Abstract
Cd-enriched cadmium telluride (CdTe) polycrystalline films were grown on corning glass substrates by close spaced sublimation (CSS) technique. To our knowledge, Cd-enriched CdTe thin films by CSS have not been reported earlier. The structural investigations performed by means of X-ray diffraction (XRD) technique, scanning electron microscope (SEM), and energy dispersive X-ray spectroscopy (EDX) showed that the deposited films exhibit a polycrystalline structure with 〈111〉 as preferred orientation. The structural, optical, and electrical properties of these films were analyzed as a function of the Cd concentration. For the films having an excess of Cd, the electrical resistivity dropped several orders of magnitude. The deposited films also showed that the value of resistivity decreased with increasing temperature manifesting the semiconducting behavior of the films. The results showed that using this deposition technique, n-type Cd-enriched CdTe polycrystalline film could be produced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CdTe is II–VI semiconductor material quite appropriate for applications to solar energy conversion devices.1–3 The employment of this semiconductor as solar cell material has been limited basically by economical factors. An important reduction in the cost of solar cells can be achieved by preparing doped polycrystalline thin films of appropriate quality. CdTe thin films can be prepared by several growth techniques.4–19 However, the best CdTe thin-film-based solar cells have been fabricated using CSS method; this is one of the various techniques with large area manufacturing potential due to its high throughput and efficient material utilization.20 Thin films prepared by close spaced sublimation technique have distinct novel features, such as large grain sizes, well-defined preferential orientation, and relatively high absorption coefficients needed in the fabrication of solar cells material. This article deals with the analysis of some physical properties of Cd-rich CdTe thin films prepared by CSS method.
Experimental
CdTe thin films were deposited on the corning glass substrates. The deposition of CdTe thin films by the CSS technique is based on the following reversible dissociation of CdTe at high temperature:
A very small distance of about 4 mm separates a CdTe source and a substrate. The source is maintained at a higher temperature than the substrate. At a high temperature, the source CdTe dissociates into its constituent elements (Cd and Te), and then they recombine on the substrate surface, which is at a lower temperature.
The source material (CdTe 99.999% purity) was placed in a graphite sublimation cast. The substrate was first cleaned with IPA bath in an ultrasonic cleaner for 60 min and then cleaned by lint free tissue with the help of tweezers and finger cots to avoid fingerprints on glass. It was then kept in the desiccators for drying. The substrate was then supported by the cast and kept in a close proximity to the source material. The vacuum chamber was then closed and allowed to evacuate by a rotary vane pump. The chamber was kept for evacuation for 1 h each time and vacuum reaches approximately 10−3 mbar depending on the source temperature. The source and substrates could be maintained at the desired temperature as they were heated and controlled separately. Two K-type thermocouples were used to monitor the temperatures of the source and the substrate during the evaporation process. The temperatures of the source and substrate were maintained at 500 and 350°C, respectively. The deposition time was 5 min. The film was then kept at substrate temperature until the temperature of source became lower than the substrate temperature. After that the substrate heater was switched off to allow cooling to ∼40°C before opening the vacuum chamber. By using the automatic temperature controller for heating the source and substrate, the quality of the film was quite improved. After the deposition of CdTe, Cd (99.99% purity) was deposited on these films used as substrate by the same technique. The source (Cd) and substrate temperatures were 350 and 250°C respectively. After deposition the films were removed from the chamber and different properties were studied.
The compositions of the samples were determined by EDX attached with the scanning electron microscopy (SEM). The structure of the samples was studied by X-ray diffraction (XRD) technique. Resistivity of samples was measured (with van der Pauw geometry contacts) and with two probes method at room temperature.
Results and discussions
X-ray diffraction studies
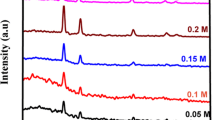
The structure of the deposited films was studied by using XRD technique with Cu-Kα radiation (λ = 1.5418 Å). The XRD patterns of the as-deposited sample and Cd-enriched CdTe samples are presented in Fig. 1. The main reflection of all the samples is the same and can be indexed based on a cubic CdTe lattice.19 The strong (111) reflection in the patterns indicates a preferential orientation of [111] in films as already reported for thermally evaporated CdTe thin films.21 The low intensity of other peaks suggests a few other oriented grains. The position of (111) crystalline line shifts slightly to higher angular position as Cd concentration is increased. The lattice constant for each sample was calculated from the diffraction spectra using Bragg law and angular position of (111) diffraction peak. From the full-width at half maximum (FWHM) of the diffraction peaks one may estimate the average grain diameter d of polycrystalline materials by applying the Scherrer formula:
where the Scherrer constant k is equal to 0.9,22 D is the full width at half maximum in radians and θ is the Bragg angle in degrees corresponding to the maximum peak intensity. In this way the average grain size in our as-deposited sample comes out to be 490 nm.
The lattice constant decreases as Cd concentration is increased. The incorporation of extra Cd reduces the lattice constant due to reduction in the bond length produced by the interstitial Cd atoms in CdTe lattice. The calculated values of lattice constants for all samples are given Table 1. It has been seen that the glass substrate and its crystallographic orientations have no effect on the growth of CdTe thin films. Similar results have been reported by Mane et al.23
Morphology studies
JEOL JSM SEM along with energy dispersive X-ray spectroscopy (EDX) attachment (INCA 200; Oxford Instruments, UK) is used to investigate the elemental composition of Cd-riched CdTe samples. Typical EDX patterns and detail of relative analysis of the samples are given in Figs. 2a and 2b. The elemental analysis was carried out only for Cd and Te. The EDX analysis of the samples to the actual contents of Cd and Te is given in Table 1. The EDX results show the Cd content in sample SI–SVII increases, which indicates that Cd has been doped into CdTe films by this technique.
The morphology and microstructure of samples were analyzed by SEM, and reveal that the Cd-enriched CdTe samples using CSS have a significant change in the shape and size. As a result, different microstructure and morphology are created. SEM images of as-deposited and Cd-enriched CdTe samples are given in Figs. 3a–3c.
Optical analysis
The deposition parameters were selected to reduce the surface roughness. However, it is known that the CSS method results in rough surface films when compared with other methods. The transmittance T spectra (in the range 350–2500 nm) were recorded for as-deposited CdTe and Cd-enriched CdTe samples using Perkin–Elmer, Lambda 900, UV–VIS/NIR spectrophotometer with UV-Winlab software. The examination of the deposited samples in the large wavelength span of the spectrum is possible because our substrates show more than 90% transmission for visible to far infrared wavelengths. Usually CdTe films have a high absorption in the region of visible and near infrared.18 Indeed, the same was observed in the films deposited during this study. The transmittance spectrum of the enriched samples did not change significantly after the optimization of heat treatment for 30 min, which ensured the diffusion of Cd in the samples.
The transmittance decreases with the increase of Cd content as shown in Figs. 4a–4c. It is clear from the figure that the Cd has strongly affected the transmittance. The Cd present in the samples shows high absorption in the respective region.
Electrical analysis
The electrical properties of semiconductor thin films strongly depend on the factors like film compositions, ambient conditions like temperature and exposure to light. We have checked dark electrical resistivity as a function of Cd concentration for the SI–SVII samples. The direct method of measuring electrical resistivity, or sheet resistance of a prepared rectangular sample is described.24 For electrical measurement, very low resistance silver wires and silver paste for ohmic contacts were used and all measurements were performed at room temperature.
Due to the formation of Cd vacancies in CdTe lattice acting as acceptor centers, CdTe films prepared by different techniques have generally high electrical resistivity (107–109 Ωcm), and with slightly p-type conductivity, on the other hand, an excess of Cd atoms located interstitially can produce n-type CdTe samples.19
Figure 5 shows the dark electrical resistivity as a function of Cd concentration for all the samples. It is clear from the figure that the resistivity of the samples having an excess of Te is around 108 Ωcm, which drops to about 105 Ωcm on Cd concentration approximately reaching 52 at.%. The result shows that samples having excess of Te contain a small amount of crystalline Te which is embedded in the CdTe structure and gradually disappears with the increasing of Cd. It is also known that the CdTe structure in these samples contains Cd vacancies, which account for the p-type conductivity and therefore, making it difficult for the n-type doping of this material. The electrical resistivity of these samples changes with increasing Cd concentration. It is clear that the incorporation of Cd first prevents the formation of crystalline Te and then inhibits the formation of Cd vacancies. At about 50.5 at.% of Cd, the vacancies have been drastically reduced and the incorporation of more Cd probably goes to the interstitial positions in the CdTe lattice. It means that the electrical parameters change as soon as the Cd exceeds the stoichiometric composition indicating an effective enriching by the interstitial Cd.
The temperature-dependent dark resistivity of Cd-enriched CdTe thin films was measured and found that the dark resistivity decreases with increasing temperature, which shows the semiconducting behavior of the films.
Conclusions
The present work demonstrates the preparation of Cd-enriched CdTe thin films by CSS technique and their characterization. The experimental results show that the position of (111) crystalline line shifts slightly to higher angular position as Cd concentration is increased. The lattice constant decreases as Cd concentration is increased. The incorporation of extra Cd reduces the lattice constant due to reduction in the bond length, which is produced by the interstitial Cd atoms in CdTe lattice. The Cd-enriched CdTe samples using CSS have a significant change in the shape and size. As a result, different microstructure and morphology are created. The EDX results show the Cd content in samples increases, which indicates that Cd has been doped into CdTe films by this technique. The Cd has strongly affected the transmittance. The transmittance decreases with the increase of Cd content. The Cd present in the samples shows high absorption in the respective region. It also shows that as-deposited CdTe films have high electrical resistivity ≈3.48 × 108 Ωcm and are slightly p-type conductivity, due to the formation of Cd vacancies in the CdTe lattice acting as acceptor centers. The electrical resistivity of CdTe samples with an excess of Cd atoms drops several orders of magnitude, i.e. 1.03 × 105 Ωcm and the conductivity goes from p-type to n-type when the composition of the films goes from Te rich to Cd rich. The results presented here indicate that n-type doping can be achieved when interstitial Cd atoms are incorporated in the CdTe lattice.
References
Zweibel, K, Ullal, HS, Mitchell, RL, “Polycrystalline Thin Film Photovoltaics.” 21st IEEE Photov. Spec. Conf. Kissimee (1990)
Abbas Shah N, Ali A, Aqili AKS, Maqsood A, Physical Properties of Ag Doped Cadmium Telluride Thin Films Fabricated by Closed Space Sublimation Technique. J. Crys. Growth, 290, 452–458 (2006) 10.1016/j.jcrysgro.2006.01.051
Bashrat M, Hannan MA, Shah NA, Ali A, Arif M, Maqsood A Structural, Optical and Electrical Characterization of Hg x Cd1−x Te Polycrystalline Films Fabricated by Two-Source Evaporation Technique. J. Cryst. Res. Technol., 42 817–821 (2007) 10.1002/crat.200710911
Bube RH (1998) Photovoltaic Materials. Imperial College Press, London
Wu, X, Keane, JC, Dhere, RG, Dehart, C, Albin, DS, Duda, A, Gessert, TA, Asher, SD, Levi, H, Sheldon, P, “Seventeenth European Photovoltaic Solar Energy Conference.” Proceedings from the International Conference, Vol. 1, p. 995. WIP-Munich and ETA-Florence (2001)
Chou HC, Rohatgi A, Jokerst NM, Kamra S, Stock SR, Lowrie SL, Ahrenkiel RK, Levi DH Approach Toward High Efficiency CdTe/CdS Heterojunction Solar Cells. Mater. Chem. Phys., 43 178–182 (1996) 10.1016/0254-0584(95)01626-6
Chu TL, Chu SS, Ferekides C, Britt J, Wu Q Cadmium Telluride Films by Metalorganic Chemical Vapor Deposition. J. Appl. Phys., 69 7651–7655 (1991) 10.1063/1.347536
Rohatgi, A, Summers, CJ, Erbil, A, “A Study of Polycrystalline Cd(Zn, Mn)Te/Cds Films and Interfaces.” Proceedings of the Polycrystalline Thin Film Program Review Meeting, 45 (1989)
Yamaguchi K, Nakayama N, Matsumoto H, Ikegami S CdS–CdTe Solar Cell Prepared by Vapor Phase Epitaxy. J. Appl. Phys., 16 (7) 1203–1211 (1977) 10.1143/JJAP.16.1203
Uda, H, Matsumoto, H, Komatsu, Y, “Interdiffusion of CdS and CdTe in Screenprinted and Sintered CdS.” 16th IEEE Photovoltaics Specialists Conference, p. 801 (1982)
Matsumoto H, Kuribayashi K, Uda H, Komatsu Y, Nakano A, Ikegami S Screen-Printed CdS/CdTe Solar Cell of 12.8% Efficiency for an Active Area of 0.78 cm2. Solar Cells, 11 367–373 (1984) 10.1016/0379-6787(84)90100-5
Basol, BM, Tseng, ES, Rod, RL, Ou, SS, Stafsudd, OM, “Development and Analysis of Vapor Transport Deposition of Cadmium Telluride Thin Film.” 16th IEEE Photovoltaic Specialists Conference, pp. 801–804 (1982)
Meyers PV Preparation of Dilute Magnetic Semiconductor Films by Metalorganic Chemical Vapor Deposition. Solar Cells, 24 35 (1988) 10.1016/0379-6787(88)90034-8
Serreze, HB, Lis, S, Squillante, MR, Turcotte, R, Talbot, M, Entine, G, “Spray Pyrolysis Prepared CdTe Solar Cells.” 15th IEEE Photovoltaics Specialists Conference, p. 1068 (1981)
Yi X, Wang L, Mochizuki K, Zhao X (1988) CdTe Crystals Grown by Chemical Vapour Deposition. J. Phys. D Appl. Phys. 21 1755 (1988) 10.1088/0022-3727/21/12/015
Rusu M, Nicolaescu II, Rusu GG Influence of Deposition Conditions on the Structural Characteristics of Sublimated CdTe Thin Films. Appl. Phys. A, 70 561 (2000) 10.1007/s003390051081
Rose, D, Powell, R, Jayamaha, U, Maltby, M, Giolando, D, McMaster, A, Kormanyos, K, Faykosh, G, Klopping, J, Dorer, G, “Solar Energy Materials and Solar Cells.” 28th IEEE Photovoltaic Specialists Conference, p. 428 (2000)
McCandless, BE, Birkmire, RW, Buchanan, WA, “Contact Wetting Angle as a Characterization Technique for Processing CdTe/CdS Solar Cells.” 29th IEEE Photovoltaics Specialists Conference, p. 547 (2002)
Abbas Shah N, Ali A, Ali Z, Maqsood A, Aqili AKS Properties of Te-rich Cadmium Telluride Thin Films Fabricated by Closed Space Sublimation Technique. J. Crys. Growth, 284 477 (2005) 10.1016/j.jcrysgro.2005.08.005
Abbas Shah N, Ali A, Maqsood A Preparation and Characterization of CdTe for Solar Cells, Detectors and Related Thin Film Materials. J. Electron. Mater., 37(2) 145–151 (2008) 10.1007/s11664-007-0338-7
Ali A, Abbas Shah N, Aqili AKS, Maqsood A, Impact of Evaporation Rates of Cd and Te on Structural, Morphological, Optical and Electrical Properties of CdTe Thin Films Deposited by a Two-Sourced Evaporation Technique. J. Cryst. Growth Des., 6, 2149–2154 (2006)
Kapaklis V, Poulopoulos P, Karoutsos V, Manouras T, Politis C Growth of Thin Ag Films Produced by Radio Frequency Magnetron Sputtering. Thin Solid Films, 510 138–142 (2006) 10.1016/j.tsf.2005.12.311
Hannan MA, Basharat M, Shah NA, Ali A, Arif M, Maqsood A Preparation and Physical Properties of Cd x Hg1−x Te Thin Films Fabricated by Close Spaced Sublimation Technique. Mater. Lett. 62 1400–1402 (2008) 10.1016/j.matlet.2007.08.065
Schroder DK Semiconductor Materials and Device Characterization, 2nd edn. John Wiley & Sons Inc., New York, (1988)
Acknowledgments
The authors would like to thank HEC for financial support through project 20-694(2006) and COMSATS Institute of Information Technology Islamabad Pakistan for providing facilities. The authors would like to thank Dr. Adil Syed, Manzar Abbas and Dr. Ashraf Atta of CIIT, Islamabad, for valuable discussion. This work was partially done at the Thermal Physics Laboratory, Quid-e-Azam University, Islamabad.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arif Khan, M., Shah, N.A., Ali, A. et al. Fabrication and characterization of Cd-enriched CdTe thin films by close spaced sublimation. J Coat Technol Res 6, 251–256 (2009). https://doi.org/10.1007/s11998-008-9111-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-008-9111-y