Abstract
In this study, crack free, well-adhered and transparent zein coatings were obtained on 316L stainless steel substrates by electrophoretic deposition (EPD) employing varying deposition voltages and times. Obtained films were studied by Fourier transform infrared spectroscopy and scanning electron microscopy, and it was shown that the obtained coatings exhibit homogeneous and smooth surfaces. The deposition yield was investigated at various EPD conditions; the highest yield was found at 10 V and 10 min deposition time. The deposition mechanism was discussed by considering chemical reactions occurring during EPD. The EPD method developed here is attractive for the surface modification of metal implants by zein layers aiming at functionalizing surfaces for biomedical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Significant research efforts are currently being devoted to the development and characterization of biopolymers for use in the food, agriculture, and biomedical industries.1 Among the large range of natural polymers available,1,2 zein, an alcohol-soluble protein from corn, as a raw material, has an important place in food processing, biomedical, and pharmaceutical applications due to the fact that it is biodegradable, biocompatible, and derived from renewable sources. Zein is particularly rich in glutamic acid (21–26% w), leucine (20% w), proline (10% w), and alanine (10% w) aminoacids.3 It mainly consists of amine (NH2) and carboxyl (COOH) chemical groups.
Zein-based coatings are being used for several purposes like reducing oil uptake by deep-fat fried foods, protecting active ingredients in chewing gum, achieving controlled release of active ingredients in pharmaceutical tablets, and masking the taste of orally administered drugs.4 Zein can also be combined with hydrophilic and hydrophobic polymers to produce novel materials with improved properties. In the tissue engineering field, zein has been mixed with inorganic materials, such as hydroxyapatite, to produce scaffolds for bone tissue regeneration.5 Porous zein–bioactive glass (BG) scaffolds have been also developed for potential bone tissue engineering applications.6 A straightforward traditional way to make zein films is covering hard surfaces with zein solutions and allowing the solvent to evaporate, forming rigid and glossy, water resistant and protective zein coatings that are resistant to microbial attack.7 To improve the processability and mechanical properties of zein films, a plasticizer such as glycerol, polyol, or fatty acid, is usually needed. Chen et al.8 showed that the tensile strength and the maximal elongation of zein films were improved when glycerol (20 wt%) was added to the initial solution of zein.
In the last twenty years, more than 2000 papers have been published, as recorded in the data base Web of Science™, using the word “zein” as “topic” keyword, of which 772 articles were published in the last 5 years. This indicates the increase in interest, both in the technological and academic fields, on the science and applications of this material. However, there is a significant lack of research efforts regarding the application of electrophoretic deposition (EPD), as a convenient coating technology for biomaterials,9 to develop zein films and coatings for biomedical purposes. EPD is a colloidal processing method which can be used to produce self-standing films and coatings from a wide variety of materials, including ceramics, polymers, and metals and their combinations.10 EPD continues to gain increasing interest both in academia and industry as a coating processing technique, given its high versatility, being cost-effective and not necessitating complicated apparatus.9,10
In the field of natural polymers, research has been carried out on EPD of chitosan and alginate,11–14 while other polymers such as hyaluronic acid and cellulose have been also considered.15,16 To the best knowledge of the authors, EPD of zein has not been widely investigated. In a recent study, the effect of EPD voltage on the physicochemical properties of zein films was considered;17 however, the mechanism of electrophoretic deposition of zein has not been reported. In this contribution, we present a basic study on the EPD of zein and discuss the mechanism behind the EPD technique to obtain zein coatings on 316L stainless steel substrates. The EPD parameters were optimized in order to produce adhesive, homogeneous, and uniform zein coatings with potential applications in the biomedical field.
Experimental methods and materials
Materials
Zein from corn maize in powder form purchased from Sigma-Aldrich (Germany, CAS number 9010-666), ethanol with 99.5% purity (Merck Millipore Germany, CAS number 64-17-5), and glycerol (Sigma-Aldrich Germany, CAS Number 56-81-5) were used without further purification.
Preparation of zein films
Glycerol as a plasticizer with 20 wt% of zein amount was dissolved in a 90 vol% aqueous ethanol solution. Zein powder with a concentration of 0.15 g/mL was gently added to 50 mL of 90 vol% aqueous ethanol–glycerol solution and magnetically stirred for 30 min at room temperature. The pH of the solution was measured with a pH meter (Mettler-Toledo AG FiveEasyTM FE20, 8603 Schwerzenbach, Switzerland). The pH meter was inserted into the solution, and it was waited until a stable pH value was reached. Commercially available 316 L stainless steel (SS) foil (thickness = 0.3 mm) was cut to 15 × 30 mm2 size substrates which were used as both working and counter electrodes. The deposition area was fixed as 15 × 20 mm2, and the distance between the electrodes was 10 mm for all experiments. Before EPD, the substrates were cleaned with ethanol, acetone, and high-purity water in an ultrasonic bath (25 kHz, 100% sweep; Elma, Fisher Bioblock Scientific) for 5 min and were then dried in air at room temperature. Via direct current (DC) EPD, deposition voltages of 3, 5, and 10 V and deposition times of 30 s, 1, 2, 5, and 10 min were considered. The deposition yield was evaluated using an analytical balance with a precision of 0.0001 g. Coated substrates were dried for 24 h in a desiccator at room temperature prior to mass determination and further characterization.
Characterization of zein films
Zein deposits (films) were firstly visually inspected to assess their macroscopic appearance and to determine qualitatively their homogeneity. The deposit weight per area with changing voltages and deposition times was calculated. Bending tests were performed manually in order to qualitatively evaluate the deformation ability of the coatings and the adhesion between the substrate and the deposited zein films. Adhesive tape test was conducted using a cross-hatch cutter (model Elcometer 107) according to the ASTM D3359-B standard method. This is a tape-peel off test wherein lattice patterns with 11 cuts in two orthogonal directions are made in the coating. Pressure-sensitive tape is applied over the lattice and then removed by pulling it out in direction parallel to the substrate in a single procedure. The detached areas of patterned coatings are assigned six quality degrees: 5B for 0% removal, 4B for less than 5% removal, 3B for 5–15% removal, 2B for 15–35% removal, 1B for 35–65% removal, and 0B for more than 65% removal, respectively.18,19
The surfaces of deposited coatings were observed using scanning electron microscopy (SEM, ZEISS model “Ultra Plus” and LEO435VP). Samples were fixed onto stubs with silver paste and investigated under varying voltages and magnifications. To verify the presence of zein and to determine the chemical groups of the films in the wavenumber region 4000–500 cm−1, Fourier Transform Infrared Spectroscopy (FTIR, LabX Thermo Scientific Nicolet 6700, Canada) was carried out. The deposited coatings were scratched and mixed thoroughly with KBr at a concentration of 1% (w/w) and compressed into pellets prior to the measurement in the IR region.
Results and discussion
Microstructure and macroscopic features
Zein powder used in the experiments was characterized under SEM. As shown in Fig. 1, the particles exhibit irregular shape with length varying in the range 40–60 μm and width in the range 10–30 μm.
It is clear from SEM images of the obtained zein coatings (Figs. 2a–c) using EPD under 3, 5, and 10 V at 10 min deposition time that the coatings were homogeneous exhibiting fairly smooth surfaces. It was found that as the voltage increased the surface appearance of the films became coarser, as more material was deposited, as evident when comparing Figs. 2a–c. In fact the deposits obtained at low voltages (3 and 5 V) were very thin and the underlying topography of the stainless steel substrate could be observed (Figs. 2a and 2b). As the voltage increased to 10 V, more deposition occurred and the surface of the stainless steel substrate was fully covered with zein (Fig. 2c).
It was also observed that the coatings obtained were continuous and “transparent” for applied voltages of 3, 5, and 10 V. Even if the optical properties of the coatings were not measured, visual inspection indicated that light could be transmitted through the coatings and the underlying stainless steel substrate was visible. All coatings had qualitative good adhesion to the 316L stainless steel substrates. Figures 3a and 3b show the cross section of coatings deposited on stainless steel substrates at two magnifications. The thickness of such films was typically ~1 μm. The coatings are seen in optical microscope images in Fig. 4. Deposits obtained at 10 V for 10 min without the addition of glycerol could be detached from the stainless steel substrate to form self-standing films, as shown in Fig. 5. The possibility of fabricating homogeneous thin films of zein by EPD suggests potential applications of such films in the biomedical field including drug delivery, wound dressings, nanofluidic devices, cell adhesive surfaces, antibacterial coatings, and tissue engineering.20,21
A typical FTIR spectrum of a zein coating obtained at 10 V and 10 min deposition time is shown in Fig. 6.
The FTIR spectrum shows four bands characteristic of the zein structure and two bands of glycerol. The band corresponding to the stretching of the N–H and O–H bonds of the amino acids appears between 2800 and 3500 cm−1; this band is the amide A band.4 In the 2930 cm−1 region, the stretching band of C–H group is observed that possibly belongs to CH −3 , CH −2 , or CH−.4 Glycerol has stretching bands of C–H groups in the 2881 and 2930 cm−1 regions.22,23 The band occurring between 2240 and 2280 cm−1 belongs to the stretching of nitrile (C≡N) groups.24,25 Another band appears at 1650 cm−1, corresponding to stretching of the carbonyl (C=O) of amide groups belonging to the peptide groups (amide I).4 The band at 1540 cm−1 is amide II4 and corresponds to the angular deformation vibrations of the N–H bond, and lastly, the band at 1230 cm−1 corresponds to the axial deformation vibrations of the C–N bond.4 At 1106 cm−1, the peak corresponding to the stretching of C–O group is observed, which can be ascribed to glycerol.22,23
EPD kinetics
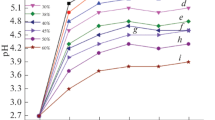
Figure 7a shows the deposit weight per area of the coatings as a function of applied voltage and deposition time. As expected, 10 V, 10 min deposition conditions gave the highest yield in terms of weight gain per surface area.
Zein molecules acquire a positive charge when dispersed in the aqueous ethanol solution at pH 5.3. The hydrophilic part of the zein protein is attracted to the cathode, and deposition occurs on this electrode. The positively charged molecular species present in the solution can be explained considering the pH of the solution at which the amine and carboxyl functional groups would be both protonated as discussed below. As seen in Fig. 7b, the solution used for EPD was uniformly dispersed and stable.
Adhesion between coatings and substrate
In order to qualitatively evaluate the adhesion strength between the coating and the stainless steel substrate, zein-coated substrates were extensively bent and the surfaces were visually inspected. Typical optical images are shown in Fig. 8.
It is observed that after the bending of the substrates coated by zein, no visible flaking or cracking appeared in the films. The bonding between zein layers and the 316L stainless steel substrate was assessed qualitatively by the adhesive tape test, as discussed above. Two specimens obtained at EPD conditions of 10 V and 10 min were tested.
Both samples exhibited intact surfaces after the adhesive test. No peeled areas which would indicate detachment of the zein coating were observed. The adhesion grade for these coatings was found to be “5B”, according to ASTM D3359-B standard.19 This value is similar to those found on other EPD produced biopolymer coatings, e.g., alginate and chitosan. For example, in a study on the EPD of alginate/bioactive glass coatings on stainless steel substrates,12 it was shown that the samples exhibited intact surfaces after adhesive test with no peeled areas, indicating a “5B” scale, according to ASTM D3359-B standard. However, as the number of EPD cycles increased, the extension of peeled areas caused by the tape test increased steadily and at the 3 rd EPD cycle, the maximum value of peeled area was estimated to be ~15% which refers to “3B” scale, according to ASTM D3359-B standard.12 Similarly, for chitosan/hexagonal boron nitride and chitosan/hexagonal boron nitride/titania composites deposited on 316L stainless steel substrates by EPD, the adhesion test values according to ASTM D3359-B standard were 3B and 4B, respectively.26
The mechanism of Zein EPD
Zein’s defining characteristic is to be insoluble in water except in the presence of alcohol, high concentrations of urea, high concentrations of alkali (pH 11 or above), or anionic detergents.3 This is due to its amino acid composition,3 which is responsible for the solubility behavior of zein.
A previous study27 done on the rheology of zein–aqueous ethanol solutions (50–90 vol% ethanol) showed that regardless of zein concentration, the viscosity of the solution decreased, as Kim and Xu28 investigated the size variation of zein particles by a dynamic light scattering technique in order to understand the aggregation behavior of zein in aqueous ethanol solution. It was found that at ethanol concentrations greater than 90 vol%, precipitation of zein occurred. At around 90 vol% ethanol, the aggregation number of zein particles was at the minimum point.
According to these studies, 90 vol% aqueous ethanol solution provides high solubility for zein powders, so this composition of solvent was selected for the present study. The electrical characteristics of zein molecules depend on the pH of the solution, being cationic at low pH due to protonation of amine and carboxyl groups (\NH3 + and \COOH) and anionic at high pH due to de-protonation of these groups (\NH2 and \COO−). In this study, the starting pH of the zein/90 vol% aqueous ethanol solution was 5.3 which is acidic, thus protonation of the amine and carboxyl groups of zein should occur in the solution after dissolution of zein powder. Under the action of an electric field, positively charged zein molecules can move toward the cathode. In aqueous solutions, the cathodic reduction in water results in increasing pH at the cathode surface.11 It is known that the increase in pH results in precipitation of zein at pH 5–6.29 Below the isoelectric point (pH 5.5), zein molecules exhibit positive charge.30 Therefore, it is likely that the deposition of zein is achieved via electrophoretic motion of zein molecules to the cathode and neutralization of the protonated zein macromolecules in the increased pH (5.5) region at the cathode surface. The critical precipitation limit for zein in acetate or phosphate buffer made up in 60 vol% ethanol solution was found to be in the neighborhood of pH 5.4.29 Moreover, the deposition process should result in the formation of insoluble zein films, suggested by the following reaction:

The suggested electrophoretic deposition process of zein molecules on the cathode electrode is illustrated schematically in Fig. 9.
This mechanism proposed for the electrophoretic deposition of zein is similar to that put forward for EPD of chitosan.11 Chitosan is insoluble in water and in organic solvents. However, protonated chitosan can be dissolved in water, water–ethanol, and water–methanol mixtures at low pH and the protonation of the amine groups of chitosan can be achieved in acidic solutions.11,13,14
Conclusions
We have demonstrated the capability of EPD to obtain crack free, well-adhered, robust and transparent zein coatings on 316L stainless steel substrates at varying voltages and deposition times. The mechanism of zein EPD was suggested by considering expected chemical reactions during deposition in acidic pH. Similarly as in chitosan, due to the protonation of amine and carboxyl functional groups in zein under acidic pH, cathodic EPD takes place. The characterization tools helped to investigate the microstructure of zein coatings and the adhesion of zein films to the metallic substrate. Zein coatings have potential applications in the biomedical field to provide a biocompatible, soft and compliant surface for implant/tissue interfaces.
References
Ghanbarzadeh, B, Almasi, H, Biodegradable Polymers, Biodegradation - Life of Science. Agricultural and Biological Sciences, InTech, ISBN 978-953-51-1154-2 (2013)
Rehm, BHA, Microbial Production of Biopolymers and Polymer Precursors: Applications and Perspectives. Caister Academic Press, ISBN: 978-1-904455-36-3 (2009)
Shukla, R, Cheryan, M, “Zein: The Industrial Protein from Corn.” Ind. Crops Prod., 13 171–192 (2001)
Gennadios, A, “Protein-based Films and Coatings.” CRC Press LLC., ISBN: 978-1- 58716-107-9 134-149 (2002)
Corradini, E, Curti, P, Meniqueti, A, Martins, A, Rubira, A, Muniz, E, “Recent Advances in Food-Packing, Pharmaceutical and Biomedical Applications of Zein and Zein-Based Materials.” Int. J. Mol. Sci., 15 (12) 22438–22470 (2014)
Naseri, S, Hum, J, Lepry, WC, Miri, AK, Nazhat, SN, Boccaccini, AR, “Fabrication and Characterization of Zein-bioactive Glass Scaffolds.” Bioinspired Biomim. Nanobiomaterials, 3 (BBN4) 1–6 (2014)
Reiners, R, Wall, AJS, Inglett, GE, “Corn Proteins: Potential for Their Industrial Use. In Industrial Uses of Cereals.” American Association of Cereal Chemists, 285-298 (1973)
Chen, Y, Ye, R, Liu, J, “Effects of Different Concentrations of Ethanol and Isopropanol on Physicochemical Properties of Zein-Based Films.” Ind. Crops Prod., 53 140–147 (2014)
Boccaccini, AR, Keim, S, Ma, R, Li, Y, Zhitomirsky, I, “Electrophoretic Deposition of Biomaterials.” J. R. Soc. Interf., 7 S581–S613 (2010)
Besra, L, Liu, M, “A Review on Fundamentals and Applications of Electrophoretic Deposition (EPD).” Prog. Mater. Sci., 52 (1) 1–61 (2007)
Zhitomirsky, I, Hashambhoy, A, “Chitosan-mediated Electrosynthesis of Organic–inorganic Nanocomposites.” J. Mater. Process. Technol., 191 68–72 (2007)
Chen, Q, Cordero-Arias, L, Roether, JA, Cabanas-Polo, S, Virtanen, S, Boccaccini, AR, “Alginate/Bioglass Composite Coatings on Stainless Steel deposited by Direct Current and Alternating Current Electrophoretic Deposition.” Surf. Coat. Technol., 233 49–56 (2013)
Zhitomirsky, D, Roether, JA, Boccaccini, AR, Zhitomirsky, I, “Electrophoretic Deposition of Bioactive Glass/Polymer Composite Coatings with and without HA Nanoparticle Inclusions for Biomedical Applications.” J. Mater. Process. Technol., 209 1853–1860 (2009)
Wu, LQ, Gadre, AP, Yi, H, Kastantin, MJ, Rubloff, GW, Bentley, WE, Payne, GF, Ghodssi, R, “Voltage-Dependent Assembly of the Polysaccharide Chitosan onto an Electrode Surface.” Am. Chem. Soc., 18 8620–8625 (2002)
Deen, I, Zhitomirsky, I, “Electrophoretic Deposition of Composite Halloysite Nanotube–Hydroxyapatite–Hyaluronic Acid Films.” J. Alloys Compd., 586 (1) S531–S534 (2014)
Chen, Q, de Larraya, UP, Garmendia, N, Lasheras-Zubiate, M, Cordero-Arias, L, Virtanen, S, Boccaccini, AR, “Electrophoretic Deposition of Cellulose Nanocrystals (CNs) and CNs/alginate Nanocomposite Coatings and Free Standing Membranes.” Coll. Surf. B. Biointerfaces, 118 41–48 (2014)
Chen, Y, Ye, R, Wang, J, “Effect of Voltage On The Mechanical and Water Resistance Properties of Zein Films by Electrophoretic Deposition.” Food Bioprocess Technol., 8 (2) 486–491 (2015)
Lakshmi, RV, Basu, BJ, “Fabrication of Superhydrophobic Sol-gel Composite Films Using Hydrophobically Modified Colloidal Zinc Hydroxide.” J. Coll. Interf. Sci., 339 (2) 454–460 (2009)
Standard Test Methods for Measuring Adhesion by Tape Test Designation: D3359-09 ASTM, http://tankpaint.com/wp-content/uploads/D3359-Substrate-Adhesion.pdf
Larkin, AL, Davis, RM, Rajagopalan, P, “Biocompatible, detachable, and free-standing polyelectrolyte multilayer films.” Biomacromolecules, 11 2788–2796 (2010)
Caridade, SG, Monge, C, Gilde, F, Boudou, T, Mano, JF, Picart, C, “Free-standing polyelectrolyte membranes made of chitosan and alginate.” Biomacromolecules, 14 1653–1660 (2013)
Glavcheva-Laleva, Z, Kerekov, St, Pavlov, D, Glavchev, Iv, “Obtaining of Modifiers for Reduced Friction by Esterification of Waste Glycerol from Biodiesel Production and Sylfat 2.” Chemical Engineering and Science, 3 1-6 (2015)
Tsang, SCE, Oduru, WO, Redman, DJ, “Methanol production process.” WO2009130452 A1 Patent (2009)
Derrick, MR, Stulik, D, Landry, JM, Infrared Spectroscopy in Conservation Science. Scientific Tools for Conservation. The Getty Conservation Institute, Los Angeles (1999)
Silverstein, RM, Webster, FX, Kiemle, DJ, Bryce, DL, Spectrometric Identification of Organic Compounds. John Wiley & Sons (2014)
Raddaha, NS, Cordero-Arias, L, Cabanas-Polo, S, Virtanen, S, Roether, JA, Boccaccini, AR, “Electrophoretic Deposition of Chitosan/h-BN and Chitosan/h-BN/TiO2 Composite Coatings on Stainless Steel (316L) Substrates.” Materials, 7 ISSN 1996-1944 1814-1829 (2014)
Fu, D, Weller, C, “Rheology of Zein Solutions in Aqueous Ethanol.” J. Agric. Food Chem., 47 2103–2108 (1999)
Kim, S, Xu, J, “Aggregate Formation of Zein and Its Structural Inversion in Aqueous Ethanol.” J. Cereal Sci., 47 1–5 (2008)
Watson, CC, Arrhenius, S, Williams, JW, “Physical Chemistry of Zein.” Nat. Publ., 137 322–323 (1936)
Juttulapa, M, Sriamornsak, P, “Effect of pH on Stability of Oil-in-Water Emulsions Stabilized by Pectin-Zein Complexes.” Adv. Mater. Res., 506 319–322 (2012)
Acknowledgments
The authors thank Dirk Dippold (University of Erlangen–Nuremberg) for help with FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaya, S., Boccaccini, A.R. Electrophoretic deposition of zein coatings. J Coat Technol Res 14, 683–689 (2017). https://doi.org/10.1007/s11998-016-9885-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-016-9885-2