Abstract
A series of ultraviolet (UV)-curable waterborne hyperbranched polyurethane dispersions (WHPUDs) have been successfully synthesized by modifying the hyperbranched polyester H10, which was prepared using pentaerythritol as a core molecule and dimethylolpropionic acid as monomers, with succinic anhydride, toluene diisocyanate (TDI), and hydroxypropyl acrylate (HPA). The H10 was characterized by 1H nuclear magnetic resonance spectroscopy and matrix-assisted laser desorption/ionization time of flight mass spectrometry. The properties of the WHPUDs with different content of succinic anhydride and TDI–HPA have been investigated by measuring the stability, the particle size, and the rheological behavior. The effects of the content of succinic anhydride and TDI–HPA were studied in terms of UV-curing rate, water resistance, and thermogravimetric behaviors of WHPUD coatings. The WHPUDs showed good appearance, particle size, viscosity, and storage stability. The WHPUD films showed superior photosensitivity and the percent conversion of C=C bonds reached about 80% when the radiation time was 50 s. Moreover, the UV-cured films had good water resistance and thermostability, which can benefit a waterborne polyurethane resin for waterborne coatings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ultraviolet (UV) radiation is a well-accepted technology for the fast curing of polymeric materials, whereas waterborne coatings are new safe materials that can reduce environmental pollution. Recently, waterborne coatings using UV-curing technology have gained increasing interest because they can decrease air pollution, reduce the risk of fire, improve aspects of occupational health and safety, lower energy consumption, and have high curing speed.1–3
Polyurethanes have been popularly applied in organic coatings for their prominent performance. However, ordinary polyurethanes are difficult to disperse in water. Therefore, a special modification on structures is necessary for these oligomers to be dispersible in water. This is normally done by incorporating a small amount of ionic pendant groups. However, the waterborne coatings have also been limited by several problems such as low solid content and low water resistance.4,5 To overcome those disadvantages, waterborne polyurethanes have been developed rapidly in recent years. Hyperbranched polymer is one of the most important dendritic polymers synthesized by a relatively new method with relatively low cost. Because of their unique structures, hyperbranched polymers in some cases exhibit properties that are quite different from those of linear polymers.6–8 For example, hyperbranched polymers are known to exhibit a very low degree of chain entanglement and typically have relatively low viscosities even at high molecular weights, which is a benefit to coating applications. Recently, the hyperbranched polymers have been widely used to synthesize PU and other materials.9–12
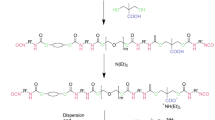
The hyperbranched polyester H10 can be prepared using pentaerythritol (PEL) as a core molecule and dimethylolpropionic acid (DMPA) as monomers, and the UV-curable, waterborne dispersions can be prepared by modifying some of the hydroxyl groups of H10 as a polyol to acidic groups and capping the remaining hydroxyl groups with diisocyanate and acrylic functionalities, such as toluene diisocyanate (TDI) and hydroxypropyl acrylate (HPA), respectively, as shown in Fig. 1.13–15 The present paper describes the synthesis and structure–property relationship of a new UV-curable, waterborne, hyperbranched ionomer based on H10 that was characterized by 1H nuclear magnetic resonance (1H NMR) spectroscopy, and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). A variety of UV-curable, waterborne dispersions have been synthesized with different content of succinic anhydride and TDI–HPA in H10-based prepolymer. The properties of waterborne hyperbranched polyurethane dispersion (WHPUD), such as appearance, storage stability, viscosity, and particle size, have been investigated and the water resistance and thermal properties of UV-cured films were determined. In addition, Fourier transform infrared spectroscopy (FTIR) was used to characterize the structure and study the UV-curing rate of WHPUDs.
Experimental
Materials
DMPA and TDI were purchased from J&K Scientific Ltd., China, and used without further purification. p-Toluenesulfonic acid (p-TSA), 1,4-dioxane, stannous chloride dihydrate (SnCl2·2H2O), HPA, succinic anhydride, and triethylamine (TEA) were purchased from Shanghai Linfeng Chemical Co., China. Di-n-butyltindilaurate (DBTDL), PEL, and p-hydroxyanisole were purchased from Shanghai Guoyao Chemical Co., China. 2-Hydroxyl-2-methyl-1-phenyl-1-propanone (Darocur 1173) was supplied by Ciba Co., Switzerland, and used as a photoinitiator.
All the above materials were used as-received without further purification except HPA and 1,4-dioxane, which were dried over CaH2 and distilled.
Synthesis
H10 synthesis
The synthesis was carried out in a 250 mL three-necked, round-bottomed flask equipped with a mechanical stirrer, thermometer, and condenser with a drying tube. The reaction temperature was controlled between 140 and 160°C, and p-TSA was added as catalyst. A resin with, theoretically, eight hydroxyl groups was yielded after evaporating the small molecule of water, as shown in Fig. 1.16–18
PU prepolymer synthesis
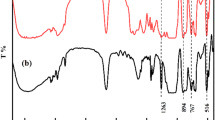
A schematic presentation for the reaction procedure is shown in Fig. 2. The synthesis was carried out in a 250 mL four-necked, round-bottomed flask equipped with a mechanical stirrer, thermometer, condenser with a drying tube, and N2 gas was aspirated for 10 min to eliminate residual moisture. The reaction temperature was controlled by using a constant-temperature oil bath. In the first step, 23.6 g (39.24 mmol, 313.86 mmol hydroxyl groups) hyperbranched aliphatic polyester H10 was dissolved in 1,4-dioxane, and then 8.57 g (85.6 mmol) succinic anhydride and a catalytic amount of SnCl2·2H2O were added. The reaction was conducted at 100°C to insure an acceptable rate of reaction until the anhydride peak at 1786 cm−1 in the FTIR spectrum disappeared, as shown in Fig. 3a. Finally, 1,4-dioxane was evaporated to obtain a light yellow, highly viscous resin that has, theoretically, three hydroxyl groups conjugated to carboxyl groups. The first step was performed at a relatively low temperature (100°C) to suppress unwanted side reactions such as etherification and transesterification.
In the second step, 2.484 g (14.26 mmol) TDI and 15 mL of 1,4-dioxane were poured into a 250 mL four-necked, round-bottomed flask equipped with a mechanical stirrer, thermometer, and dropping funnel, and N2 gas was aspirated for 10 min to eliminate residual moisture. After dissolving 0.1 wt% DBTDL, 0.1 wt% p-hydroxyanisole, and 2.06 g (15.85 mmol) HPA in 10 mL dioxane, the mixture was dropped slowly into the flask under efficient stirring at 0°C and kept for another 1 h. The temperature was then increased to 30°C and maintained for 4 h. Half of NCO groups remained, as shown in Fig. 3b. The change of isocyanate (NCO) value during the reaction was determined by the di-n-butylamine back titration method to find out the end point of this step.
In the third step, the prepolymer synthesized in the first step, which was cooled to 60°C, was added dropwise into the above reaction vessel containing TDI-terminated HPA by the dropping funnel and stirred vigorously at 40°C until the peak at 2270 cm−1 for NCO groups completely disappeared, as shown in Fig. 3c. The end point was also determined by the change of NCO value by using the di-n-butylamine back titration method.
Neutralization of the prepolymer
Finally, the residual –COOH were neutralized with an appropriate amount of TEA (8.66 g, 85.6 mmol). The temperature was maintained at 40°C for 2 h to insure completion of the reaction.
Dispersion formation
After neutralization, the waterborne, hyperbranched polyurethane dispersion was obtained by evaporating the 1,4-dioxane under vacuum, dispersed by adding deionized water to the prepolymer at 35–40°C. The stirring speed was controlled in 1000–1200 rpm and the dispersion was prepared at 30% solid content.
For comparison, a series of waterborne, hyperbranched polyurethane dispersions have been prepared. Therefore, in the first step, the stoichiometry for the modification of hydroxyl groups into carboxylic acid groups and acrylate groups was adjusted as shown in Table 1.
Measurements
FTIR spectra were recorded on a NICOLET 5700 infrared spectrometer by averaging 32 scans using a KBr disk. The 1H NMR spectra were recorded on a Bruker Avance 500 spectrometer and the chemical shifts were referenced to the residual proton signals of solvent. The molecular weight was determined by an Applied Biosystems Voyager 4418 MALDI-TOF MS spectrometer.
The stability of WHPUD was determined by the centrifugal settling method in the speed of 3000 r/min. If it was still stable after 15 min, it could be determined that the emulsion had 6 months storage stationary phase. The average particle sizes and the distribution of WHPUDs were measured by the laser light scattering method. The viscosity of WHPUDs was measured by a Brookfield-DV-II+ viscometer with LV-1 spindle at 25°C. The rheological behavior of the final products was determined with variable shear rates.
The UV-curing properties of the WHPUD films were monitored by FTIR. The films were exposed to 365 nm UV light of 23 mw/cm2 and the distance of the sample to the focal point of the UV lamp was 10 cm.
Thermogravimetric (TG) analysis was carried out with a TG-60 thermoanalyzer (Schimadu Co., Japan). Approximately 5 mg of each sample was used at a heating rate of 20°C/min under N2 from 30 to 650°C.
The water absorption behavior of UV-cured films was measured at 25°C and 100% relative humidity. The films were first dried in a vacuum oven at 100°C for 12 h. Then the membrane was kept in a humidified chamber with deionized water and the weight change of the films was recorded by using the thin film diffusion analyzer (model D-200; CAHN Instruments, Inc.) until no further water uptake took place. The water uptake (S) was calculated using the following equation:
where m 1 and m 2 are the sample weight at the dried state and the sample weight at the saturated state, respectively.
Preparation of UV-cured films
WHPUDs were first mixed with 2 wt% Darocur 1173, and then the mixture was coated on a tin plate by an applicator with a 100 μm gap, and cured for 60 s by exposing it to a UV lamp (365 nm, 23 mw/cm2).
Results and discussion
Characterizations of H10
In the experiment, the H10 was prepared using PEL as a core molecule and DMPA as monomers, and it was characterized by 1H NMR and MALDI-TOF MS.
1H NMR of H10
The 1H NMR spectra of the H10 is shown in Fig. 4. Four groups of characteristic peaks are obviously observed, which prove the molecular structure of the H10. The peaks of a (1.00–1.10 ppm), b (3.35–3.56 ppm), and d (4.65–5.20 ppm) are assigned to the hydrogen atom of DMPA. The peaks of c (4.00–4.22 ppm) are assigned to the hydrogen atom of PEL. The peak at about 2.5 ppm is attributed to the residual hydrogen protons of the solvent (DMSO). All the above analysis confirms the formation of H10, which is accordance with the ideal structure of H10.
MALDI-TOF MS
MALDI-TOF MS is regarded as an excellent method for characterizing the molecular weight of H10. The MALDI-TOF MS spectra of H10 are presented in Fig. 5. The MALDI spectrum shows that the molecular weights of H10 range from 0 to 3000. And the intense peak is in 507.17–855.34 g/mol, which contains those calculated by the theory. The theoretical value is 600.25 g/mol, (136.15 + 4×134.03) − 4 × 18.01 = 600.23 g/mol.
Appearance, stability, and particle size of WHPUDs
The appearances, stabilities, and particle sizes of WHPUDs were examined at room temperature. These results are summarized in Table 2. The results in Table 2 show that all WHPUDs have good stabilities except for WHPUD 2.5-10.
The average particle size could be controlled to some extent by such emulsification conditions as stirring speed or dispersing temperature, but it is mostly governed by the concentration of hydrophilic groups. Consequently, the average particle size decreases as the acid content increases. It can be seen in Fig. 6 and Table 2 that the particle sizes follow the order: WHPUD 2.5-1.0 > WHPUD 3.0-1.0 > WHPUD 3.5 > 1.0; and WHPUD 3.0-1.5, WHPUD 3.0-0.5 are close to WHPUD 3.0-1.0. The asymptotic decrease of particle size with increasing acid content is due to the stabilizing mechanism of ionomer dispersions.19
In this study, the carboxyl content and the TDI–HPA content on the average of particle size and stability of WHPUDs were investigated. Only when three or more hydroxyl groups of H10 have been modified to carboxyl groups do WHPUDs show a better storage stability and a lower particle size.
Rheological behaviors
The rheological property is a very important factor determining the processibility and end-use property of a polymer material. The chain structure and topology of a polymer determine its rheological properties. The viscosity arises from the interactions between the particles of emulsion. Therefore, many factors, such as molecular architecture, molecular weight, solid content, and dispersion medium, affect the rheological behaviors of emulsion. Moreover, the viscosity of a polymer is related to the dynamic extension and the segment density within the volume of a molecule and intermolecular chain entanglement. As a result of the structures of hyperbranched polymers, which is relatively branched and sphere-like, there will be few entanglements among molecular chains, resulting in a rather lower viscosity.19,20
Figure 7 shows the rheological behavior of WHPUD 3.5-1.0, WHPUD 3.0-1.5, WHPUD 3.0-1.0, WHPUD 3.0-0.5, and WHPUD 2.5-1.0. It can be observed that the viscosity for WHPUDs decreases drastically when the shear rate increases from 10 to 30 min−1, whereas it decreases gradually from 30 to 60 min−1. When the shear rate increases, the physical crosslinks between the particles of emulsion are broken down, leading to the drastic decrease in viscosity. This implies that WHPUDs exhibit shear thinning or pseudoplastic behavior, which arises in common polymer emulsion. As seen in Fig. 7, WHPUDs exhibit pronounced deviation from Newtonian flow behavior.
The effect of the carboxyl content of WHPUD on the viscosity is also illustrated in Fig. 7. The viscosity of these emulsions follows the order WHPUD 2.5-1.0 < WHPUD 3.0-1.0 < WHPUD 3.5-1.0. As the concentration of ionic groups in the PU molecule increases, the mutual repulsion of the same charges causes the expansion of chains, which may result in increasing the viscosity.
The hard segment of prepolymer also plays an important role in determining the viscosity. The viscosity of WHPUDs emulsion follows the order of WHPUD 3.0-0.5 < WHPUD 3.0-1.0 < WHPUD 3.0-1.5. The chain entanglement and hydrogen bonding between urethane and urea linkages can be more rapidly formed in the polymer because of the flexibility of the chains. As the content of prepolymer TDI–HPA increases, the chain entanglement and hydrogen bonding increases, which may result in increasing the viscosity.
UV curing
The UV-curing properties of the synthesized WHPUDs were measured by FTIR. In order to stimulate the photoinitiator, the film was exposed to 23 mW/cm2 of 365 nm light for an increasing amount of time. Figure 8 shows the FTIR spectra of WHPUD 3.0-1.0 in the absence of photoinitiator at various curing time(s) (0, 10, 20, 30, 40, 50).
The percent conversion of C=C bond is calculated from the decay of the absorption bands of the double bond group (CH=CH2) twisting vibration at 810 cm−1, and the characteristic absorption peak of carbonyl group (C=O) stretching mode at 1720 cm−1 is used as the internal standard peak by integration of the peak areas. Hence, the double bond conversion (c) after a given exposure time (t) of CH=CH2 can be readily obtained from the ratio of the FTIR absorbance areas before (A 0) and after UV irradiation (A t ) by equation (2).21–23
Figure 9 illustrates the relationship between the double bond conversion (%) and irradiation time (s). It can be found that, in the initial stage of UV radiation, the percent conversion of C=C bond increases quickly with the increase of radiation time. The maximum conversion value of C=C bond is about 80% when exposure time is 50 s. The conversion increases slightly when the radiation time exceeds 30 s, indicating that WHPUDs have superior photosensitivity.
As shown in Fig. 9, five types of WHPUDs with the increase of irradiation time follows the order of WHPUD 3.0-1.5 > WHPUD 3.0-1.0 > WHPUD 3.0-0.5, and the values of c of WHPUD 3.5-1.0 and WHPUD 2.5-1.0 are close to WHPUD 3.0-1.0. The results can be explained with the double bond concentration. In general, the higher concentration of double bonds of the acrylic end group located at the outer layer of the sphere-like structure may lead to higher conversion.
Water resistance
Waterborne polyurethanes have a large number of hydrophilic terminal groups, so the water resistance of waterborne polyurethane is lower than that of solvent polyurethanes. This factor becomes the restriction of the development of waterborne polyurethane. To overcome this disadvantage, the present paper uses the unique hyperbranched polymer (H10) and UV-curing technology to improve the water resistance of waterborne polyurethane.2,5,24
The water absorption of UV-cured films is shown in Table 3. As seen in Table 3, the water absorption of UV-cured films decreases with increasing double bond concentration (the content of TDI–HPA),WHPUD 3.0-0.5 > WHPUD 3.0-1.0 > WHPUD 3.0-1.5. The films with higher double bond concentration exhibited better water resistance, and this may be ascribed to the higher crosslinking density between crosslinking points after UV curing. In the same crosslinking density, along with the carboxyl content increasing, the water absorption gradually increases, WHPUD 2.5-1.0 < WHPUD 3.0-1.0 < WHPUD 3.5-1.0. The film with fewer hydrophilic groups has a better water resistance.
Therefore, hyperbranched polyurethane has water resistance that is superior to linear polyurethane. In addition, after UV curing, it is able to form a three-dimensional chemical bonding between PU polymer molecules, leading to the increasing crosslinking density of the polymer and decreasing free volume of the molecule chain. Thus, the water molecules are difficult to penetrate into the films, and therefore the water resistance increases.
Heat resistance
The heat resistance investigation of polyurethanes allows the determination of proper conditions to obtain high-performance products which are stable and free of undesired by-products. Besides, TG provides a method for accelerating the lifetime testing of polyurethanes so it can be used to predict in-use lifetime.
Earlier work has shown that the thermal degradation initiates at the urethane group of hard segment. Polyurethane has at least two stages of degradation, where the initial stage is mainly due to the decomposition of hard segment. TG curves of UV-cured films are shown in Fig. 10 and Table 4, which present the degradation temperatures at a weight loss of 10%.
As seen in Fig. 10, all samples have a similar process of thermal decomposition. It is obvious that the initial temperature of decomposition and the temperature of complete decomposition for WHPUD films were about 200 and 315°C.
The degradation in the early stage plays an important role in the overall thermal stability of WHPUDs films. As seen in Table 4, the temperature of 10% weight loss is WHPUD 3.0-1.5 > WHPUD 3.0-1.0 > WHPUD 3.0-0.5, and the temperatures of WHPUD 3.5-1.0 and WHPUD 2.5-1.0 are close to WHPUD 3.0-1.0. The heat resistance of UV-cured films is improved with the increasing of hard segment. Samples with higher hard segment result in films with higher crosslinking density after being cured by UV and the possibility of hydrogen bonding formation, and possess better heat resistance. These results are coincident with the paper reported by Asif et al.25
Conclusions
In conclusion, we have successfully synthesized a series of UV-curable waterborne hyperbranched polyurethane by modifying the hyperbranched polyester H10, which was prepared using PEL as a core molecule and DMPA as monomers, with succinic anhydride, TDI, and HPA. The rheological behavior of WHPUDs suggested that all WHPUDs belong to pseudoplastic fluids and have a lower viscosity. The investigation of UV-curing kinetics indicates that these WHPUDs films are photosensitive, and that the percent conversion of C=C bond reaches about 80% when the exposure time was 50 s. TG analysis indicated that the heat resistance of the UV-cured films increased with an increase of the content of TDI–HPA, which is attributed to the formation of a highly crosslinked network through UV curing. Moreover, the UV-cured films showed excellent water resistance properties. In the field of overall properties, WHPUD 3.0-1.0 is the best sample with good storage stability, low viscosity, and small particle size. Moreover, the UV-cured film of WHPUD 3.0-1.0 has good water and heat resistance.
References
Ates, S, Aydogan, B, Torun, L, Yagci, Y, “Synthesis and Characterization Oftriptycene Type Crosslinker and Its Use in Photoinduced Curing Applications.” Polymer, 51 825–831 (2010)
Han, WS, Lin, BP, Yang, H, Zhang, XQ, “Synthesis and Properties of UV-Curable Hyperbranched Polyurethane Acrylate Oligomers Containing Carboxyl Groups.” Polym. Bull., 68 1009–1022 (2012)
Tasic, S, Bozic, B, Dunjic, B, “Synthesis of New Hyperbranched Urethane-Acrylates and Their Evaluation in UV-Curable Coatings.” Prog. Org. Coat., 51 321–328 (2004)
Gao, QZ, Li, HQ, Zeng, XR, “Preparation and Characterization of UV-Curable Hyperbranched Polyurethane Acrylate.” J. Coat. Technol. Res., 8 (1) 61–66 (2011)
Han, WS, Lin, BP, Zhou, YD, Song, JG, “Synthesis and Properties of UV-Curable Hyperbranched Polyurethane Acrylate Oligomers Containing Photoinitiator.” Polym. Bull., 68 729–743 (2012)
Gregorowicza, J, Fras, Z, Parzuchowski, P, Rokicki, G, Kusznerczukb, M, Dziewulski, S, “Phase Behaviour of Hyperbranched Polyesters and Polyethers with Modified Terminal OH Groups in Supercritical Solvents.” J. Supercrit. Fluid., 55 786–796 (2010)
Zhao, XF, Liu, LX, Dai, HX, Ma, CX, Tan, XH, Yu, RH, “Synthesis and Application of Water-Soluble Hyperbranched Poly(ester)s from Maleic Anhydride and Glycerol.” J. Appl. Polym. Sci., 113 3376–3381 (2009)
van Benthem Rolf, ATM, “Novel Hyperbranched Resins for Coating Applications.” Prog. Org. Coat., 40 203–214 (2000)
Cheng, XE, Huang, ZG, Liu, JH, Shi, WF, “Synthesis and Properties of Semicrystalline Hyperbranched Poly(ester-amide) Grafted with Long Alkyl Chains Used for UV-Curable Powder Coatings.” Prog. Org. Coat., 59 284–290 (2007)
Foix, D, Fernández-Francos, X, Ramis, X, Serra, A, Sangermano, M, “New Pegylated Hyperbranched Polyester as Chemical Modifier of Epoxy Resins in UV Cationic Photocuring.” React. Funct. Polym., 71 417–424 (2011)
Morgan, S, Ye, ZB, Zhang, KJ, Subramanian, R, “One-Pot Synthesis of Hyperbranched Polyethylenes Tethered with Pendant Acryloyl Functionalities by Chain Walking Copolymerizations.” Macromol. Chem. Phys., 209 2232–2240 (2008)
Deka, H, Karak, N, “Bio-based Hyperbranched Polyurethanes for Surface Coating Applications.” Prog. Org. Coat., 66 192–198 (2009)
Miao, H, Cheng, LL, Shi, WF, “Fluorinated Hyperbranched Polyester Acrylate Used as an Additive for UV Curing Coatings.” Prog. Org. Coat., 65 71–76 (2009)
Florian, P, Jena, KK, Allauddin, S, Narayan, R, Raju, KVSN, “Preparation and Characterization of Waterborne Hyperbranched Polyurethane-Urea and Their Hybrid Coatings.” Ind. Eng. Chem. Res., 49 4517–4527 (2010)
Maji, PK, Guchhait, PK, Bhowmick, AK, “Effect of the Microstructure of a Hyperbranched Polymer and Nanoclay Loading on the Morphology and Properties of Novel Polyurethane Nanocomposites.” ACS Appl Mater Interfaces, 1 (2) 289–300 (2009)
Du, YY, Chen, QL, Shen, L, Xing, YJ, Dai, JJ, “Synthesis and Application of Long-Chain Alkyl Quaternary Ammonium-Functionalized Hyperbranched Polyester.” J. Appl. Polym. Sci., 121 2927–2935 (2011)
Zhang, XL, “Synthesis and Characterization of Hyperbranched Polyesters Based on Isophthalic Acid and Trimethylolpropane.” J. Macromol. Sci. A, 48 128–134 (2011)
Murillo, EA, Cardona, A, Lopez, BL, “Rheological Behavior in the Molten State and Solution of Hyperbranched Polyester of Fourth and Fifth Generation.” J. Appl. Polym. Sci., 119 929–935 (2011)
Asif, A, Huan, CY, Shi, WF, “Structure–Property Study of Waterborne Polyurethane Acrylate Dispersions Based on Hyperbranched Aliphatic Polyester for UV-Curable Coatings.” Colloid Polym. Sci., 283 200–208 (2004)
Asif, A, Hu, LH, Shi, WF, “Synthesis, Rheological, and Thermal Properties of Waterborne Hyperbranched Polyurethane Acrylate Dispersions for UV Curable Coatings.” Colloid Polym. Sci., 287 1041–1049 (2009)
Yin, WH, Zeng, XR, Li, HQ, Hou, YJ, Gao, QZ, “Synthesis, Photopolymerization Kinetics, and Thermal Properties of UV-Curable Waterborne Hyperbranched Polyurethane Acrylate Dispersions.” J. Coat. Technol. Res., 8 (5) 577–584 (2011)
Gao, QZ, Li, HQ, Zeng, XR, “UV-Curing of Hyperbranched Polyurethane Acrylate-Polyurethane Diacrylate/SiO2 Dispersion and TGA/FTIR Study of Cured Films.” J. Cent. South. Univ., 19 63–70 (2012)
Wang, SJ, Liu, X, Kong, J, Tian, W, Fan, XD, Xu, H, Lu, JR, “Synthesis and UV Curing Kinetics of Rapidly UV-Curable Hyperbranched Polycarbosiloxanes.” Polym. Int., 59 1323–1330 (2010)
Qiao, LG, Shi, WF, “Synthesis and Characterization of Hyperbranched Polyurethane-Benzyltetrazole.” Chin. J. Polym. Sci., 6 (29) 670–683 (2011)
Asif, A, Hu, LH, Shi, WF, “Synthesis, Rheological, and Thermal Properties of Waterborne Hyperbranched Polyurethane Acrylate Dispersions for UV Curable Coatings.” Colloid. Polym. Sci., 287 (9) 1041–1049 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Lin, X., Zhang, S. & Qian, J. Synthesis and properties of a novel UV-curable waterborne hyperbranched polyurethane. J Coat Technol Res 11, 319–328 (2014). https://doi.org/10.1007/s11998-013-9520-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-013-9520-4