s [21] for use as resorbable sutures. During the past decades, degradable polymers have been used successfully in many fields of surgery such as craniomaxillofacial surgery, neurosurgery, general surgery, and orthopaedic surgery, including sports medicine for the repair of menisci and rotator cuff tears, foot and ankle surgery, and recently, spine surgery [5, 9, 10, 14, 16, 18, 21, 23, 30, 38]. However, little is known regarding the use of biodegradable implants in pediatric patients. Therefore, we studied a series of patients of this age group in whom implants made of the TMC-PLLA-PDLLA (Inion OTPS™) biodegradable copolymer were used for fixation of fractures and management of malformations.
The small number of patients included in our study and the short-term followup may be considered important limitations. However, the young age of the patients, the variable diagnoses for surgical treatment using biodegradable implants, and the inadequate immobilization and poor compliance with postoperative management of this age group increase the value of the study. Moreover, biodegradable implant-related reactions appear during the period of implant degradation. According to the manufacturer, the biocopolymer mass of the implants used here starts to reduce at 18 to 36 weeks, which is within the period of this study.
Major complications of biodegradable implants include the potential for aseptic inflammatory reaction from wear debris generated during implant resorption, sterile sinus tract formation, osteolysis, synovitis, hypertrophic fibrous encapsulation [4, 6–9, 11, 31], infection, and failure of fixation [31]. In 2528 orthopaedic applications of pins, rods, bolts, and screws made of PGA and PLA homopolymers and copolymers, the rate of a clinically significant local inflammatory, sterile tissue reaction was 4.3% (108 patients); this complication was higher in the PGA group compared with the PLA group of patients (5.3% versus 0.1%) [11]. Localized swelling resulting in a sterile discharging sinus, osteolytic areas next to the absorbable devices, and foreign body reaction occurred within 2 to 4 months after surgery [11]. In a review of more than 2500 fracture fixation cases in which biodegradable implants were used, a 3.6% incidence of bacterial wound infection, 2.3% of nonspecific foreign body reaction, and 3.7% of fixation failure were reported [31]. Others have shown, compared with metallic fixation, biodegradable fixation is associated with a lower incidence of infection [34] and no statistically significant difference in operative or postoperative complications [13]. In our study, although a small series, at a mean followup of 17 months, which is adequate for implant degradation to begin, none of these reactions were observed.
Biodegradable implants in children have shown satisfactory results, especially for treatment of distal humerus physeal fractures [9, 23]. In the current study, we successfully used biodegradable implants for supplementary bone fixation in rapidly healing patients such as children and adolescents in a relatively mechanical stable environment such as the hand and the forearm. In all these patients, protective casting was applied postoperatively for 4 to 6 weeks (Table 1). In the patients with a distal forearm fracture, the biodegradable implant fixation was used supplementary to metallic percutaneous fixation and protective casting for 6 weeks postoperatively. Also, we used a biodegradable screw in the intercondylar notch region of the tibial plateau; although this is not a weightbearing zone, protective weightbearing and bracing also was suggested for 4 weeks postoperatively. Given the young age of the patients and relative stability of these fractures and osteotomies, biodegradable implant fixation should not be intended to be used without appropriate immobilization in load-bearing bones.
In our study, six biodegradable screws of the 52 implants used broke during insertion; this complication occurred in early patients in whom biodegradable implants were used and was attributed to improper technique (inadequate drilling and tapping) before insertion of the screws as recommended by the manufacturer. Also, three biodegradable screws had to be replaced because of damage to the screw head during assembly with the screwdriver. All broken and damaged screws were replaced intraoperatively, and stable fixation was obtained. Fragility of the biodegradable implants may be a problem before the surgeon becomes familiar with the handling characteristics of these implants. Although the number of broken biodegradable screws and screw heads is high when compared with equivalent metallic implants, we believe the major advantages of these implants outweigh these disadvantages.
We have successfully used biodegradable implants for supplementary bone fixation in rapidly healing patients such as children and adolescents in a relatively mechanically stable environment. These implants seem to be biocompatible, because no inflammatory reaction, infection, or other complications were observed during this study. Adequate handling, drilling, and tapping are recommended before inserting the screw. The assembly with the screwdriver to the screw head may be considered a manufacturing design flaw that may be revised.
References
Agins HJ, Alcock NW, Bansal M, Salvati EA, Wilson PD Jr, Pellicci PM, Bullough PG. Metallic wear in failed titanium-alloy total hip replacements: a histological and quantitative analysis. J Bone Joint Surg Am. 1988;70:347–356.
Ambrose CG, Clanton TO. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann Biomed Eng. 2004;32:171–177.
Andriano KP, Pohjonen T, Tormala P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater. 1994;5:133–140.
Bergsma JE, de Bruijn WC, Rozema FR, Bos RR, Boering G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials. 1995;16:25–31.
Böstman OM. Absorbable implants for fracture fixation. J Bone Joint Surg Am. 1991;73:148–153.
Böstman OM. Osteolytic changes accompanying degradation of absorbable fracture fixation implants. J Bone Joint Surg Br. 1991;73:679–682.
Böstman OM. Intense granulomatous inflammatory lesions associated with absorbable internal fixation devices made of polyglycolide in ankle fractures. Clin Orthop Relat Res. 1992;278:193–198.
Böstman OM, Hirvensalo E, Mäkinen J, Rokkanen P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J Bone Joint Surg Br. 1990;72:592–596.
Böstman OM, Mäkelä EA, Södergärd J, Hirvensalo E, Törmälä P, Rokkanen P. Absorbable polyglycolide pins in internal fixation of fractures in children. J Pediatr Orthop. 1993;13:242–245.
Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21:2615–2621.
Böstman OM, Pihlajamäki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop Relat Res. 2000;371:216–227.
Brodke DS, Gollogly S, Alexander Mohr R, Nguyen BK, Dailey AT, Bachus AK. Dynamic cervical plates: biomechanical evaluation of load sharing and stiffness. Spine. 2001;26:1324–1329.
Bucholz RW, Henry S, Henley MB. Fixation with bioabsorbable screws for the treatment of fractures of the ankle. J Bone Joint Surg Am. 1994;76:319–324.
Ciccone WJ 2nd, Motz C, Bentley C, Tasto JP. Bioabsorbable implants in orthopaedics: new developments and clinical applications. J Am Acad Orthop Surg. 2001;9:280–288.
Claes LE. Mechanical characterization of biodegradable implants. Clin Mater. 1992;10:41–46.
Cordewener FW, Schmitz JP. The future of biodegradable osteosyntheses. Tissue Eng. 2000;6:413–424.
El-Amin SF, Attawia M, Lu HH, Shah AK, Chang R, Hickok NJ, Tuan RS, Laurencin CT. Integrin expression by human osteoblasts cultured on degradable polymeric materials applicable for tissue engineered bone. J Orthop Res. 2002;20:20–28.
Hollinger JO, Battistone GC. Biodegradable bone repair materials: synthetic polymers and ceramics. Clin Orthop Relat Res. 1986;207:290–305.
Jorgenson DS, Mayer MH, Ellenbogen RG, Centeno JA, Johnson FB, Mullick FG, Manson PN. Detection of titanium in human tissues after craniofacial surgery. Plast Reconstr Surg. 1997;99:976–981.
Kim YK, Yeo HH, Lim SC. Tissue response to titanium plates: a transmitted electron microscopic study. J Oral Maxillofac Surg. 1997;55:322–326.
Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966;93:839–843.
Liu JG, Ma WH, Xu XX. Orthopaedic applications for biodegradable and absorbable internal fixation of fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2002;16:209–211.
Mäkelä EA, Böstman O, Kekomäki M, Södergård J, Vainio J, Törmälä P, Rokkanen P. Biodegradable fixation of distal humeral physeal fractures. Clin Orthop Relat Res. 1992;283:237–243.
Mäkelä EA, Vainionpää S, Vihtonen K, Mero M, Helevirta P, Törmälä P, Rokkanen P. The effect of a penetrating biodegradable implant on the growth plate: an experimental study on growing rabbits with special reference to polydioxanone. Clin Orthop Relat Res. 1989;241:300–308.
Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopaedic devices. Biomaterials. 2000;21:2335–2346.
Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36:333–338.
Orringer JS, Barcelona V, Buchman SR. Reasons for removal of rigid internal fixation devices in craniofacial surgery. J Craniofac Surg. 1998;9:40–44.
Papagelopoulos PJ, Giannarakos DG, Lyritis GP. Suitability of biodegradable polydioxanone materials for the internal fixation of fractures. Orthop Rev. 1993;22:585–593.
Pietrzak WS, Sarver DR, Verstynen ML. Bioabsorbable polymer science for the practicing surgeon. J Craniofac Surg. 1997;8:87–91.
Raghoebar GM, Liem RS, Bos RR, van der Wal JE, Vissink A. Resorbable screws for fixation of autologous bone grafts. Clin Oral Implants Res. 2006;17:288–293.
Rokkanen P, Böstman O, Vainionpää S, Mäkelä EA, Hirvensalo E, Partio EK, Vihtonen K, Pätiälä H, Törmälä P. Absorbable devices in the fixation of fractures. J Trauma. 1996;40(suppl):S123–S127.
Rokkanen PU, Böstman O, Hirvensalo E, Mäkelä EA, Partio EK, Pätiälä H, Vainionpää S, Vihtonen K, Törmälä P. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21:2607–2613.
Schmidt BL, Perrott DH, Mahan D, Kearns G. The removal of plates and screws after Le Fort I osteotomy. J Oral Maxillofac Surg. 1998;56:184–188.
Sinisaari I, Pätiälä H, Böstman O, Mäkelä EA, Hirvensalo E, Partio EK, Törmälä P, Rokkanen P. Wound infections associated with absorbable or metallic devices used in the fixation of fractures, arthrodeses, and osteotomies. Eur J Orthop Surg Traumatol. 1995;5:41–43.
Stendel R, Krischek B, Pietila TA. Biodegradable implants in neurosurgery. Acta Neurochir (Wien). 2001;143:237–243.
Sullivan PK, Smith JF, Rozzelle AA. Cranio-orbital reconstruction: safety and image quality of metallic implants on CT and MRI scanning. Plast Reconstr Surg. 1994;94:589–596.
Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation: a 5-year experience. Minerva Stomatol. 2005;54:199–206.
Vaccaro AR, Singh K, Haid R, Kitchel S, Wuisman P, Taylor W, Branch C, Garfin S. The use of bioabsorbable implants in the spine. Spine J. 2003;3:227–237.
Wu HC, Shen FW, Hong X, Chang WV, Winet H. Monitoring the degradation process of biopolymers by ultrasonic longitudinal wave pulse-echo technique. Biomaterials. 2003;24:3871–3876.
Yaremchuk MJ, Fiala TG, Barker F, Ragland R. The effects of rigid fixation on craniofacial growth of rhesus monkeys. Plast Reconstr Surg. 1994;93:1–15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Mavrogenis, A.F., Kanellopoulos, A.D., Nomikos, G.N. et al. Early Experience with Biodegradable Implants in Pediatric Patients. Clin Orthop Relat Res 467, 1591–1598 (2009). https://doi.org/10.1007/s11999-008-0537-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-008-0537-4