Abstract
This study focuses on synthesis of waterborne epoxy (WBE) derived from epoxidized soybean oil (ESO) and its compatibility with water-dispersible curing agent Pripol 1009, which is a bioderived long-chain dimer acid. The reaction parameters involved in the synthesis of WBE from ESO have been optimized based on physicochemical properties like hydroxyl value, epoxy equivalent value and degree of solubility of WBE. The WBE obtained after 5 and 6 h of reaction time was found to be of optimum composition with balanced physicochemical properties. The mechanical, thermal and physicochemical properties of WBE obtained after 6 h of reaction time revealed relatively better performance characteristics as compared to ESO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased environmental concerns have fostered the development of solvent-free coating technology in order to reduce the volatile organic compound (VOC) content in the environment. For decades, conventional polymeric materials generated from petroleum-based sources have been utilized in widespread applications ranging from automotive, building construction to aerospace industries and many more. Various polymers such as alkyds, polyurethanes, epoxys and polyols have been among the materials of greatest choice for several applications. Among all the systems, epoxy has the immense potential for adhesion to almost all substrates such as metals, concrete, glass, ceramics and leather that provides good dimensional stability to the coating. It represents a major share of global demand for paints and coatings industry.1 A novel biobased epoxy resin has been studied recently in order to develop materials with excellent mechanical properties and low flammability to serve as a better alternative to DGEBA.2 Similarly in another study by Maiorana et al., a thermally stable biobased thermoset that possessed comparatively better properties than its petroleum-based counterparts was synthesized.3 The prime threat associated with conventional epoxy is often related to the crosslinker and the solvent used which causes skin sensitization and asthma. Hence, the necessity for a solvent-free or water-based coating has become a prime requirement. In addition to that, awareness over global warming and high risks of hazardous carbon emission issues have caused a shift towards renewable resource-based material as a source of monomers as well as curing agents for polymeric coatings. Hence, waterborne epoxys synthesized from renewable resources are receiving tremendous attention due to their abundance as well as low cost, less toxicity, low viscosity, ease of cleaning and environmental benefits. The epoxidized oils derived from renewable feedstock like canola oil, cottonseed oil, linseed oil, castor oil and tung oil are the major sources of raw materials of waterborne biobased epoxy resins. The most adopted method of waterborne epoxy synthesis is the structural modification of hydrophobic backbone into hydrophilic by incorporation of polar groups. These newly formed groups in the polymer backbone further act as sites for crosslinking with the curing agent. Furthermore, petro-based conventional curing agents like amines and anhydrides and solvents have health hazard issues to human life. This has resulted in a great demand for exploring plant-based curing agents to develop a complete water-soluble biobased coating formulation.4,5 In a study, Songqi et al. reported on the synthesis and characterization of a hard and flexible degradable thermoset derived from epoxidized sucrose soyate (ESS) and a dicarboxylic acid that had explored the applicability of naturally occurring acids as a crosslinker for renewable resource-based epoxy systems.6 The utilization of plant and plant-derived products in coatings and adhesives is decades old, but today the scientific community is emphasizing various approaches towards synthesizing a completely water-soluble biobased epoxy system with enhanced novel properties. It has been previously reported that the thermosets obtained from ESS and natural fatty acids derived from citrus fruits have achieved excellent thermal and mechanical properties, especially in coatings.7 This approach provides a significant chance to lower the dependency on petroleum-based raw materials, solvents and curing agents and provides sustainable and green solutions to the polymer industry.
In view of the above observations, the present work is aimed at developing a waterborne epoxy (WBE) from epoxidized soybean oil cured with water-dispersible curing agent Pripol 1009. The physicochemical, mechanical and thermal properties along with chemical resistance and swelling characteristics of the synthesized WBE were evaluated, and a comparative study of these properties with respect to ESO was also established.
Experimental
Materials and methods
ESO was purchased from M/s Makwell Plasticizers Pvt Ltd., India, and diethanolamine, O-phosphoric acid, 2-methyl imidazole and ethanol were supplied by Sigma-Aldrich, USA. Fatty acid dimer (Pripol 1009) was kindly supplied by M/s Croda, India.
Synthesis of waterborne epoxy (WBE)
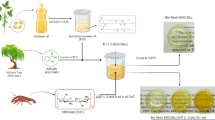
WBE was synthesized from epoxidized soybean oil (ESO) under controlled reaction conditions.8 An Erlenmeyer flask fitted with thermometer, magnetic stirrer and nitrogen gas inlet was charged with 0.1 mol ESO, and subsequently 0.1 mol diethanolamine was added dropwise with continuous stirring at 50°C followed by gradual increase in temperature to 80°C. The epoxy equivalent and hydroxyl value were calculated every hour to monitor the progress of the reaction. After the desired value was obtained, the reaction was terminated by being neutralized with phosphoric acid and then cooled. As a result, highly viscous yellowish-brown liquid of waterborne epoxy (WBE) was obtained. Then, 50 ml of water and ethanol mixture mixed in equal proportion was added to it, and finally a uniform homogenous mixture was prepared. The sample codes of WBE based on the reaction parameters are displayed in Table 1.
Preparation of cured ESO and WBE
The curing agent (hydrogenated fatty acid dimer) was mixed with ESO and WBE in stoichiometric molar ratio of 1:1. Subsequently, catalyst 2-methyl imidazole (1w% of the total formulation) was added to it and vigorously stirred at 120°C for 2 min. For WBE, the prereaction was carried out after mixing by placing it in a vacuum oven at 60°C for 2 h to avoid the occurrence of any kind of phase separation due to solvent evaporation and to obtain a clear and a transparent solution. The solution was then poured into a rectangular-shaped Teflon mold of dimension 10 cm x 5 cm and subjected to cure initially at 120°C for 2 h and finally at 160°C for 2 h.
Characterization
The hydroxyl value (OHV) and epoxide equivalent value (EEW) of ESO and WBE series were determined according to ASTM D 1957-86 and ASTM D 1652, respectively. The changes in viscosities were observed as per ASTM D 1824 with the Lv.3 spindle of Brookfield viscometer.
Solubility test
The solubility of WBE was analyzed in a mixture of equal volume of water and ethanol at room temperature. The density of the cured films was evaluated based on sinker method according to ASTM 792.
Particle size analysis
The average particle size of both WBE-5 and WBE-6 was determined using Malvern particle size analyzer Mastersizer 2000 with a measurement size range of 0.100–1000 µm.
Storage stability
The stability of the WBE systems was evaluated as per ASTM D 1849-95 in a sealed glass bottle under room temperature for 1 month to determine the sound storage period which is an important criterion of a high-quality waterborne coating system.
Solid content
The solid content of WBE dispersion samples was determined according to ASTM D 2834. A sample of 2 g was taken in a petri dish for analysis, and the percentage of solid content was calculated after heating at 100°C in oven for 60 min. The solid content was calculated using the following formula:
W o, weight of empty petri dish; W b, weight of WBE before heating; W a, weight of WBE after heating.
Spectral analysis
FTIR spectra were recorded using thermoscientific FTIR (ATR mode 4000–400 cm−1, M/S Nicolet 6700, USA) spectrophotometer to observe the generations of newly formed as well the disappearance of the chemical bonds. The proton NMR spectra of WBE and ESO were recorded using spectrometer (M/s Bruker 500-NMR, UK) using CDCl3 as solvent to confirm the synthesis of WBE.
Chemicals resistance
The chemical resistance behaviors of both ESO and WBE samples were studied in different acid and alkali exposure medium for a period of 1 week.
Water uptake
The percentage of water absorption value was determined using equation (2).
W d, initial dry weight of the film; W t, weight of the sample at time ‘t’ of immersion.
Furthermore, water diffusion coefficient of ESO and WBE films was determined using the following equation:
D, diffusion coefficient; b, thickness of the specimen; W ∞, maximum water absorption value.
Swelling and crosslink density determination
The swelling experiment for both ESO and WBE samples was performed at room temperature to calculate the effective crosslinking density. The interaction parameter between the solvent used and the polymer was calculated as per the equation reported by Gopalakrishnan et al.9 The average molecular weight between two crosslinks and crosslinking density of both ESO and WBE-6 samples were determined as per the following modified Flory–Rehner equation (3).
V e, volume fraction of epoxy in swollen network; χ, interaction parameter; V s, molar volume of the diffusing agent; M c, average molecular weight between two crosslinks.
Mechanical analysis
-
(a)
Tensile strength
The tensile properties of the dumb-bell-shaped films of dimension 60 mm × 10 mm were analyzed as per ASTM D 882, a universal testing machine (M/s) Instron 3382, UK) fitted with 1 KN capacity load cell at a crosshead speed of 2 mm/min. The samples were conditioned at room temperature and RH 55% for 24 h prior to testing. Ten samples of each formulation were analyzed, and the average value of it is reported in Table 2.
Five coated MS strips of both formulation (ESO and WBE) were prepared using draw-down method (ASTM D 41470-93) of thickness 100–110 μm, and their coating properties viz. adhesion strength (ASTM standard D 3359), scratch hardness (ASTM D 5178), abrasion resistance (ASTM D 4060) and gloss (ASTM D 2457) were evaluated. The average values of it are reported in Table 3.
-
(b)
Scratch hardness
The scratch hardness of the coated samples (70 mm × 25 mm × 1 mm) was evaluated to measure the resistance of the specimens to indentation of scratching using the scratch hardness tester. The coated panels were subjected to loads of different weights until a scratch showing a clear and bare substrate surface was observed.
-
(c)
Abrasion resistance
The abrasion resistance of the coated samples (10 cm × 10 cm) was carried out using M/s. Taber Model 530 Abraser as per ASTM D 4060 method. For every 200 cycles of samples rotation, the loss in weight was recorded. The abrasion resistance values resulted in terms of weight loss per 1000 cycles with abrasive wheel No. CS 10 along with a load of 500 g each has been reported in Table 3.
-
(d)
Adhesion strength
The adhesion strength of coated ESO and WBE samples was determined by crosshatch method using crosscut adhesion tester. The adhesion strength of the coated specimens was calculated using the following equation:
S intact, number of squares remaining intact to the tape applied area; S total, total number of squares present on the tape applied area.
-
(e)
Gloss
The gloss of the coated ESO and WBE samples was evaluated using glossmeter at 45° angle.
Differential scanning calorimetry analysis
The DSC thermograms of both the formulations were recorded out using DSC Q-20 at a heating rate of 10°C min−1 under N2 atmosphere to determine the glass transition temperature (T g) and temperature range of curing.
Results and discussion
Physicochemical analysis of WBE
Physicochemical properties of synthesized formulations were investigated in terms of epoxide equivalent weight, hydroxyl value, viscosity and solubility, and the results thus obtained are summarized in Table 1. The synthesized WBE exhibited an increasing trend in OHV and EEW as a function of reaction over 6 h. This reveals the formation of hydroxyl functionalities and consumption of epoxide ring towards formation of waterborne epoxy.10 Further, it is noticed that the rate of increase in EEW was relatively higher beyond 4 h of reaction time suggesting a higher rate of reaction and hence generating greater number of free hydroxyl groups due to rapid consumption of epoxide groups.11 An enhancement in the viscosity of WBE was also noticed with the increase in reaction time. The reason for such transformation is the increase in hydroxyl content from WBE-1 to WBE-6.12 Moreover, the solubility of all the formulations in water/ethanol mixture has also been assessed and is reported in Table 1. The analysis showed that WBE-5 and WBE-6 were soluble in the water/ethanol blend revealing the presence of sufficient polar hydroxyl and amide groups which induced polarity, thereby resulting in dissolution of WBE-5 and WBE-6.13 No alterations in the physical state like appearance, separation and viscosity were noticed in either the WBE-5 and WBE-6 system until 30 days of storage, confirming that the synthesized WBE has a good shelf life and hence meets the essential requirement of a high standard coating system.14 The solid content value of the resulting WBE dispersion was found to be 45% and the remaining part can be ascribed to solvent (water/ethanol blend) or solvent dissolved substances. In waterborne coatings high solid content implies rapid drying and hence more efficient coating.15,16 Particle size of the WBE was evaluated to compare the stability of both the WBE dispersions and the results are reported in Fig. 1. The observation indicated a drop in mean particle size from 49 µm of WBE-5 to 36 µm of WBE-6 with increase in hydroxyl value. In this WBE dispersion, the average particle size was affected solely by the hydrophilicity of the system as the constituents of WBE-5 and WBE-6 are same. The higher hydroxyl content in the WBE-6 film plays a key role in increasing the compatibility of the polymer with aqueous medium and hence forms smaller dispersed particles.17,18 The lower particle size of WBE-6 and uniform distribution curve indicated a relatively better dispersion stability as compared to that of WBE-5 which has got a broader and bimodal particle size distribution curve. The thickness of the solid films was maintained to be in the range of 1–1.5 mm.
Fourier transform infrared spectroscopic (FTIR) analysis
The synthesis of waterborne epoxy was confirmed using FTIR as is shown in Figs. 2 and 3. In the case of ESO, the characteristic peaks at 822 cm−1 and 1750 cm−1 corresponded to oxirane ring and C=O ester linkage, respectively. In the case of waterborne epoxy, these peaks were found to be unaltered until reaction time of 4 h. However, beyond 5 h of reaction time, intensity of these peaks was found to be reduced, revealing the consumption of oxirane groups and ester groups. This was further confirmed by the appearance of an additional peak at 3322 cm−1 corresponding to OH functionalities in the case of WBE obtained beyond 5 h of reaction time due to the opening of oxirane rings and thereby forming hydroxyl functionalities.19 Additionally, a peak at 1625 cm−1 for WBE samples corresponding to C = O stretching of tertiary amides was observed beyond 3 h of reaction time. This indicated the occurrence of reaction between diethanolamine and ester functionalities present in ESO resulting in the formation of diethanolamides.20 Moreover, the peak at 822 cm−1 had not completely disappeared in the case of WBE-5 and WBE-6 indicating the presence of oxirane group that remained unreacted. These unreacted oxirane groups might contribute towards curing of WBE. 21 The peak at 1750 cm−1 indicated the presence of aliphatic ester in the ESO and WBE chains. The peak corresponding to hydroxyl functionalities at 3322 cm−1 was relatively more prominent in the case of WBE-6 and WBE-7 as compared to that of WBE-5, which revealed the fact that WBE-5 contained a relatively lower number of hydroxyl groups. However the peak intensity corresponding to the oxirane ring at 822 cm−1 was nearly similar for WBE-5 and WBE-6 which confirmed the presence of oxirane group required to assist the crosslinking, whereas in the case of WBE-7 the peak at 822 cm−1 completely disappeared which proves the complete consumption of oxirane groups. The presence of oxirane groups along with hydroxyl groups was required in order to facilitate the crosslinking; hence due to complete disappearance of epoxy band, WBE-7 was not considered for further evaluation as already highlighted in the earlier section. Therefore, based on the higher number of hydroxyl content present in WBE-6 as compared to WBE-5, the former was considered to be the optimum formulation of waterborne epoxy for further evaluation and shall be denoted as WBE in further discussions. The schematic representation of synthesis of WBE is illustrated in Fig. 4.
The FTIR pattern and curing mechanism of ESO and WBE cured with carboxylic groups containing curing agent (Pripol 1009) is presented in Fig. 5. The cured samples of ESO and WBE did not show the presence of the oxirane peak at 822 cm−1 confirming the complete utilization and ring-opening of the epoxy rings during curing. The opening of the oxirane group occurred via nucleophilic substitution reaction between epoxy and curing agent in presence of a catalyst. A band at 1560 cm1 was observed in both cured ESO and WBE which can be attributed to the formation of carboxylate intermediate due to the attachment of epoxy resin with the imidazole catalyst. This result was found to be in accordance with the previous report.22 The catalyst might have attacked the carboxylic groups of the curing agent resulting in the formation of carboxylate intermediate that acted as a nucleophile and attacked the electrophilic carbon thereby initiating the crosslinking reaction. The nucleophile might attack the oxirane ring or the ester linkage in both the cases of ESO and WBE. However, the carboxylate intermediate attacked the very active oxirane ring due to its high strain. The oxygen present in the oxirane ring served as the leaving group that attracted the proton released from curing agent (carboxylic groups) and became protonated.23 The reaction continued until the whole oxirane groups were utilized. Moreover, the peak at 1750 cm−1 present in uncured ESO and WBE was found to have shifted to 1735 cm−1 in cured ESO and WBE. This showed the formation of new ester linkage due to the interaction between aliphatic ester groups of ESO/WBE and carboxylic groups of Pripol. Hence, it can be concluded that both oxirane groups and ester groups act as crosslinking sites in ESO and WBE. Furthermore, it is noticed that in cured ESO, a broad peak appeared at 3340 cm−1 revealing the formation of hydroxyl functionalities. This confirmed the fact that during interaction between oxirane groups and carboxylic groups of curing agent, OH groups were formed due to the protonation of alkoxide generated during the reaction. However, no peak corresponding to OH functionalities could be observed in WBE-6. This suggested that since the number of oxirane and ester groups was relatively less in WBE-6 as compared to ESO due to its conversion into hydroxyl functionality, relatively less amount of curing agent could be consumed towards crosslinking via interaction with the oxirane. In this case the hydroxyl groups present in WBE started to act as crosslinking sites and interacted with the carboxylic end of the remaining curing agent thereby causing consumption of hydroxyl groups which was confirmed by the absence of peak near 3340 cm−1 in cured WBE. This led to the formation of ester, represented by a peak nearly at 1730 cm−1 in the case of WBE. Figure 6 illustrates the possible reaction occurred during curing of ESO and WBE.
Proton NMR analysis
In the case of ESO the peaks at 0.9 ppm, 1.3 ppm and 1.4 ppm correspond to the characteristic protons of methyl and methylene groups present in the polymer backbone. Furthermore, the peak at 2.9 ppm represents presence of protons of oxirane ring in the resin. Moreover, in the case of WBE the peaks at 0.9 ppm, 1.3 ppm and 1.4 ppm remained unaltered indicating no change in the polymer backbone after addition of diethanolamine in the ESO resin as shown in Fig. 7. However, the intensity of the peak at 2.9 ppm was found to be lowered revealing the consumption of oxirane group during the formation of waterborne epoxy. Additionally, a peak at 5.2 ppm was noticed in the case of WBE-6, confirming the presence of hydroxyl group formed due to the ring-opening of oxirane group.24 As diethanolamine acts as a driving force in the ring-opening of epoxides, reduction in protons was observed at 2.9 ppm in WBE, and a signal due to the terminal ethylenic protons appeared at 4.7 ppm.12 The observations were in good accordance with the FTIR analysis and physicochemical properties confirming the synthesis of WBE from ESO.
Mechanical analysis
Mechanical properties of ESO and WBE were evaluated in terms of tensile strength, scratch resistance, abrasion resistance, adhesion and gloss. The analysis revealed that tensile strength and modulus of WBE were relatively higher than that of ESO revealing the relatively higher degree of crosslinking in WBE as compared to ESO. However, the elongation at break for WBE was lower than that of ESO to the tune of 20%. This is attributed to the relatively higher flexibility of ESO films owing to the higher number of free hydroxyl groups in cured ESO as compared to cured WBE corroborating the findings obtained from FTIR analysis. The result was in accordance with findings reported by Mestrovic et al.25 where it was reported that tensile strength varied with the amount of free hydroxyl groups present.
Besides oxirane rings that act as crosslinking sites in uncured ESO and WBE, hydroxyl groups provided additional crosslinking sites for WBE as compared to ESO that resulted in enhanced scratch resistance. These observations were found to be well consistent with the results obtained from swelling study discussed in the earlier section. The higher adhesive strength in WBE as compared to ESO indicated its great adhesion performance, which can be attributed to the greater crosslinking density and stronger intermolecular interaction between the remaining oxirane rings, hydroxyl groups and ester groups with the carboxyl ends of Pripol 1009, thereby generating hydrogen bonding. Greater crosslinking and higher H-bonding interactions resulted in greater adhesion strength. Further, the higher abrasion resistance of WBE as compared to ESO might be due to effective crosslinking that imparts greater elasticity and resistance against deformation. The WBE coating showed higher gloss value as compared with ESO coating which might be due to the development of a highly three-dimensional crosslinked network structure in WBE. This suggests that WBE contains optimum content of reactive sites that leads to greater degree of crosslinking.
Differential scanning calorimetry (DSC) analysis
ESO as well as WBE exhibited two distinct transitions in DSC thermogram as shown in Fig. 8. The transition near −25 and −17°C in the case of ESO and WBE represented glass transition temperature of the resins. A higher Tg for WBE also indicated denser crosslinking and the absence of unreacted moieties.26,27 The second transition in case of ESO and WBE was found to be in the range of 140–268 and 146–283°C, which represented the crosslinking due to the opening up of oxirane rings. The onset curing temperature of ESO and WBE was found to be 140 and 146°C, respectively, whereas the final curing temperature of ESO and WBE was 268 and 283°C, respectively as shown in Table 4. Moreover, a single peak of the curing without any shoulder could be noticed in both the cases of ESO and WBE representing a single-stage curing process. However, the higher curing temperature of WBE might be attributed to the slow diffusion of molecular chains due to its greater low-temperature stability. The inclusion of amine groups in WBE might be responsible for the improvement in lower-temperature stability due to its antioxidant property.28,29 The enthalpy of reaction in WBE was also higher than ESO, suggesting greater crosslinking.
Chemicals resistance
Resistance of ESO and WBE was evaluated against various chemicals viz. 5% NaOH, 5% NaCl and 5% HCl. Both the systems (i.e., ESO and WBE-6) did not exhibit good alkali resistance owing to the presence of ester linkages which are susceptible to alkali attack.30 Deterioration in gloss and change in coloration were observed in the samples immersed in alkali medium which is shown in Fig. 9. Moreover, in all the chemical medium WBE showed relatively higher resistance as compared to that of ESO. This is attributed to the excess hydroxyl content in cured ESO rather than that of WBE, as observed from FTIR analysis which caused swelling of the ESO films in various media. These hydroxyl groups of cured ESO might also interact with the polar chemical media and lead to the disruption of its chemical integrity.31 The lower chemical resistance of ESO than that of WBE also suggested the possibility of the presence of voids, free volume that in turn resulted in the increased affinity towards external chemical media.
Swelling and crosslink density
The swelling property of ESO and WBE was evaluated in order to predict their crosslinking and wetting ability. The results were obtained in terms of swelling coefficient (S), crosslink density (Q) and average molecular weight between two crosslinks (M c) and are summarized in Table 5 and Fig. 10. The analysis showed higher swelling of ESO sample in all the solvents as compared to WBE that may be ascribed to the presence of a higher number of free hydroxyl groups present in ESO that act as active sites for the solvent molecules to interact. This was in accordance with the findings observed in earlier sections. Moreover, M c calculated for WBE was found to be lower as compared with ESO. This revealed the highly crosslinked network in WBE, suggesting that the crosslinking in WBE occurred via linkages between the carboxylic end of curing agent and oxirane, ester and hydroxyl groups, while in case of ESO it is crosslinked via a single path (i.e. linkages formed between oxirane groups of ESO and carboxyl end groups of curing agent). Moreover, the higher values of crosslinking density for WBE confirmed the dense crosslink network corroborating the earlier observation. The higher swelling of ESO in all the solvents can be ascribed to relatively loose packing of crosslinks as compared to WBE that allows easy migration of solvent into the ESO network (Table 5).
Water uptake
Figure 11 and Table 6 represents the water absorption characteristics of the ESO and WBE immersed in water for 20 days. The analysis showed that both ESO and WBE exhibited Fickian behavior with an increasing trend of the water uptake (%) with respect to the immersion period which subsequently attained a saturation point. Moreover, it was noticed that ESO exhibited a relatively higher water uptake percentage as compared to WBE over the entire period of experiment. Further, it was noticed that saturation point for ESO was reached after almost 9 days of immersion, whereas it was delayed to 14 days in case of WBE. This revealed a reduced water uptake tendency of WBE as compared to ESO.32,33 The higher water uptake percentage of ESO is related to the presence of the hydroxyl groups in the cured ESO unlike cured WBE. The free hydroxyl groups in ESO provided bonding sites to water molecules via dipole interactions that resulted in higher affinity to water. Further the delayed saturation point in WBE confirmed the higher degree of crosslinking in comparison with ESO counterpart corroborating the earlier observations. A higher diffusion coefficient value was also observed in the case of ESO that showed greater diffusion of water molecules into ESO film and hence less crosslinking than WBE.
Conclusion
WBE was synthesized via modification of epoxidized soyabean oil (ESO) using diethanolamine under controlled reaction conditions. The physicochemical properties and FTIR pattern of WBE obtained after 6 h of reaction time were found to be of optimum composition. Then a comparative analysis of ESO and WBE-6 was carried out. The NMR pattern revealed a successful synthesis of WBE from ESO. The FTIR pattern of cured ESO and WBE exhibited their compatibility with the carboxylic groups containing water-dispersible curing agent Pripol 1009. The higher degree of crosslinking was observed in the case of WBE as compared to ESO through mechanical property analysis. Both the samples showed excellent chemical resistance except towards alkali. WBE showed an excellent combination of coating properties with high scratch and abrasion resistance as well as outstanding adhesion. The DSC analysis revealed single-step curing of both ESO and WBE. Swelling and crosslink density analysis confirmed a higher degree of crosslinking in WBE and presence of a greater number of hydroxyl groups in ESO after curing. These results were further supported by water uptake analysis where a delayed saturation, lower water uptake (%) and lower diffusion coefficient value of WBE were obtained as compared to ESO.
References
Pradhan, S, Pandey, P, Mohanty, S, Nayak, SK, “Insight on the Chemistry of Epoxy and Its Curing for Coating Applications: A Detailed Investigation and Future Perspectives.” Polym. Plast. Technol. Eng., 55 (8) 862–877 (2016)
Wan, J, Gan, B, Li, C, Molina-Aldareguia, J, Li, Z, Wang, X, Wang, DY, “A Novel Biobased Epoxy Resin with High Mechanical Stiffness and Low Flammability: Synthesis, Characterization and Properties.” J. Mater. Chem. A, 3 (43) 21907–21921 (2015)
Maiorana, A, Spinella, S, Gross, RA, “Bio-Based Alternative to the Diglycidyl Ether of Bisphenol A with Controlled Materials Properties.” Biomacromolecules, 16 (3) 1021–1031 (2015)
Ding, C, Matharu, AS, “Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use.” ACS Sustain. Chem. Eng., 2 (10) 2217–2236 (2014)
Baroncini, EA, Kumar Yadav, S, Palmese, GR, Stanzione, JF, “Recent Advances in Bio-Based Epoxy Resins and Bio-Based Epoxy Curing Agents.” J. Appl. Polym. Sci., 133 (45) 44103 (2016). doi:10.1002/app.44103
Ma, S, Webster, DC, Jabeen, F, “Hard and Flexible, Degradable Thermosets from Renewable Bioresources with the Assistance of Water and Ethanol.” Macromolecules, 49 (10) 3780–3788 (2016)
Ma, S, Webster, DC, “Naturally Occurring Acids as Cross-Linkers to Yield VOC-Free, High-Performance, Fully Bio-Based, Degradable Thermosets.” Macromolecules, 48 (19) 7127–7137 (2015)
Shah, MY, Ahmad, S, “Waterborne Vegetable Oil Epoxy Coatings: Preparation and Characterization.” Prog. Organ. Coat., 75 248–252 (2012)
Gopalakrishnan, S, Fernando, TL, “Bio-Based Thermosetting Tough Polyurethanes.” Der. Chem. Sin, 2 (5) 54–64 (2011)
Wakita, K, Kuwabara, H, F, N, Tatebe, C, Sato, K, Akiyama, H, “A Comparative Study of the Hydroxyl and Saponification Values of Polysorbate 60 in International Food Additive Specifications.” Am. J. Anal., 5 199–204 (2014)
Dinda, S, Patwardhan, AV, Goud, VV, Pradhan, NC, “Epoxidation of Cottonseed Oil by Aqueous Hydrogen Peroxide Catalysed by Liquid Inorganic Acids.” Bioresour. Technol., 99 3737–3744 (2008)
Biswas, A, Adhvaryu, A, Gordon, SH, Erhan, SZ, Willett, JL, “Synthesis of Diethylamine-Functionalized Soybean Oil.” J. Agric. Food Chem., 53 (24) 9485–9490 (2005)
Liu, M, Mao, X, Zhu, H, Lin, A, Wang, D, “Water and Corrosion Resistance of Epoxy–Acrylic–Amine Waterborne Coatings: Effects of Resin Molecular Weight, Polar Group and Hydrophobic Segment.” Corros. Sci., 75 106–113 (2013)
Singh, AP, Gunasekaran, G, Suryanarayana, C, Baloji Naik, R, “Fatty Acid Based Waterborne Air Drying Epoxy Ester Resin for Coating Applications.” Prog. Organ. Coat., 87 95–105 (2015)
Zhaoying, Z, Yuhui, H, Bing, L, Guangming, C, “Studies on Particle Size of Waterborne Emulsions Derived from Epoxy Resin.” European Polym. J., 37 (6) 1207–1211 (2001)
Lee, SK, Kim, BK, “High Solid and High Stability Waterborne Polyurethanes via Ionic Groups in Soft Segments and Chain Termini.” J. Colloid Interface Sci., 336 (1) 208–214 (2009)
Saalah, S, Abdullah, LC, Aung, MM, Salleh, MZ, Biak, DRA, Basri, M, Jusoh, ER, “Waterborne Polyurethane Dispersions Synthesized from Jatropha Oil.” Ind. Crops Products, 64 194–200 (2015)
Bao, LH, Lan, YJ, Zhang, SF, “Synthesis and Properties of Waterborne Polyurethane Dispersions with Ions in the Soft Segments.” J. Polym. Res., 13 (6) 507–514 (2006)
Sharmin, E, Ashraf, SM, Ahmad, S, “Synthesis, Characterization, Antibacterial and Corrosion Protective Properties of Epoxies, Epoxy-Polyols and Epoxy-Polyurethane Coatings from Linseed and Pongamia Glabra Seed Oils.” Int. J Biol. Macromol., 40 (5) 407–422 (2007)
Lee, CS, Ooi, TL, Chuah, CH, “The Effect of Reaction Temperature on Retaining Oxirane Oxygen Contents in the Synthesis of Epoxidized Diethanolamides.” Am. J. Appl. Sci., 6 (1) 72 (2009)
Lopez Tellez, G, Vigueras Santiago, E, Hernandez Lopez, S, Bilyeu, B, “Synthesis and Thermal Cross-Linking Study of Partially Aminated Epoxidized Linseed Oil.” Des. Monomers Polym., 11 435–445 (2008)
Shikha, D, Kamani, PK, Shukla, MC, “Studies on Synthesis of Water-Borne Epoxy Ester Based on RBO Fatty Acids.” Prog. Organ. Coat., 47 (2) 87–94 (2003)
Matejka, L, Lovy, J, Pokorny, S, Bouchal, K, Dusek, K, “Curing Epoxy Resins with Anhydrides. Model Reactions and Reaction Mechanism.” J. Polym. Sci.: Polym. Chem. Ed., 21 (10) 2873–2885 (1983)
Sharmin, E, Ashraf, SM, Ahmad, S, “Synthesis, Characterization, Antibacterial and Corrosion Protective Properties of Epoxies, Epoxy-Polyols and Epoxy-Polyurethane Coatings from Linseed and Pongamia Glabra Seed Oils.” Int. J. Biol. Macromol., 40 (5) 407–422 (2007)
Petrović, ZS, “Polyurethanes from Vegetable Oils.” Polym. Rev., 48 (1) 109–155 (2008)
Supanchaiyamat, N, Hunt, AJ, Shuttleworth, PS, Ding, C, Clark, JH, Matharu, AS, “Bio-Based Thermoset Composites from Epoxidised Linseed Oil and Expanded Starch.” RSC Adv., 4 (44) 23304–23313 (2014)
Tan, SG, Chow, WS, “Thermal Properties, Curing Characteristics and Water Absorption of Soybean Oil-Based Thermoset.” Expr. Polym. Lett., 5 (6) 480–492 (2011)
Kocaman, S, Ahmetli, G, “A Study of Coating Properties of Biobased Modified Epoxy Resin with Different Hardeners.” Prog. Organ. Coat., 97 53–64 (2016)
Tan, SG, Chow, WS, “Curing Characteristics and Thermal Properties of Epoxidized Soybean Oil Based Thermosetting Resin.” J. Am. Oil Chem. Soc., 88 (7) 915–923 (2011)
Xia, C, Wang, L, Dong, Y, Zhang, S, Shi, SQ, Cai, L, Li, J, “Soy Protein Isolate-Based Films Cross-Linked by Epoxidized Soybean Oil.” RSC Adv., 5 (101) 82765–82771 (2015)
Enns, JB, Gillham, JK, “Effect of the Extent of Cure on the Modulus, Glass Transition, Water Absorption, and Density of an Amine-Cured Epoxy.” J. Appl. Polym. Sci., 28 (9) 2831–2846 (1983)
Zlatanić, A, Lava, C, Zhang, W, Petrović, ZS, “Effect of Structure on Properties of Polyols and Polyurethanes Based on Different Vegetable Oils.” J. Polym. Sci. Part B: Polym. Phys., 42 (5) 809–819 (2004)
Zanni-Deffarges, MP, Shanahan, MER, “Diffusion of Water into an Epoxy Adhesive: Comparison Between Bulk Behaviour and Adhesive Joints.” Int. J. Adhes. Adhes., 15 (3) 137–142 (1995)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pradhan, S., Pandey, P., Mohanty, S. et al. Synthesis and characterization of waterborne epoxy derived from epoxidized soybean oil and bioderived C-36 dicarboxylic acid. J Coat Technol Res 14, 915–926 (2017). https://doi.org/10.1007/s11998-016-9884-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-016-9884-3