Abstract
Protection against bacterial contamination remains a demand for healthcare textiles such as wound dressings to reduce or eliminate hospital-acquired infections related to antibiotic-resistant bacteria. We report herein a simple and straightforward in situ approach to deposit copper oxide and titanium oxide nanoparticles onto cotton fabric using a sonochemical-mediated sol–gel method. Modification of the cotton surface was achieved by incorporation of citric acid (CA) and polyethylene glycol (PEG) to improve the attachment of the nanoparticles and reduce the attachment of bacteria to the cotton surface, respectively. The resultant cotton fabric was used against Escherichia coli as a Gram-negative bacterium and Staphylococcus aureus as a Gram-positive bacterium in dark condition as an in vitro model for treatment of bacterial wound infection. The effects of different treatment parameters including duration and frequency of ultrasonic irradiation, surface modification with PEG and/or CA, and cotton chemical composition with different metal oxide molar ratios on the antibacterial activity of the treated cotton fabric were studied. All treated cotton fabrics showed antibacterial activity, with higher efficiency for those coated with CuO or CuO/TiO2 (1:1 molar ratio) among the single metal oxide and composite-modified cotton fibers, respectively. Our results show that such functionalized cotton fibers could actively fight the spread of bacterial infections by preventing bacterial adhesion, enabling more efficient bonding, and ultrasonically promoting generation of nanoparticles and their strong adhesion to the fabric surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Substantial effort has led to the use of antimicrobial finishes for production of antibacterial fabrics to reduce or eliminate hospital-acquired infections (HAIs) related to antibiotic-resistant bacteria. Many conventional antimicrobial agents have been employed to provide textiles with antibacterial activity, including inorganic salts, antibiotics, phenolics, formaldehyde, urea, and amine derivatives, many of which are toxic to humans.1
Therefore, there is currently interest in the development of efficient, nontoxic, durable, and cost-effective antimicrobial finishes for textiles. Nanoparticles are currently being investigated as antibacterial agents against both Gram-negative and Gram-positive microorganisms. Among these, nano-TiO2-based materials have attracted significant attention for application on self-cleaning, hygienic textiles or as antibacterial coatings, owing to their nontoxicity, low cost, and environmentally benign nature.2–4 Most results on titania-based antimicrobials indicate enhanced antimicrobial activity in the ultraviolet (UV) region.5 However, the wide bandgap of TiO2 makes this semiconductor inactive under visible-light irradiation. Therefore, new research has focused on modification of the electronic and optical properties of TiO2 to expand its catalytic activity for practical use in medical applications under visible-light irradiation or in dark conditions6,7 through incorporation of coupled, doped, and hybrid TiO2-based materials.8–11
In addition to modification of the optical properties of TiO2, use of more than one antimicrobial agent (known as combination antimicrobial therapy) is another approach based on incorporation of other nanoparticles, to prevent or reduce emergence of resistant strains. Among such incorporated nanoparticle materials, CuO has also attracted significant attention due to the extreme susceptibility of microorganisms to copper,12 the fact that copper is essential to human health,13 the use of wound-healing treatments containing copper,14 the low sensitivity of human tissue to copper,15 the extremely low risk of adverse reactions from dermal exposure to copper,12 and the adsorption of copper or copper oxide through the skin.16 Copper is an essential trace element involved in numerous human physiological and metabolic processes,12 including skin formation17 and wound healing.18 The improved antibacterial activity of such Cu/TiO2 composites in the dark19 and under visible light7,20 has been well reported, especially at higher copper content. To the best of our knowledge, there are no reports on the antibacterial properties of CuO/TiO2/cotton nanocomposites. However, Schmidt et al. studied a solvothermally prepared copper-modified TiO2 composite for the realization of photocatalytically active and antimicrobial viscose fabrics.21
Since the initial reports on the ultrasonic-mediated deposition technique from Bar Ilan University,22 sonochemical coating of cotton with copper oxide, zinc oxide, and titanium oxide nanoparticles to produce valuable functional textiles with high quality, stability, and antibacterial activity has been reported.23 Ultrasound has been widely used in chemistry, dyeing, finishing, and cleaning industries because of its obvious advantages such as no requirement for posttreatment,24 short processing time,25 increased high-velocity interparticle collisions to avoid formation of larger particles,26 simplicity, and energy efficiency.27
According to some reports, nosocomial infections are estimated to occur in at least 5% of all hospitalized patients,28 and 1.4 million people are affected by HAIs throughout the world each day.29 The most common wound-infection- and HAI-mediated pathogens include staphylococci (especially Staphylococcus aureus), Pseudomonas, and Escherichia coli. These bacteria can also develop multidrug resistance, so single-compound therapy will not be effective against them. Moreover, due to the development of various bacterial strains resistant to antibiotics,30 use of antibiotics to control acquisition of infections is not cost-effective and thus not reasonable.
Wound dressings and devices form an important segment of the medical and pharmaceutical wound care market worldwide. Currently, much research effort is being focused on acceleration of wound healing through the use of systematically designed dressing materials with antibacterial activity to treat wounds infected with antibacterial-resistant microorganisms. In this regard, application of nanotechnology has been extensively studied to introduce antimicrobial activity into medical textiles as well as to control the growing problem of multidrug-resistant bacteria-related infections.31 However, due to the lack of attraction between inorganic particles and polymeric materials such as textiles,32 a problem exacerbated for nanoparticles with high specific surface area, surface modification of textiles with nanoparticles is not permanent. Therefore, structural modification of the textile surface is performed by utilizing different approaches such as introduction of a variable density of negative groups, such as –COO−. Crosslinking of cellulose is an important textile chemical process which can be defined as stabilization of cellulosic fibers through chemical reaction. Besides, some natural (e.g., collagen, honey)33 or synthetic-based products (e.g., polyethylene glycol, PEG)34 can be regenerated for use as effective antimicrobial components in combination therapy to prevent local infections. There are some reports on use of PEG-based composites as wound dressings.35–37
As a chemical raw material, cellulose has been used for about 150 years.38 Cellulose-based materials with amphiphobicity and adhesion-inhibition effects on bacteria could be used to develop novel nanostructured materials with designed functionalities. The combination of the specific chemical properties of the guest substrate and the unique physical features of cellulose materials as a template thus holds great promise for use in various fields such as self-cleaning surfaces, antifouling surfaces, and intelligent membranes.39 The chemical inertness of the surface of cellulose fibers, which represents a serious obstacle to modification of the functional surface by coating, could be addressed by using metal oxide as a guest substrate. Hydroxyl groups on the surface of cellulose fibers provide a suitable substrate for deposition of metal oxide thin films via a surface sol–gel process.40
We report herein an easy method for ultrasonic sol–gel in situ synthesis of a novel TiO2/CuO/PEG nanocomposite with subsequent impregnation on a cotton bandage to achieve superior bacterial growth inhibition under dark conditions compared with single TiO2- and CuO-treated cotton bandages. The effect of pretreatment of the coated bandage cotton using citric acid as a crosslinking agent on the efficiency of the resultant antimicrobial textiles and on their durability against washing was also examined. To evaluate the efficiency of the biocidal-coated cottons, two methods including the disk diffusion method and dynamic shake method were used against Escherichia coli (E. coli, ATCC 25922) as model Gram-negative bacterium and Staphylococcus aureus (S. aureus, ATCC 6538) as model Gram-positive bacterium. The phase composition and surface morphology were investigated by scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis, respectively. The obtained results lead to a new direction in the design of multicomponent nanomaterials for enhanced bactericidal effect, which might help combat emerging pathological bacteria that cause wound infections.
Experimental
Materials
Titanium(IV) isopropoxide, triethylamine, ethanol, copper acetate, citric acid (CA), sodium hypophosphite, and hydrochloric acid were purchased from Sigma-Aldrich. Poly(ethylene glycol) was purchased from Fluka. Raw woven cotton fabric (100%) was used with mass of 146 g/m2, weft yarn density of 25 yarns/cm, 10 × 10 cm2 size, and thickness of 0.7 mm.
Sol preparation and application onto cotton fabric
The sol–gel technique provides a new way to functionalize fabrics, improving their chemical composition and physical properties.41 For textile materials, this technique can modify the surface and thereby improve the antibacterial finishing.
Two single metal oxide sols were prepared as follows: As in other typical TiO2 sol syntheses, titanium isopropoxide (0.01 mol) was added to ethanol (50 ml) under vigorous stirring, then triethylamine (0.005 mol) was added as solution stabilizer followed by stirring (200 rpm) for 15 min under inert environment. Another solution was then prepared separately as follows: dilute hydrochloric acid (2 M, 1 ml) and water (0.5 ml) were added to ethanol (50 ml) and mixed well using a magnetic stirrer for 10 min. This solution was then added dropwise into the above mixture consisting of titanium isopropoxide, ethanol, and triethylamine. This mixture was stirred vigorously at room temperature for hydrolysis. Subsequently, after continuous stirring for 2 h, a yellowish transparent sol was obtained (solution A).
As in other typical CuO sol syntheses, 2 mmol Cu(Ac)2·2H2O was added into 20 ml double-distilled water. Secondly, ammonium hydroxide (NH3·H2O) was dropped into the aqueous solution slowly until the pH reached 7 (solution B). After adding the ammonium solution, the color of the reaction mixture changed from light blue to deep blue, then became dark brown. To achieve binary metal oxide composite sols with TiO2:CuO molar ratio of 1:1, 1:2, and 2:1, volumes of solution A and B at ratios of 1:1, 1:2, and 2:1 were added together, respectively. All prepared sols were kept for one day for aging.
Before coating, the cotton fibers were washed first by water and detergent (Soap Liquid, detergent free of optical brighteners and softeners) at 50°C for 30 min to remove impurities such as wax and fat, then washed several times using a large amount of deionized water. They were further cleaned in acetone for 10 min and dried at room temperature. The prepared two single sols and three composite sols with different molar ratios were coated onto freshly cleaned cotton fabric using an ultrasonic-mediated dip-coating technique as follows: 1 piece of 10 × 10 cm2 bandage (~0.7 g) was added to each prepared sol in a 100-ml sonication flask. The flask was placed in an ice–water cooling bath, maintaining a constant temperature of 30°C during sonication. The reaction mixture was irradiated for different time intervals of 1, 2, and 5 h using a high-intensity ultrasonic horn (Ti horn, 20 kHz, 750 W at 70% efficiency). This method serves as a simple green route for homogeneous coating of the cotton fibers without significant damage to their structure. The fabric was then dipped in ethanol, washed with water to remove any excess detergent, and finally dried in an oven at different temperatures including 160 and 200°C for 10 and 7 min, respectively. At the end of the coating application, the color of the fabric changed depending on the treatment condition as seen in Fig. 1.
In another experiment, cleaned cotton fabric was first pretreated with 10% aqueous poly(ethylene glycol) (PEG 600) solution at 25°C for 60 min. Then, the PEG-pretreated cotton fabric was treated in an ultrasonic bath containing separately the aforementioned five sols plus citric acid (CA) as crosslinking agent (6%, o.w.f = on weight of fabric) and sodium hypophosphite (SHP) as catalyst (4%, o.w.f). PEG/CA/metal oxide-modified and metal oxide-modified cottons were obtained according to the previous procedure and used for antimicrobial testing.
For convenience, the cotton fabrics treated under the different conditions are denoted using the acronyms presented in Table 1.
Characterization
The surface morphology of cotton fibers in untreated form and when loaded with CuO and TiO2 nanoparticles was studied using a Philips XL30 scanning electron microscope (SEM). The crystallinity and average particle size were analyzed based on X-ray diffraction (XRD) analysis using an X-ray diffractometer (Bruker, D8 Advance, Cu Kα). The patterns were recorded in the 2θ diffraction range from 10° to 80° at scan speed of 2°/min in steps of 0.040°. The hydrophobicity of the treated cotton was also evaluated by measuring the water droplet contact angle under a Philips UV-C lamp with intensity of 4 mW cm−2, centered at 365 nm in atmospheric air and at room temperature. Contact angles were determined from corresponding pictures [obtained from a charge-coupled device (CCD) camera–lens optical system, OCA 15 plus] using an image-processing algorithm. Electron microscopy studies were carried out by transmission electron microscopy (TEM, Philips CM 10).
To quantify the amount of nanoparticles present on the fibers, one of the simplest methods is to burn the organic support and weigh the final inorganic residue. The amounts of TiO2 and CuO loaded on the cotton fabric were determined by atomic absorption spectrometry (AAS, Unicam 939). Approximately 1 g of each sample was burned. The nanoparticle support was eliminated after burning the CuO- and TiO2-coated cotton fibers at 500°C for 3 h, leaving behind the nanoparticles in powder form. During this process, the cellulose support was completely removed before reaching temperature of 400°C.42 Thereafter, the burnt samples were cooled in desiccators and the weight of ash recorded. Then, 3 ml hot concentrated H2SO4 was added to dissolve all TiO2 and/or CuO content. Finally, the concentration of each solution sample was determined by AAS.
Microbiological tests
Several test methods have been developed to determine the efficacy of antimicrobial textiles, generally falling into two categories: agar diffusion test (qualitative method) and dynamic shake test (quantitative method). These two methods were used to evaluate the antibacterial properties of the different modified cotton fibers against two bacterial species: Escherichia coli (Gram negative, E. coli, ATCC 25922) and Staphylococcus aureus (Gram positive, S. aureus, ATCC 6538).
Agar diffusion test
The antibacterial activity of the fabrics was demonstrated using the Kirby agar diffusion method by the diameter of the inhibition zone.43 The agar diffusion method is a qualitative, relatively quick, and easily executed test when a large number of samples have to be screened to determine the antibacterial activity of diffusible antimicrobial agents on treated textile material.
The initial bacterial concentration in the vial was approximately 107 CFU/ml (colony-forming units, working dilution). To ensure that any decrease in bacterial number was likely to be due to exposure to the coated bandage treatment, two 0.9% NaCl solutions without any fabric and with uncoated bandage were included separately in the experiment as additional controls. Prior to testing, these samples were sterilized in covered glass by autoclaving them for 15 min at 120°C and 103 kPa. The treated cotton fabrics and control sample were cut into small pieces (1 × 1 cm2) and transferred into Petri dishes containing bacterial strains cultured on Luria–Bertani medium solid agar. They were then incubated for 24 h at 37°C, and the inhibition zone was recorded.
Dynamic shake tests
Dynamic shake testing provides quantitative values for antimicrobial activity but is more time-consuming than agar diffusion testing. Incubated test culture in nutrient broth was diluted with sterilized 0.3 mM phosphate buffer (pH 7.2) to concentration of ~107 CFU/ml. Each small (1 × 1 cm2) piece of fabric was transferred to a flask containing 30 ml of working dilution. All flasks were shaken for different contact times at 150 rpm. After obtaining a dilution series of the bacterial solutions using buffer solution, 1 ml of solution was plated in nutrient agar. The inoculated plates were incubated at 37°C for 24 h. Colonies on the nutrient agar plates were counted to determine the number of viable cells of each diluted sample, reported as colony-forming units (CFU)/ml. The antimicrobial activity is expressed as the number of surviving organisms after contact for different times with the test specimen compared with the number of bacterial cells surviving after contact with the control. For each sample, the colony number was taken as the average of the values from three experiments.
Washing durability test
The treated cotton samples were washed in a bath containing 2 g/l nonionic detergent with liquor-to-good (L:G) ratio of 50:1 at 50°C for 15 min. Next, they were rinsed with distilled water and dried at 100°C for 5 min. The antimicrobial activity was assessed using the same procedure as described above after 5–10 washing cycles, reported as the percentage reduction of bacteria calculated using the following formula:
where R is the percentage reduction, N 0 is the number of bacteria in the broth inoculated with the treated test fabric sample immediately after inoculation, i.e., at zero contact time, and N t is the number of bacteria recovered from the broth inoculated with the treated test fabric sample after the desired contact period (3 h).
Results and discussion
Structural and morphological characterization
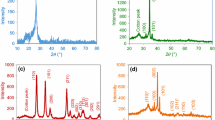
XRD analysis was used to investigate changes of the phase structure of cotton samples before and after treatment. The diffraction pattern for untreated cotton showed characteristic broad peaks at 17–19° and an intense one at 26° indicating cellulose crystallinity44 (Fig. 2a). Additionally, the XRD pattern for sample i (TiO2/CA/PEG) (Fig. 2b) indicated presence of TiO2 as anatase [Joint Committee on Powder Diffraction Standards (JCPDS) 84-1286] with peaks assigned at ~25° (1 0 1), ~38° (0 0 4), and ~48° (2 0 0). The characteristic peaks for monoclinic CuO (JCPDS 80-1917), located at ~35.7° (\(\bar{1}\) 1 1), ~38° (1 1 1), ~48° (\(\bar{2}\) 0 2), and ~58° (2 0 2), were found for sample a (CuO/CA/PEG) (Fig. 2c). Figure 2d shows characteristic peaks of both TiO2 and CuO for sample k (Cu1–TiO2/CA/PEG). Due to the unique hot spots generated under ultrasound irradiation, synthesis of nanoparticles may occur under milder operating conditions such as low temperature and pressure. Furthermore, sonochemical irradiation leads to the formation of crystalline phase without needing to heat the coated textile, as a consequence of the formation, growth, and collapse of cavitation bubbles. This plays a critical role in deposition of nanoparticles, particularly metal oxides, onto textiles.45
XRD patterns for (a) untreated cotton fabric, (b) sample i (* cotton peak), (c) sample a, and (d) sample k (# TiO2 peak). Samples assigned as in Fig. 1
The crystallinity and average particle size were calculated using the Debye–Scherrer equation for the most intense XRD peaks: (1 0 1) for TiO2/cotton, (\(\bar{1}\) 1 1) and (1 1 1) for CuO/cotton, and (1 0 1), (\(\bar{1}\) 1 1), and (1 1 1) for Cu1–TiO2/cotton, being found to be ~65, ~87, and ~45 nm, respectively.
To investigate the morphology of all the cotton samples, comparative SEM images are shown in Fig. 3. As seen, the clean and smooth surface of the uncoated cotton fibers (Fig. 3a) changed to a homogeneously coated surface with nanoparticles of size ranging from 40 to 95 nm after sonication. In situ sonosynthesis of CuO/TiO2 on the cotton was achieved without significant damage to the yarn’s structure by ultrasonic irradiation even up to 5 h as an important and serious issue as shown in Fig. 3b. The smaller and more homogeneous particle size distribution could be considered as favoring the antibacterial activity of the Cu1–TiO2 sample (Fig. 3g and 3h). EDAX analysis of the cotton samples (Fig. 4) confirmed presence of TiO2 and CuO nanoparticles on the cotton surface.
Figure 5 shows a TEM image of the Cu1–TiO2/CA/PEG cotton fiber after burning in oxygen to eliminate the cotton fiber, indicating particles with nanoscale size.
The percentage of TiO2 and CuO nanoparticles incorporated into the cotton fabrics was measured by AAS. The results revealed that the TiO2 and CuO loading was in the range of 10–12 wt% for the metal oxide loaded cotton fibers.
Antibacterial activity
Agar diffusion test results
The results of the antibacterial efficacy tests carried out against E. coli using the disk diffusion method with the different coated cotton fabrics are shown in Fig. 6. The inhibition zone for the tested samples was measured in millimeters (mm) as a qualitative measure of antimicrobial activity. The influence of the different treatments with different duration and frequency of ultrasonic irradiation, surface modification with PEG and/or CA, and cotton chemical composition with different metal oxide molar ratios on the antibacterial activity of the cotton fabrics was studied and is shown in Fig. 6. From these results, the following conclusions can be drawn: (1) No bacterial reduction was found on the untreated cotton sample even after 6 h of contact time (not shown in Fig. 6), indicating that the tested bacteria may use cotton as a nutrient.44 (2) All the single metal oxide treated cottons showed antibacterial activity, with higher efficiency for the CuO-coated cottons. This may be related to the lower bandgap energy of CuO compared with TiO2 (~1.7 eV vs ~3.2 eV, respectively), causing higher antibacterial activity for the former under dark condition. It has been suggested that TiO2 and CuO nanoparticles may use at least four antimicrobial mechanisms: intimate contact between the nanoparticle surface and bacteria, causing physical damage to the bacterial cell wall;45,46 reactive oxygen species generated on the surface of the particles, inhibiting both DNA replication and amino acid synthesis in microbes and leading to cell death;47,48 ion release;46 and nanoparticle internalization.48,49 (3) The cottons treated with crosslinker showed improved antibacterial activity compared with those without crosslinker. The bonding mechanism of the nanoparticles with the cotton fibers includes both chemical bonding and physical adsorption of nanoparticles onto the fiber surface. Citric acid (CA), a trifunctional carboxylic acid, is known as a cost-effective and environmentally friendly cotton crosslinking agent. Use of this crosslinking agent may enable more effective bonding of CuO and TiO2 to functional groups on the cellulose. In addition, the more acidic pH on the CA-treated cotton surface (~pH 4) would lower the growth rate of microorganisms compared with untreated fabric.50 (4) Greater improvement of the antibacterial activity was obtained by cotton modification using both PEG and CA. Bacterial adhesion leading to biofouling is a widespread problem that affects the functioning of a variety of engineered systems. Both bacterial (surface charge and membrane composition) and solid surface properties (roughness, solid surface chemical structure, and hydrophobicity) govern the initial adhesion phase of bacteria to a surface. Functionalized microbicidal coatings can actively fight the spread of bacterial infections by preventing bacterial adhesion, killing adherent bacteria, and inhibiting biofilm formation as three principal strategies for antibacterial surface design.51–53 The effect of substrate wettability on bacterial adhesion has been known for a long time.54 To better understand the effect of the hydrophobicity or hydrophilicity of the PEG-modified cotton fabrics on their antibacterial property, contact angle analysis was performed for convenient assessment of surface wettability. The static contact angles were obtained from measurements of deionized water droplets to characterize the hydrophilic or hydrophobic properties. As shown in Fig. 7, the contact angle changed significantly for the PEG/CA-treated Cu1–TiO2 (~51°) cotton as compared with Cu1–TiO2 (~83°) cotton. Surface modification to prevent bacterial colonization using hydrophilic poly(ethylene glycol), which reduces bacterial adhesion, has been previously reported.55–57 Park et al. reported decreased E. coli adhesion on PEG-modified surfaces.58 Overall, E. coli adheres more to hydrophobic than hydrophilic surfaces.59 (5) the TiO2/CuO/CA/PEG nanocomposite-treated cotton fabrics showed even more improved antibacterial activity depending on the drying temperature and sonication frequency (the higher the temperature or the frequency, the greater the activity). These results might be explained based on a combination of the different mechanisms of action of the two metal oxide components and also a change in the nanoparticle surface chemistry. The improved anchorage of the nanoparticles to the fabric due to the use of CA as a crosslinking agent, the ultrasonic promotion of generation, fast migration, and strong adhesion of the nanoparticles to the fabric surface, the antiadherence offered by the hydrophilic, polymer-modified surface, as well as synergistic improvement of the antibacterial activity through the use of the two metal oxide components resulted in the highest efficiency of the resultant antimicrobial cotton bandages. The only drawback of TiO2 as a bactericide is its near-UV bandgap, resulting in creation of electron–hole pairs that initiate photocatalytic processes only under UV light. Therefore, obviously any modification of the TiO2-based photocatalyst resulting in narrowing of the bandgap could be a useful development. Most recently, copper-doped TiO2 nanoparticles exhibited greater antibacterial activity in comparison with equivalent levels of Cu2+ 60 or pristine TiO2.61 Cu is abundant in the Earth, being an essential trace element to most living organisms, environmentally benign, and relatively inexpensive.62 Therefore, Cu is a good candidate to replace noble-metal materials in antibacterial applications. CuO nanoparticles are also very efficient in imparting antibacterial effect to fabric.63 Cu2+ ions released from copper-based materials in the presence of water and oxygen form complexes with bacterial cell-wall compounds, resulting in damage to the cell wall and proteins. Moreover, copper ions can interact with DNA, preventing bacterial reproduction.64 The negative charge of bacterial cells at biological pH due to the excess number of carboxylic and other groups results in electrostatic forces with Cu2+ ions released from TiO2/CuO nanocomposites, which may be the reason for adhesion and bioactivity.65 Finally, the XRD and SEM analyses reveal that the ultrasonically treated TiO2/CuO/CA/PEG nanocomposite fabrics contained smaller particles which would have improved bacteria contact on the higher surface area of the treated cotton.
Dynamic shake test results
In general, our results indicated that the coated CuO/TiO2 nanocomposite cotton fibers exhibited excellent antimicrobial activity against both Gram-positive and Gram-negative bacteria. The shake test results presented in Fig. 8 show that the antibacterial activity exhibited the following trend: TiO2 < CuO < Cu1–TiO2/CA < Cu1–TiO2/CA/PEG for the cotton samples prepared using the same treatment conditions, i.e., drying at temperature of 160°C for 10 min and sonication two times for 30 min each. The lower resistance of the E. coli bacterium to the antimicrobial action of all the coated cotton samples in comparison with S. aureus may be due to the thicker cell wall of the former, consistent with previous reports.66,67 Gram-positive and Gram-negative bacteria have different membrane structures. Gram-negative bacteria such as E. coli contain only a thin peptidoglycan layer between the cytoplasmic membrane and outer membrane, whereas Gram-positive bacteria have a thick wall composed of multilayers of peptidoglycan.
Antibacterial efficiency of different coated cotton fabrics as CFU/ml against (a) E. coli and (b) S. aureus: (A) TiO2, (B) CuO, (C) Cu1–TiO2/CA, and (D) Cu1–TiO2/CA/PEG cottons prepared under the same treatment conditions, i.e., drying at temperature of 160°C for 10 min and sonication two times for 30 min each
All of the above results suggest that the antibacterial activity of the nano-TiO2-modified cottons was greatly improved by incorporation of CuO nanoparticles to form a combinative therapy system, even in the absence of light irradiation.
Wash durability results
Although washing durability and reusability should not be as important for antimicrobial textiles such as bandages compared with healthcare workers’ uniforms or hospital sheets, antibacterial tests were carried out on the treated cottons after 10 washing cycles.
As seen in Table 2, it was observed that, after application of TiO2/CuO nanoparticles to the cotton surface, the antimicrobial activity of the fabric against either E. coli or S. aureus bacteria was improved up to 100%. Furthermore, the obtained results indicate that the efficacy of the nanoparticles against the microorganisms decreased with increasing number of washing cycles due to physical desorption of the coated nanoparticles. The stability of the CA-modified Cu1/TiO2 cotton against the washing process was higher than for the Cu1/TiO2 cotton.
Thus, application of citric acid as a crosslinking agent allowed the bactericidal effect of the modified fibers to be maintained. In the presence of the crosslinking agent, hydroxyl groups of cellulose can form covalent bonds with carboxyl groups of polycarboxylic acid in an esterification reaction as one end with metal oxide hydroxyl groups created by alkaline hydrolysis on the other, effectively embedding the nanoparticle metal oxides into the fabric and resisting their removal during the washing cycles.68–70 After six washes, the bacterial growth inhibition was low, and no antibacterial activity was found for the fabrics after 10 washes.
Conclusions
There is continuous effort to develop more suitable wound-dressing materials. Improving the antimicrobial activity of textile-based nanocomposites is a key issue for the development of useful wound dressings. In this study, through a synergistic combination of CA, PEG, and sol–gel-derived TiO2 and CuO nanostructures, various features such as wash durability, hydrophilicity, and antimicrobial action were obtained and improved for the modified cotton fabrics, respectively. Sonochemical treatment of the cotton fibers during immersion resulted in coating with nanoparticles via physical adsorption onto the surface, and more efficient chemical bonding of the nanoparticles entrapped in crosslinked cellulose using CA. Furthermore, surface modification was achieved using hydrophilic PEG to prevent bacterial colonization and reduce bacterial adhesion. The results demonstrate that higher antibacterial activity was found against S. aureus than E. coli, in both qualitative and quantitative tests, for the ultrasonically PEG/CA-modified TiO2/CuO/cotton nanocomposite.
The combination of copper with TiO2 synergistically improved the electronic properties for antibacterial activity in dark condition, and also increased the antibacterial activity against Gram-positive and Gram-negative bacteria that cause wound infections. Furthermore, the proved absorption of copper or copper oxide through the skin could be considered an important feature for wound healing. The washing durability of the nanoparticles applied on the cotton surface was evaluated up to 10 consecutive washing cycles. The achieved results indicate that, with increasing washing cycles, the efficacy of the nanoparticles against the microorganisms decreased, with less reduction for the CA-treated cottons. The results obtained in this work will lead to a new direction in the design of multicomponent nanomaterials as model hydrophilic wound dressings with enhanced bactericidal effect, which might help combat emerging pathological bacteria that cause wound infections. However, before introduction of such systems into consumer products, their toxicological potential should be considered.
References
Lim, SH, Hudson, SM, “Application of a Fiber-Reactive Chitosan Derivative to Cotton Fabric as an Antimicrobial Textile Finish.” Carbohydr. Polym., 56 227–234 (2004)
Fei, B, Deng, Z, Xin, JH, Zhang, Y, Pang, G, “Room Temperature Synthesis of Rutile Nanorods and Their Applications on Cloth.” Nanotechnology, 17 1927–1931 (2006)
Nonami, T, Hase, H, Funakoshi, K, “Apatite-Coated Titanium Dioxide Photocatalyst for Air Purification.” Catal. Today, 96 113–118 (2004)
Qi, K, Daoud, WA, Xin, JH, et al., “Self-Cleaning Cotton.” J. Mater. Chem., 16 4567–4574 (2006)
Foster, HA, Ditta, IB, Varghese, S, Steele, A, “Disinfection Using Titanium Dioxide: Spectrum and Mechanism of Antimicrobial Activity.” Appl. Microbiol. Biotechnol., 90 847–868 (2011)
Karunakaran, C, Abiramasundari, G, Gomathisankar, P, et al., “Cu-Doped TiO2 Nanoparticles for Photocatalytic Disinfection of Bacteria Under Visible Light.” Colloid Interface Sci., 352 68–74 (2010)
Pham, T-D, Lee, B-K, “Cu Doped TiO2/GF for Photocatalytic Disinfection of Escherichia coli in Bioaerosols Under Visible Light Irradiation: Application and Mechanism.” Appl. Surf. Sci., 296 15–23 (2014)
Khan, MM, Ansari, SA, Pradhan, D, et al., “Band Gap Engineered TiO2 Nanoparticles for Visible Light Induced Photoelectrochemical and Photocatalytic Studies.” J. Mater. Chem. A, 2 637–644 (2014)
Yadav, HM, Otari, SV, Bohara, RA, et al., “Synthesis and Visible Light Photocatalytic Antibacterial Activity of Nickel-Doped TiO2 Nanoparticles Against Gram-Positive and Gram-Negative Bacteria.” J. Photochem. Photobiol. A Chem., 15 130–136 (2014)
Ashkarran, AA, Hamidinezhad, H, Haddadi, H, et al., “Double-Doped TiO2 Nanoparticles as an Efficient Visible-Light-Active Photocatalyst and Antibacterial Agent Under Solar Simulated Light.” Appl. Surf. Sci., 301 (15) 338–345 (2014)
Ananpattarachai, J, Boonto, Y, Kajitvichyanukul, P, “Visible Light Photocatalytic Antibacterial Activity of Ni-Doped and N-Doped TiO2 on Staphylococcus aureus and Escherichia coli Bacteria.” Environ. Sci. Pollut. Res., (2015). doi:10.1007/s11356-015-4775-1
Hostynek, JJ, Maibach, HI, “Copper Hypersensitivity: Dermatologic Aspects—An Overview.” Rev. Environ. Health, 18 (3) 153–183 (2003)
Uauy, R, Olivares, M, Gonzalez, M, “Essentiality of Copper in Humans.” Am. J. Clin. Nutr., 67 952S–959S (1998)
Pereira, CE, Felcman, E, “Correlation Between Five Minerals and the Healing Effect of Brazilian Medicinal Plants.” J. Biol. Trace Elem. Res., 65 251–259 (1998)
Micheals, HT, “Anti-microbial Characteristics of Copper.” Stand. News, 34 28–31 (2006)
Hostynek, JJ, Dreher, F, Maibach, HI, “Human Stratum Corneum Penetration by Copper: In vivo Study After Occlusive and Semi-Occlusive Application of the Metal as Powder.” Food Chem. Toxicol., 44 1539–1543 (2006)
Pickart, L, “The Human Tri-peptide GHK and Tissue Remodeling.” J. Biomater. Sci. Polym. Ed., 19 969–988 (2008)
Borkow, G, Gabbay, J, Zatcoff, RC, “Could Chronic Wounds Not Heal Due to Too Low Local Copper Levels?” Med. Hypotheses, 70 610–613 (2008)
Chen, S, Guo, Y, Chen, S, et al., “Fabrication of Cu/TiO2 Nanocomposite: Toward an Enhanced Antibacterial Performance in the Absence of Light.” Mater. Lett., 83 154–157 (2012)
Baghriche, O, Rtimi, S, Pulgarin, C, et al., “Innovative TiO2/Cu Nanosurfaces Inactivating Bacteria in the Minute Range Under Low-Intensity Actinic Light.” ACS Appl. Mater. Interfaces, 10 5234–5240 (2012)
Schmidt, F, Fischer, A, Haufe, H, Leisegang, T, Mahltig, B, “Solvothermally Prepared Copper Modified TiO2 Composite Sols—A Coating Agent for Textiles to Realize Photocatalytic Active and Antimicrobial Fabrics” Chapter 13 in: Textiles: Types, Uses and Production Methods, pp. 439–466. Nova Science Publishers Inc., (2012)
Ramesh, S, Koltypin, Y, Prozorov, R, et al., “Sonochemical Impregnation of Submicron Silica Spheres with Ni Nanoparticles.” Chem. Mater., 9 546–551 (1997)
Sadr, FA, Montazer, M, “In situ Sonosynthesis of Nano TiO2 on Cotton Fabric.” Ultrason. Sonochem., 21 681–691 (2014)
Prasad, K, Pinjari, DV, Pandit, AB, et al., “Phase Transformation of Nanostructured Titanium Dioxide from Anatase-To-Rutile via Combined Ultrasound Assisted Sol–Gel Technique.” Ultrason. Sonochem., 17 409–415 (2010)
Prasad, K, Pinjari, DV, Pandit, AB, et al., “Synthesis of Titanium Dioxide by Ultrasound Assisted Sol–Gel Technique: Effect of Amplitude (Power Density) Variation.” Ultrason. Sonochem., 17 697–703 (2010)
Ghows, N, Entezari, MH, “Fast and Easy Synthesis of Core-Shell Nanocrystals (CdS/TiO2) at Low Temperature by Micro-Emulsion Under Ultrasound.” Ultrason. Sonochem., 18 629–634 (2011)
Guo, J, Zhu, S, Chen, Z, et al., “Sonochemical Synthesis of TiO2 Nanoparticles on Graphene for Use as Photocatalyst.” Ultrason. Sonochem., 18 1082–1090 (2011)
Nguyen, QV, “Hospital-Acquired Infections” (2006). http://www.emedicine.com/ped/topic1691.htm. Accessed 21 April 2008
Hospital-Acquired Infections-Trends Across Europe, Frost and Sullivan,/http://www.reportlinker.com/p0249335-summary/Hospital-acquired-infections-trends-across-Europe.htmlS; June 2010. Accessed 1 Nov 2012
Kyriacou, SV, Brownlow, WJ, Xu, XH, “Using Nanoparticle Optics Assay for Direct Observation of Function of Antimicrobial Agents in Single Live Bacterial Cells.” Biochemistry, 43 140–147 (2004)
Selvam, S, Sundrarajan, M, “Functionalization of Cotton Fabric with PVP/ZnO Nanoparticles for Improved Reactive Dyeability and Antibacterial Activity.” Carbohydr. Polym., 87 1419–1424 (2012)
Cheng, Q, Li, C, Pavlinek, V, et al., “Surface-Modified Antibacterial TiO2/Ag Nanoparticles: Preparation and Properties.” Appl. Surf. Sci., 252 4154–4160 (2006)
Boekema, BKHL, Pool, L, Ulrich, MMW, “The Effect of a Honey Based Gel and Silver Sulphadiazine on Bacterial Infections of In Vitro Burn Wounds.” Burns, 39 754–759 (2013)
Gupta, B, Arora, A, Saxena, S, et al., “Preparation of Chitosan–Polyethylene Glycol Coated Cotton Membranes for Wound Dressings: Preparation and Characterization.” Polym. Adv. Technol., 20 58–65 (2009)
Shingel, KI, Di Stabile, L, Marty, JP, et al., “Inflammatory Inert Poly(ethylene glycol)–Protein Wound Dressing Improves Healing Responses In Partial- and Full-Thickness Wounds.” Int. Wound J., 3 332–342 (2006)
Sinha, M, Banik, RM, Haldar, C, et al., “Development of Ciprofloxacin Hydrochloride Loaded Poly(Ethylene Glycol)/Chitosan Scaffold as Wound Dressing.” J. Porous Mater., 20 799–807 (2013)
Bader, RA, Herzog, KT, Kao, WJ, “A Study of Diffusion in Poly(Ethyleneglycol)-Gelatin Based Semi-Interpenetrating Networks for Use in Wound Healing.” Polym. Bull., 62 381–389 (2009)
Klemm, D, Heublein, B, Fink, H-P, et al., “Cellulose: Fascinating Biopolymer and Sustainable Raw Material.” Angew. Chem. Int. Ed., 44 3358–3393 (2005)
Jin, C, Jiang, Y, Niu, T, Huang, J, “Cellulose-Based Material with Amphiphobicity to Inhibit Bacterial Adhesion by Surface Modification.” Mater. Chem., 22 2562–12567 (2012)
Huang, J, Gu, Y, “Self-Assembly of Various Guest Substrates in Natural Cellulose Substances to Functional Nanostructured Materials.” Curr. Opin. Colloid Interface Sci., 16 470–481 (2011)
Alongi, J, Ciobanu, M, Tata, J, et al., “Thermal Stability and Flame Retardancy of Polyester, Cotton, and Relative Blend Textile Fabrics Subjected to Sol-Gel Treatments.” J. Appl. Polym. Sci., 119 1961–1969 (2011)
Uddin, MJ, Cesano, F, Bonino, F, et al., “A Tailoring the Activity of Ti-Based Photocatalysts by Playing with Surface Morphology and Silver Doping.” J. Photochem. Photobiol. A Chem., 196 165–173 (2008)
Bauer, W, Kirby, WM, Sherris, JC, et al., “Antibiotic Susceptibility Testing by a Standardized Single Disk Method.” Am. J. Clin. Pathol., 45 493–496 (1966)
Gao, Y, Cranston, R, “Recent Advances in Antimicrobial Treatments of Textiles.” Text. Res. J., 8 60–72 (2008)
Perelshtein, I, Applerot, G, Perkas, N, et al., “A One-Step Process for the Antimicrobial Finishing of Textiles with Crystalline TiO2 Nanoparticles.” Chem. Eur. J., 18 4575–4582 (2012)
Applerot, G, Lellouche, J, Lipovsky, A, et al., “Understanding the Antibacterial Mechanism of CuO Nanoparticles: Revealing the Route of Induced Oxidative Stress.” Small, 8 3326–3337 (2012)
Gunawan, C, Teoh, WY, Marquis, CP, et al., “Cytotoxic Origin of Copper (II) Oxide Nanoparticles: Comparative Studies with Micron-Sized Particles, Leachate, and Metal Salts.” ACS Nano., 5 7214–7225 (2011)
Ludmil, T, Benov, S, Fridovich, I, “Escherichia coli Expresses a Copper- and Zinc Containing Superoxide Dismutase.” J. Biol. Chem., 269 25310–25314 (1994)
Hu, XK, Cook, S, Wang, P, Hwang, HM, “In Vitro Evaluation of Cytotoxicity of Engineered Metal Oxide Nanoparticles.” Sci. Total Environ., 407 3070–3072 (2009)
Orhan, M, Kut, D, Gunesoglu, C, “Improving the Antibacterial Activity of Cotton Fabrics Finished with Triclosan by the Use of 1,2,3,4-Butanetetracarboxylic Acid and Citric Acid.” J. Appl. Polym. Sci., 111 1344–1352 (2009)
Banerjee, I, Pangule, RC, Kane, RS, “Antifouling Coatings: Recent Developments in the Design of Surfaces that Prevent Fouling by Proteins, Bacteria, and Marine Organisms.” Adv. Mater., 23 690–718 (2011)
Kenawy, E-R, Worley, SD, Broughton, R, “The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review.” Biomacromolecules, 8 1359–1384 (2007)
Wach, J-Y, Bonazzi, S, Gademann, K, “Antimicrobial Surfaces Through Natural Product Hybrids.” Angew. Chem. Int. Ed., 47 7123–7126 (2008)
Dexter, SC, Sullivan, JD, William, J, Watson, SW, “Influence of Substrate Wettability on the Attachment of Marine Bacteria to Various Surfaces.” Appl. Microbiol., 30 298–308 (1975)
Adams, AP, Santschi, EM, Mellencamp, MA, “Antibacterial Properties of a Silver Chloride-Coated Nylon Wound Dressing.” Vet. Surg., 28 219–225 (1999)
Kingshott, P, Griesser, HJ, “Surfaces that Resist Bioadhesion.” Curr. Opin. Solid State Mater. Sci., 4 403–412 (1999)
Liu, Y, Kim, H-I, “Characterization and Antibacterial Properties of Genipin-Crosslinked Chitosan/Poly(Ethylene Glycol)/ZnO/Ag Nanocomposites.” Carbohydr. Polym., 89 (1) 111–116 (2012)
Park, KD, Kim, YS, Han, DK, “Bacterial Adhesion on PEG Modified Polyurethane Surfaces.” Biomaterials, 19 (7) 851–859 (1998)
Harris, JM, Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications. Plenum, New York (1992)
Wu, B, Huang, R, Sahu, M, Feng, X, Biswas, P, Tang, YJ, “Cu-Doped TiO2 Nanoparticles Enhance Survival of Shewanella oneidensis MR-1 Under Ultraviolet Light (UV) Exposure.” Sci. Total Environ., 408 1755–1758 (2010)
Hassan, MS, Amna, T, Kim, HY, Khil, M-S, “Enhanced Bactericidal Effect of Novel CuO/TiO2 Composite Nanorods and a Mechanism Thereof.” Compos. Part B, 45 904–910 (2013)
Guo, YG, Cao, FF, Xin, S, Wan, LJ, “Wet Chemical Synthesis of Cu/TiO2 Anocomposites with Integrated Nano-Current-Collectors as High-Rate Anode Materials in Lithium-Ion Batteries.” Phys. Chem. Chem. Phys., 13 2014–2020 (2011)
Perelshtein, I, Applerot, G, Perkas, N, et al., “CuO-Cotton Nanoparticles: Formation, Morphology and Antibacterial Activity.” Surf. Coat. Technol., 204 54–57 (2009)
Borkow, G, Gavia, J, “Copper as a Biocidal Tool.” Curr. Med. Chem., 12 2163–2175 (2005)
Stoimenov, PK, Klinger, RL, Marchin, GL, Klabunde, KJ, “Metal Oxide Nanoparticles as Bactericidal Agents.” Langmuir, 18 6679–6686 (2002)
Gao, Y, Cranston, R, “Recent Advances in Antimicrobial Treatments of Textiles.” Text. Res. J., 78 (1) 60–72 (2008)
Han, S, Yang, Y, “Antimicrobial Activity of Wool Fabric Treated with Curcumin.” Dyes Pigm., 64 157–161 (2005)
Meilert, KT, Laub, D, Kiwi, J, “Photocatalytic Self-Cleaning of Modified Cotton Textiles by TiO2 Clusters Attached by Chemical Spacers.” J. Mol. Catal. A Chem., 237 101–108 (2005)
Yuranova, T, Laub, D, Kiwi, J, “Synthesis, Activity and Characterization of Textiles Showing Self-Cleaning Activity Under Daylight Irradiation.” Catal. Today, 122 109–117 (2007)
Nazari, A, Montazer, M, Yazdanshenas, ME, et al., “Nano TiO2 Photo-Catalyst and Sodium Hypophosphite for Cross-Linking Cotton with Poly Carboxylic Acids Under UV and High Temperature.” Appl. Catal. A Gen., 371 10–16 (2009)
Acknowledgments
The authors are grateful to Islamic Azad University, Shahreza Branch for financial support to carry out this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khani, A., Talebian, N. In vitro bactericidal effect of ultrasonically sol–gel-coated novel CuO/TiO2/PEG/cotton nanocomposite for wound care. J Coat Technol Res 14, 651–663 (2017). https://doi.org/10.1007/s11998-016-9870-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-016-9870-9