Abstract
The curing kinetics of UV-curable powder coatings based on commercial unsaturated polyesters were monitored using photo-DSC, Real-Time FTIR-ATR and a modified rheometer equipped with a UV source. The effect of physical and chemical factors on curing such as type of photoinitiator, photoinitiator concentration, temperature and atmosphere of curing were evaluated. Coatings containing amounts of photoinitiator from 0.5 to 10 wt% were cured at different temperatures in less than 10 s reaching conversions approximately of 60%. The increase of the temperature of curing reduces the final conversion and also the rate of polymerization due to the chain transfer process and depolymerization that dominates the photopolymerization at high temperatures. The reactivity of the photoinitiators was similar for all the studied photoinitiators apart from benzophenone that was found to be the slowest initiator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, high-quality industrial coatings with both superior mechanical and decorative properties are often solvent-based and cured through stoving, i.e., at high temperatures. This practice is environmentally unfriendly and does not comply with the EU solvent directive. In addition, stoving, which is necessary for powder coatings, requires energy consuming heating. Typical stoving temperatures for powder coatings are in the range of 150–200°C. The powder coatings have been optimized at these high temperatures in order to prevent thermal curing before a good leveling is achieved. The high temperatures required restrict the use of these coatings to substrates that can withstand these temperatures such as metals. The theoretical lowest curing temperature possible for conventional amorphous powder coating systems is approximately 110°C. This temperature is determined by the combination of two different factors; storage stability of the powder and the process of film formation. Powder coatings must be storable at 30°C without fusing of the particles. Therefore, the glass transition temperature (T g) of the binders must be 60°C or higher in order to insure this storage stability. On the other hand the process of film formation for amorphous material requires a temperature of at least 50°C above T g. Taking into account these factors, the resulting minimum curing temperature for a stable powder coating should be approximately 110°C, although finding a system reactive at 110°C and still extrudable at 90°C seems to be complicated. Hence it is clear that meeting these requirements with conventional coatings is difficult. However, some systems have been described in the literature, e.g., systems that cure at temperatures starting at 130°C that are based on blocked isocyanates.1
Low-temperature curable coatings require an initiator that is activated at the curing temperature but stable at storage temperature,2 which is difficult to achieve using conventional thermal initiators as we have already explained. At this point UV curing offers a possible solution to this problem since it allows separation of the melting and the film formation stage from the curing stage. In UV-curable powder coatings, the melt and the flow are controlled by the application of heat to the substrate and the final curing stage is then only activated by the presence of UV light leading to the crosslinking reaction of the coating. The powder is melted in general by IR sources and afterwards the curing reaction is initiated by UV light. Using this technology, coatings with smooth finishes can easily be obtained in a very short period of time without overheating the samples. By lowering the curing temperature, the application of these formulations can be extended to heat sensitive substrates like preassembled metal objects containing heat sensitive parts, MDF boards for furniture applications, wood, and even plastics. This makes powder coatings interesting for many different industries. UV-curable powder coatings have recently gone from what might be called “laboratory curiosities” to a new industrial technology.
The photoinitiator (PI) is well known as the key factor that governs the curing speed and the through cure of the final coating. The performance of a photoinitiator in a UV-curable coating can be evaluated using different techniques. The kinetics of the fast photopolymerization reaction can be analyzed by photo-DSC and Real-Time Infrared spectroscopy (RTIR). The viscoelastic properties during curing and the mechanical properties of the cured coatings can be evaluated by rheological studies.
Photo-DSC has been used extensively to evaluate the kinetics of the curing of UV systems,3,4 and the technique is ideally suited to compare different PIs and various curing conditions. Nevertheless, photo-DSC has some limitations such as long response times, so in some cases it is not possible to determine the kinetics of reactions which occur in less than 10 s.5 Also it is necessary to know the theoretical value of the enthalpy of the reaction, and that value is not always available for complex systems such as commercially available coating formulations that contain different types of additives. Another technique that has been widely used and has been a powerful tool to monitor UV curing reactions is RTIR spectroscopy that is capable to study reactions that can occur in a short time scale. It offers a rapid and quantitative measurement of the conversion of the selected functional group.6,7 The use of rheology to monitor the UV curing of powder coatings has not been widely studied, but a few authors describe the use of a modified rheometer to monitor the cure of fast reactions8,9 and are able to determine gel points within less than 1 s.8
In this article we describe a complete study of the kinetics of a UV-curable powder coating by these different techniques. Commercially available unsaturated polyester-based powder coatings formulations are cured by means of UV radiation at relatively low temperatures. The kinetics of the reaction is studied by means of photo-DSC, rheology, and real-time FTIR. The effect of various parameters such as temperature, photoinitiator concentration, and the type of initiator are evaluated.
Experimental part
Materials
The polypropylene sheets (10 cm * 10 cm * 2 mm) are supplied by Vink co. (Didam, The Netherlands) and the surface was modified using different photoinitiators by a photografting method as described previously.10 The unsaturated dimethacrylate polyesters, Uvecoat 1000 and Uvecoat 9010, were supplied by UCB Chemicals (Drogenbos, Belgium) and used as received. The photoinitiators, Benzohenone (BP), 1-Hydroxy-cyclohexyl-phenylketone (HCHPK), 2,2-dimethoxy-2-phenylacetophenone (DMPA), Bis(2,4,6-trimethylbenzoyl)-phenylphosphine-oxide (BTPPO) and 1-[4-(2-Hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (HEPHMP), were supplied by Ciba Speciality Chemicals (Groot-Bijgaarden, Belgium) and used without any further purification (Table 1).
The absorption spectrum of the initiators is shown in Fig. 1.
Preparation of the powder
Both resins were mixed using the proportion described in the literature as the one that offers the best performance; 75 wt% Uvecoat 1000 and 25 wt% Uvecoat 9010.11
In order to mix the two resins and the different initiators the components were ground using an Alpine 100 UPZ pinmill and extruded at 80°C and 250 rpm using a twin-screw extruder from APV. After extrusion the mixture was ground again and sieved to obtain a powder with a particle size below 150 μm.
Photo-DSC
The photo-differential scanning calorimetry (Photo-DSC) measurements were carried out by means of a modified Perkin Elmer DSC 7 equipped with an Osram F mercury lamp (450 W). A uniform UV light intensity is delivered across the DSC cell to the sample and reference pans. The wavelength of the UV radiation was selected with the use of a monochromator (350 ± 1 nm). The intensity of the UV light at the measuring cells was evaluated using two graphite cylinders 1 mm thick as described12 and the value obtained was approximately 1.60 mW/cm2. The measurements were performed with equal sample amounts, i.e., approximately (but precisely weighted) 10.0 mg. Nitrogen was used as purge gas in a flux of 20 mL/min. Indium and tin were used in the temperature and enthalpy calibration of the DSC. The sample was held at the isothermal temperature for 5 min under inert conditions prior starting the measurement. The measurement started maintaining the samples at the isothermal temperature for 2 min, at that time the shutter was opened and the sample was exposed to UV light for 5 min. The electronic shutter was controlled by the software of the DSC and thus enables synchronization between the irradiation and the measurement of the evolved heat. The shutter was closed after the irradiation time and the temperature was held for an additional 1 min to recover the baseline. All the exotherms obtained were plotted as heat flow vs. irradiation time. The peaks were integrated to yield the enthalpy evolved per mass unit during the UV curing process. Each experiment was repeated at least three times.
Real-time infrared spectroscopy (RTIR)
The infrared spectra were recorded with attenuated total reflectance (ATR) in real time using a Bio Rad FTS 3000MX Excalibur spectrometer with a MTC detector. The spectrometer was equipped with a heatable Golden GateTM Diamond ATR Top-Plate (Specac) that permits recording spectra up to 200°C. The temperature is controlled by a separate 3000 Series Temperature Controller (Specac). The spectra were recorded with a time resolution of 1 s and with a spectral resolution of 4 cm−1. For the curing experiments, the spectrometer was connected to a Spectral Illuminator (Oriel Instruments Systems) equipped with a 200 W Thermo Oriel mercury lamp with a liquid light guide (Oriel Instruments Systems). The intensity of the UV light was measured with a Thermo Oriel Radiant Power energy meter 70260. The measured intensity at the region of 350 nm was approximately 2.60 mW/cm2 on the ATR crystal. The wavelength of the incident light was 350 nm after passing through a monochromator Spectral Illuminator (Oriel Instruments Systems). The UV light was focused at the ATR crystal and the whole compartment was flushed either with air or dry nitrogen to evaluate the influence of the atmosphere on the curing reaction. In all the experiments the shutter was opened by the software of the RTIR spectrometer exactly 5 s after the start of the measurement.
The powder was applied on top of the diamond crystal and melted at the selected temperature. Films of approximately 50 μm thickness were obtained using a Doctor Blade applicator. The curing reaction was followed by monitoring the decay of the intensity of the peaks at 810 cm−1 (alkene twisting vibration)13 by integrating the peak areas. Each experiment was repeated at least three times.
Rheology
The rheology measurements were performed using a Paar Physica UDS200 equipped with a custom-built set up allowing UV irradiation during the measurement. The upper plate incorporates a removable quartz cylinder of 1 cm diameter that allows the light to reach the sample. The bottom plate heats the sample and when a homogeneous melt was obtained the measuring gap was set at 0.1 mm to insure complete filling of the measuring gap. The light was transmitted from a Macam UVLS 1000 mercury lamp to the rheometer through a liquid light guide (Macam Photometrics Ltd.). An electronic shutter was controlled through the software of the rheometer to insure a good synchronization between irradiation and measurement. The intensity of the UV light was measured using a Lightbug IL390B radiometer (International Light Inc.) and was approximately 1.0 mW/cm2.
Oscillatory measurements were performed on 0.1 mm samples at 100°C, using a frequency of 1 Hz and strain of 10%. The measurement was performed as a function of time starting with 1 min without irradiation followed by 7.5 min of UV exposure in which the curing took place. The bottom plate was cleaned after each experiment in order to maintain the reflectivity constant. All the experiments were performed under nitrogen atmosphere. Reproducibility was good as checked on some of the formulations.
Results
Photo-DSC
A problem of relevance in practical applications is the effect of temperature on the kinetics of the photopolymerization reactions and several studies report the use of photo-DSC to evaluate the effect of temperature.14–18
Effect of temperature
To study the effect of temperature on the isothermal curing of a UV-curable powder coating formulation a sample containing 2.5 wt% of a mixture of BTPPO and HEPHMP (1/1 wt/wt) as PI was cured at different temperatures in the range from 90 to 180°C. Figure 2 shows the isothermal DSC results recorded at different temperatures.
The isothermal curing at 90°C showed that the reaction already occurs at this temperature that is slightly above the melting temperature of the formulation. The enthalpy evolved at this temperature was approximately 25 J/g. This value increases slightly with temperature up to 120°C where a maximum is observed (see Fig. 2b). Temperatures higher than 120°C result in a substantial decrease in the enthalpy that decreases rapidly down to approximately 7 J/g at 170°C. No thermal polymerization is observed at these temperatures.
The curing of Uvecoat 1000 with different initiators was reported previously by Padaki et al.19 In their work they use photo-DSC to monitor the curing at different temperatures and to study the effect of pigments in the curing process. They found for unpigmented systems that the curing enthalpies are similar to those values obtained in our study, approx. 25 J/g. They describe that on increasing temperature the enthalpy reduces in a quadratic fashion as we had observed (Fig. 2b). The optimal curing temperature that they found results to be 110°C.
The effect of temperature on the UV curing of the Uvecoat resins depends on two factors. The first is that BTPPO decomposes thermally at 168°C reducing the amount of radicals generated and resulting in a less effective crosslinking at high temperatures.20 Second, at high temperatures the depolymerization reaction becomes more important, ultimately resulting in a rate of polymerization equal to zero. At these temperatures the termination process suppresses the auto-acceleration reaction resulting in lower conversions. Padaki et al.19 in their photo-DSC study demonstrated that no thermal curing took place at high temperatures and that photoinitiator loss did not occur at an appreciable rate. According to these results we can clearly conclude that the reduction of conversion at high temperatures is mainly caused by competing termination reactions and depolymerization mechanisms.
The same tendency of decreasing the conversions at high temperatures was also made by Broer et al.21 in the study of photocuring of acrylates and diacrylates.
In order to confirm the temperature dependence, formulations containing 2.5 wt% of DMPA were cured in the same range of temperatures. It was found that the enthalpy increased in the range from 90 to 120°C. Higher temperatures led to a rapid decrease in the conversion as it was found previously. The similar behavior found in these formulations helps us to confirm that the main reason for the decrease in conversion is due to the depolymerization and competing reactions at high temperatures.
Since the theoretical value of the enthalpy is unknown and the composition of the formulation is complex it is impossible to calculate the progress of reaction. It must be mentioned that no residual enthalpy can be measured in a subsequent dynamic temperature scan in the absence of UV light indicating completeness of reaction. RTIR should provide us information about the degree of conversion of the double bonds as it will be discussed later.
Effect of concentration and type of PI
In order to compare the behavior of the different initiators in the curing reaction formulations containing 1, 2.5, 5, and 10 wt% of each PI were prepared as described in the experimental section. Our main goal is to apply these formulations onto polypropylene (PP) substrates, therefore the lower the temperature the less deformation of the PP is expected. The minimum temperature at which conversion is acceptable and flow properties are good is 100°C. Therefore, it was decided to continue the study with the different PIs at 100°C.
Figure 3 shows an example of the isothermal curing of the formulation containing different concentrations of DMPA. The overall enthalpy measured in the 5 min of irradiation increases from 1 wt% (27.4 J/g) to 5 wt% (28.7 J/g). At 10 wt% the measured enthalpy decreases to 24.5 J/g indicating a decrease in conversion due to the different competing mechanisms and probably to higher absorption of the initiator in the outer layers that prevents the light to reach the whole sample. It must be mentioned at this point that the step observed at ca. 7 min when the radiation is turned off is caused by the thermal heating of the DSC pan by the UV source and it is corrected to evaluate the evolved heat. Obviously the time to reach the maximum is independent of the isothermal curing temperature, indicating that the rate of decomposition of the photoinitiator is not dependent on the curing temperature within the studied temperature range.
Figure 4 collects the values of the enthalpies measured in isothermal curing conditions at 100°C during 5 min for the selected PIs at different concentrations. From this figure we can observe that no significant differences in the enthalpy evolved are observed for the different concentrations studied. Only BP has a different behavior, it has not only lower enthalpy values, but it also increases the conversion as the concentration increases in the range studied. This can be explained by the different initiation mechanism for this PI and the lower absorption of UV light. The slower reaction rate allows the system to rearrange during curing increasing the conversion as the concentration of PI increases. In the other formulations no significant differences were observed up to contents of 5 wt%. When higher concentrations were used a negative effect was observed and the conversion decreases because of the high rate of recombination of radicals, the higher absorption of UV light of the initiator in the top layers and also as a result of some incompatibility of the PIs with the molten polymeric binder. The reaction proceeds faster and leads to some unreacted groups that are inaccessible for the radicals so that larger amounts of radicals had no further effect on conversion.
If we compare the reactivity of the different initiators we observe that the lowest enthalpy corresponds to BP. This PI is benzophenone, and it is well known that the initiation process goes via a hydrogen abstraction type mechanism.5 All the other initiators are α-cleavage PIs and have a higher absorption at 350 nm. In these formulations enthalpies between 25 and 30 J/g are observed. The time at the maximum in the exotherm observed in the isothermal curing can be related to the half-life (t 1/2) of the PIs. This t 1/2 is comparable for all α-cleavage type PIs studied and the values are approximately 9 s. In the case of BP t 1/2 is measured to be 15 s, again a clear indication that the reaction proceeds slower. Despite the fact that the evolved heat is comparable for all the concentrations studied, it can be clearly seen from the shape of the exotherm that the reaction proceeds faster during the first seconds of irradiation as the concentration of initiator increases, as mentioned before.
Summary of the photo-DSC results
Photo-DSC has been proved useful to study the isothermal UV curing of UV powder coatings. The effect of curing temperature was evaluated and it was found that temperatures higher than 120°C had a negative effect on conversion, the depolymerization and competing reactions being responsible for this decrease. The concentration of the PI in the formulation was also evaluated and it was found that the conversion increases with concentration when BP was used as PI. In the other formulations no significant differences were observed up 5 wt%. Higher concentrations had a negative effect because of the high rate of recombination of radicals, the higher absorption of UV light of the initiator in the top layers and also as a result of some incompatibility of the PIs with the molten polymeric binder. The reactivity of the various PIs was studied and only BP showed a different behavior. The lower reactivity of this PI is due to a different mechanism of generating radicals. The rest of PIs showed similar behavior as a function of concentration and temperature.
Real-Time Infrared Spectroscopy (RTIR)
Different authors have shown that RTIR spectroscopy is an excellent method for determining both the rate and extent of radiation initiated polymerization.22,23 This technique offers the opportunity to study fast curing reactions, e.g., acrylates,6 vinyl acrylates,24 acrylate oxetanes,25 by monitoring the absorption of specific bands. There are also results published in the field of electron beam curable systems using RTIR.26
In our powder coating formulation the absorption by double bonds is weak (see Fig. 5). Small peaks at 1636 and at 810 cm−1 can be associated with the unsaturated carbons.13 The band at 1636 cm−1 is overlapping with the carbonyl band. Moreover, its low intensity prevents an accurate measurement of the double bond disappearance. Traditionally, the alkene twisting vibration band at 810 cm−1 has been used to monitor the double bond conversion13 as we have done in this study.
Figure 5 also contains the IR spectra after irradiation with UV light for 30 s. The decreasing intensity of the bands at 1636 and 810 cm−1 is related to the conversion of double bonds. The band at 1160 cm−1 shows a decrease in absorption and it can be related to the C–O stretching mode of the ether linkage adjacent to the double bond indicating again the disappearance of the unsaturated groups as the reaction proceeds.
Effect of temperature
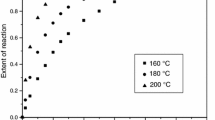
The effect of temperature on the kinetics of photopolymerization is of high practical relevance when a heat sensitive substrate is used. In order to evaluate the effect of the curing temperature on the kinetics of the polymerization, the formulation containing 2.5 wt% of a mixture of HEPHMP and BTPPO (1/1 wt/wt) was used. The isothermal studies by photo-DSC had shown that this PI had a high conversion at all the concentrations studied. The good through cure of the formulation is due to the different absorption regions of the two PIs.11 The selected temperatures range was taken guided by the photo-DSC results, from 90 to 180°C. The kinetic profiles at different temperatures give us information about the conversion vs irradiation time as shown in Fig. 6 . From these profiles we can observe that the reaction is completed between the first 5 and 10 s of irradiation at temperatures up to 160°C. Above these temperatures the time for completion is increased. Although the slope of the curve is similar at all the temperatures studied, differences in conversion can be noted from these curves. At the lower temperatures studied, between 90 and 100°C, the largest amount of double bonds are consumed (64%). The increase in curing temperature has a negative effect on the quantity of reacted double bonds resulting in lower conversions. This fact can be explained by the competition of polymerization and depolymerization processes. These results are in agreement with the previous findings using photo-DSC where, in spite of not being able to calculate the conversion, we noted the same tendency. (Fig. 7)
Comparing the results of the RTIR and photo-DSC it can be observed that the conversion profiles are related to the enthalpy measured in the DSC.
In order to confirm the dependence of the conversion with the curing temperature, formulations containing 2.5 wt% of DMPA were studied in the same range of temperatures. The curing using initiator DMPA showed the same tendency as the curing using the mixture of BTPPO and HEPHMP. The conversion decreases as the temperature increases, but in this case the differences were smaller. Only at the highest temperature (180°C) the conversion dropped to about 20%. This temperature dependence is similar as observed for DMPA and decreases as the temperature of curing increases. The maximum of the polymerization rate is measured again within the first second of irradiation but in this case with conversions that range from 15% to 40% depending on the curing temperature.
Effect of type of PI
The efficiency of different photoinitiators can be easily checked using RTIR measurements. The conversion of the double bonds in the different formulations containing 2.5 wt% of the selected PIs was monitored at 100°C on exposure to UV. The obtained conversion profiles are shown in Fig. 8. These profiles show that curing took place efficiently and that degrees of conversion between 50% and 70% are reached after irradiation. The studied initiators have similar behavior and the maximum conversion is reached after 10 s of irradiation for all the initiators apart from BP. This initiator has a slower reactivity and the maximum conversion is reached after 20 s of exposure. This lower reactivity was also observed by photo-DSC. BP has a value of maximum polymerization rate (Rp/[M 0])max of 0.02 (s−1) compared to the value of 0.10 (s−1) of HEPHMP. This maximum rate of polymerization (Rpmax) was determined making the product of the maximum slope (dα/dt)max of the conversion profiles with the initial concentration of double bonds [M 0].
The formulations cured with the mixture of initiators BTPPO and HEPHMP (1/1 wt/wt) lead to the highest conversion, 70%. From the RTIR data we can conclude that the crosslinking reaction is complete after 20 s of exposure for BP and after 10 s of exposure for the other PIs studied when the curing is performed under nitrogen atmosphere.
The curing in absence of PI was also studied because in practice it is known that UV degradation can take place. In our systems it was found that the curing reaction proceeds very slowly at 100°C without PI in the formulation, only conversions below 5% are achieved after 3 min of irradiation. The reason for this curing reaction might be the generation of free radicals in the backbone of the oligomers. Figure 9 shows the conversion of double bonds vs irradiation time in absence of initiator as well as the conversion achieved using 1 wt% of DMPA. The reaction proceeds much faster in the presence of 1 wt% of initiator where the maximum conversion reached is approximately 60%. In the case of the absence of initiator an induction time is present and at 35 s of irradiation the first change in double bond content is observed. The reaction rate is much lower; only 3% of the double bonds are reacted after 60 s of irradiation.
Effect of curing atmosphere
In practice UV curing is performed in air. It is well known that the radicals generated during UV exposure are very sensitive to the presence of oxygen. Air contains 20.8% of oxygen, which leads to an inhibition of the curing in the surface and that can go through the coating. The oxygen interrupts the chain formation resulting in lower conversions and thus to a sticky or weakly crosslinked material. To evaluate the effect of the curing atmosphere formulations containing 1 wt% of DMPA were irradiated in air and in nitrogen atmospheres. Figure 9 shows the conversion profiles vs irradiation time. It can be clearly seen in these profiles that the oxygen has an inhibition effect. The samples that were cured in air exhibit longer induction times and slower conversion profiles reaching a total conversion of approximately 35% after 60 s of irradiation. The low conversion achieved is caused by the continuous diffusion of air into the sample. When it is cured in a nitrogen atmosphere it has no induction time (lower than 1 s) and the reaction is completed after the first 5 s of exposure. The final conversion reached after 60 s is approximately 60%. The lower conversion achieved in the curing in air is explained by the inhibition effect of the oxygen.27 To overcome this negative effect higher light intensities or longer irradiation times, resulting in higher conversions, should be used, which is the case in common industrial applications.
Effect of PI concentration
Finally the effect of the PI concentration in the curing process was studied. For that purpose formulations containing 1, 2.5, 5, and 10 wt% of each PI were prepared and studied by RTIR. As an example the conversions reached for the formulations containing different amounts of HCHPK are plotted vs irradiation time in Fig. 10 . In this figure it can be observed that the final conversion achieved after 60 s is dependent on PI concentration: the higher the concentration, the higher the conversion. The effect of PI concentration is also observable in conversion rate in the first 5 s of irradiation: the higher the concentration, the faster the reaction. The same effect was observed by photo-DSC for concentrations up to 5 wt% (see Fig. 4).
The conversions obtained using different amounts of the other PIs are plotted in Fig. 11. If we take a look at these values one can observe that the conversion obtained is in the range of 50–80% depending on the type of initiator and its concentration. Formulations containing PI BP, HCHPK, and DMPA showed similar conversions and similar behavior (Fig. 11a). The conversions increase slightly or remain almost constant in the formulations containing from 1 to 5 wt% and at higher concentrations decrease. This fact was already observed by photo-DSC and was explained because of the competing reactions and the higher absorption of the initiators in the outer layers. The conversions are in the range from 50% to 55%. BP results in the slower reaction rate and also lower conversions at high concentrations of PI. At the lower concentrations of PI minor differences are noted between these PIs. The behavior of the other formulations is slightly different (Fig. 11b). Formulations containing BTPPO are almost independent of the initiator concentration but HEPHMP and the mixture of the two increases the conversion as their concentration increases. The formulations containing BTPPO as initiator lead to a maximum conversion of 60%. On the other hand formulations containing HEPHMP as initiator present a higher conversion, approximately of 65%. Finally the combination of these two initiators leads to the highest conversion observed in this study, 85%. The combination of these two PIs was already described as a good initiator. The different absorption wavelengths of the two initiators give to the formulation a good through-cure preventing the absorption in the surface that prevents the whole curing. While HEPHMP absorbs UV light near the surface and gives good cure there, BTPPO that has a longer wavelength absorption, generates radicals all the way through the coating. In our studies we observe that the highest conversions are observed when HEPHMP is used alone or in combination with BTPPO.
Summary of the RTIR results
The curing of UV-curable powder coatings can be monitored using RTIR very well. This technique allows one to follow the decrease in the absorption bands that react during the crosslinking process and to relate this data to conversion. The formulations that we have studied lead to conversions from 50% to 85% depending on the type and the concentration of PI.
The temperature effect on curing was evaluated and it was found that temperatures higher than 140°C had a negative effect on conversion, as was already observed by photo-DSC (see Fig. 7). The effect of PI concentration was also evaluated and it was found that the conversion increases with concentration. When BP, HCHPK, DMPA, and BTPPO are used as PI the increase in concentration has a low effect on conversion. The formulations with HEPHMP and a mixture of HEPHMP and BTPPO not only lead to the highest conversions but also showed remarkable increasing conversion as the concentration increases, which was not observed in the photo-DSC studies.
Rheological properties
Together with RTIR spectroscopy, rheology is another powerful tool that can be used to characterize the UV curing kinetics. Rheological properties measured during UV curing reflect the kinetics of the evolving network due to the formation of crosslinks. The extent and density of these crosslinks ultimately determine the final mechanical properties of the fully cured polymer.
From the different rheological properties the gel point is the most important one from a practical point of view because at the gel point the flow becomes restricted. There are several techniques and methods used to determine the gel point, but the most frequently used one is rheological measurements. Different definitions have been made in order to determine the gel point but the widely used one is described by Winter and Chambon28 and Chambon and Winter.29 According to their results, the gel point can be determined in a dynamic experiment by observing the frequency dependence of the loss tangent (tan δ). The gel point is defined as the point where the tan δ becomes independent of the frequency. This technique can lead to ambiguous results. It is also a common practice to take the gel point as the crossover of storage modulus G′ and loss modulus G″. It must be mentioned that the use of tan δ = 1 as an accurate gelation criterion is no longer accepted but is still used as a compromise in fast reactions.30,31 In our experiments where low frequencies are used the value of crossover of the two moduli will be used (leading to tan δ = G″/G′ = 1). In a study of Claesson et al.9 UV curing of a polyester was studied by rheology where tan δ = 1 was also used as the gel point criterion.
In our case irradiation was started after 1 min of stabilization and the whole system was purged with nitrogen in order to prevent the inhibition effect of oxygen. The effect of the different PIs and their concentration on the rheological properties of the coating formulation was evaluated. Figure 12 shows a typical plot of G′ and G″ during a UV curing experiment. During UV curing, of G′ and G″ measure the build up of the network. During the initial stages of the reaction G″ is larger than G′, indicating that the sample is still a viscous liquid. When the sample is exposed for longer periods of time the difference between G′ and G″ becomes smaller and eventually G′ equals G″ (the gel point, tan δ = 1). The gel point in a chemically crosslinking system marks the transition from a viscous liquid to a viscoelastic gel. When the sample is further exposed to UV irradiation, G′ becomes larger than G″. Note that G′ increases by up to four orders of magnitude.
Effect of type and PI concentration
Figure 13 shows the evolution of the complex viscosity of the formulation containing different amounts of DMPA. At the starting point the complex viscosity has a value approximately 1.5·102 Pa s in all the concentrations studied. Once the shutter is opened the complex viscosity increases indicating that crosslinking is taking place. An induction time is observed for the different samples, which decreases from 16 s in the 1 wt% formulation to 6.6 s in the 10 wt% formulation. Nevertheless, differences in reactivity can be observed from the shape of the curves, i.e., crosslinking proceeds faster as the initiator content increases. Also the decrease in the gel time, the time at which the gel point is observed, is an indication that the reaction proceeds faster as the concentration of PI increases. This confirms our observation by RTIR where the reaction rate was shown to be dependent on PI concentration. However, the final value of complex viscosity is comparable for all the concentrations studied, i.e., 2.5·105 Pa s. This is a clear indication that the degree of curing is comparable in the different concentrations evaluated, in agreement with the RTIR and photo-DSC results.
The efficiency of different amounts and type of PI was evaluated using the rheometer. All initiators showed a decrease in gel time with increasing concentration, indicating that a faster reaction is occurring (Fig. 14). When comparing the different PIs, it was observed that the rheological properties are comparable in all cases apart from BP and BTPPO. The reactivity of BP is lower than that of the other PIs, as we have concluded from the other experiments; the gel time in that case is almost five times higher indicating a slower reaction. This result is in good agreement with the value of the polymerization rate obtained by RTIR. BP has a value of maximum polymerization rate (Rpmax/[M 0]) of 0.02 (s−1) compared to the value of 0.10 (s−1) of HEPHMP, which was one of the most efficient initiators as demonstrated in Fig. 8. Summarizing the results obtained by rheology are in accordance with the ones obtained by RTIR: HEPHMP is five times faster than BP.
The case of formulations containing BTPPO is completely different. This initiator has a melting point of 127–133°C,20 which is the main reason for the different behavior of the formulation containing this PI. At 100°C the binder is molten but the PI is probably still solid. On UV exposure the temperature increases and the PI melts. The viscosity values before curing are surprisingly high compared to the other samples. If BTPPO is used as initiator a value around 1070 Pa s is reached before curing, compared to the value of approximately 150 Pa s in the other samples. But as soon as the UV light hits the sample the temperature increases and the viscosity drops rapidly indicating that a complete melt is obtained. After the decrease in the viscosity an increase is observed due to the crosslinking reaction initiated by the UV light. Therefore, it is not possible to accurately analyze the rheological properties in the initial stages at 100°C. Higher temperatures are needed to study formulations containing BTPPO. In this study we are interested in the minimal curing temperature which results in a good compromise between flow and cure so we will not continue the rheological study with this PI. It must be mentioned at this point that the previous studies by photo-DSC and RTIR gave apparently homogeneous melts when this initiator was used. The low mass used for these experiments and the higher UV intensity could be responsible to the formation of a homogeneous melt during the first seconds of irradiation. In Fig. 14 we can observe that almost all the PIs apart from BP exhibit similar gel times, indicating comparable reaction rates. This observation is a confirmation of the RTIR measurements.
Comparing the values of the complex viscosity for the different formulations we can observe that the initial values are comparable for all the samples apart from the ones containing BTPPO. As we have already mentioned they are substantially higher due to the probable presence of solid initiator that melts just after the light hits the sample. The values in the melt are approximately 150 Pa s and these values decrease slightly as the PI content increases. The low molecular weight PIs used had relatively low melting points, and they decreased the overall viscosity of the formulation as their content increases. Although there are some differences between the different initiators they are not significant and no further conclusions can be extracted from the initial viscosity values. When the UV light is turned on, the initial viscosity increases fast due to crosslinking and reaches its maximum value after 1 min in most of the studied samples. The final values for complex viscosity at 100°C are all in the range between 2.5·105 and 3.0·105 Pa s, indicating that a crosslinked material is obtained after irradiation. The values of complex viscosity of the cured formulations with HCHPK, DMPA, and HEPHMP are comparable for the different concentrations studied. The values for the samples cured with BP increase as the concentration of initiator increases. This fact could be explained because the lower reaction rate observed for this initiator. In the other formulations the faster reaction can leave some reacting groups unreacted in the matrix resulting in similar networks independently of the concentration used.
The ratio of G″ and G′ is a parameter that gives some information about the rigidity or quality of the network, the lower this value the more rigid the network behaves. The values of G′ are independent of the type and concentration of the PI. For BP a slight increase in G′ is observed with increasing concentration, probably due to a higher crosslinking density, thanks to the lower reactivity of the PI. In the other formulations the independency of G′ of the concentration indicates that the network obtained is similar and independent of the amount of PI used. The value of G″ decreases as the concentration of PI increases for all the initiators, except for formulations containing DMPA where G″ remains constant. This indicates again that the obtained network is similar for all the concentrations studied, probably due to the higher reactivity of the initiator.
If we observe the relation between G″ and G′ (Fig. 15) we can conclude that crosslinked networks are formed in all the formulations studied as the value G″/G′ is lower than 1. In the formulations containing DMPA this relationship is independent of the initiator content, indicating that similar networks are obtained. For the other PIs a decrease in this value is observed as the concentration of PI increases, indicating that higher crosslinked networks are obtained when higher concentrations of PI are used.
All PIs produced crosslinked materials as concluded from G′ and G″ values. From the increase in viscosity vs irradiation time formulations cured with BP were found to be the slowest as was determined by RTIR and photo-DSC. Minor differences in reaction rate were observed between the other PIs. By using different concentrations of PI the gel time can be adjusted without loss of properties.
Conclusions
The curing of an unsaturated polyester-based powder coating was monitored using photo-DSC, RTIR, and rheology. The formulations are cured within 5 s reaching conversions about 60% at 100°C. An increase of the curing temperature reduces the final conversion and also the rate of polymerization due to the chain transfer process and depolymerization that dominates the photopolymerization at high temperatures. The reactivity of the photoinitiators was similar for all the studied photoinitiators except for the slowest initiator, BP.
From the rheology measurements the gel time was determined and found to decrease with increasing initiator concentration. The values of complex viscosity were comparable between the different initiators; this value increased as concentration of initiator increased in the formulations containing BP and was constant in the other initiators. The relationship G″/G′ decreases as concentration of initiator increases indicating that a highly crosslinked network is obtained.
References
Witzeman JS, “Studies on Low Temperature Curable Powder Coatings” Prog. Org. Coat., 27, 269 (1996)
Pappas SP, Hill LW, “Kinetic Parameter Considerations for Maximizing Stability and Minimizing Cure Temperature of Thermosetting Coatings – Sulfonium Salts as Latent Thermal Initiators for Cationic Polymerization” J. Coat. Technol., 675, 43 (1981)
Scott TF, Cook WD, Fortsythe JS “Photo-DSC Cure Kinetics of Vinyl Ester Resins. I. Influence of Temperature” Polymer 43, 5839 (2002)
Scott TF, Cook WD, Fortsythe JS “Photo-DSC Cure Kinetics of Vinyl Ester Resins II: Influence of Diluent Concentration” Polymer 44, 671 (2003)
Decker, C, In: Pappas, SP (ed.) Radiation Curing: Science and Technology, p. 135. Plenum Press, New York (1992)
Scherzer T, Decker U, “Real-Time FTIR–ATR Spectroscopy to Study the Kinetics of Ultrafast Photopolymerization Reactions Induced by Monochromatic UV Light” Vibr. Spectr. 19, 385 (1999)
Scherzer T, Tauber A, Mehnert R, “UV Curing of Pressure Sensitive Adhesives Studied by Real-Time FTIR-ATR Spectroscopy” Vibr. Spectr. 29, 125 (2002)
Lee SS, Luciani A, Manson JAE, “A Rheological Characterisation Technique for Fast UV-Curable Systems” Prog. Org. Coat. 38, 193 (2000)
Claesson H, Malmstrom E, Johansson M, Hult A, Doyle M, Manson JAE, “Rheological Behaviour During UV-Curing of a Star-Branched Polyester” Prog. Org. Coat. 44, 63 (2002)
Castell P, Wouters M, Huijs F, Fischer H, de With B “Surface Modification of Poly(propylene) by Photoinitiators: Improvement of Adhesion and Wettability” J. Appl. Polym. Sci. 92, 2341 (2004)
Buysens, K, Hammerton, D, “UV Powder Coatings. A New Coating Technology.” Radtech Report, North America, Baltimore, 1999, p. 18
Phinyocheep P, Duangthans S, “Ultraviolet Curable Liquid Natural Rubber.” J. Appl. Polym. Sci. 78, 1478 (2000)
Guenzler H, Gremlich HU, Bluemich MJ, Infrared Spectroscopy Wiley VCH, Weinheim (2002)
Cook WD, “Thermal Aspects of the Kinetics of Dimethacrylate Photopolymerization” Polymer 33, 2152 (1992)
Young JS, Bowman CN, “Effect of Polymerization Temperature and Cross-Linker Concentration on Reaction Diffusion Controlled Termination” Macromolecules 32, 6073 (1999)
Cook WD, “Photopolymerization Kinetics of Oligo(ethylene oxide) and Oligo(methylene) Oxide Dimethacrylates” J. Polym. Sci. Part A: Polm. Chem. 31, 1053 (1993)
Lecamp L, Youssef B, Bunel C, Lebaudy P, “Photoinitiated Polymerization of a Dimethacrylate Oligomer: 2. Kinetic Studies” Polymer 40, 1403 (1999)
Nelson EW, Jacobs JL, Scranton AB, Anseth KS, Bowman CN, “Photo-Differential Scanning Calorimetry Studies of Cationic Polymerizations of Divinyl Ethers” Polymer 36, 4651 (1995)
Padaki, S, Buehner, RW, “Optimizing Cure of UV Powder Coatings Using Differential Photocalorimetry.” Proc. Radtech, North America, Baltimore, 2000, p. 698
Ciba datasheet of Irgacure 819, http://www.cibasc.com
Broer DJ, Mol GN, Challa G, “Temperature Effects on the Kinetics of Photoinitiated Polymerization of Dimethacrylates” Polymer 32, 690 (1991)
Decker C, Elzaouk B, Decker DJ, “Kinetic Study of Ultrafast Photopolymerization Reactions” J. Macro. Sci., Pure Appl. Chem. 33 (2) 173 (1996)
Decker C, “The Use of UV Irradiation in Polymerization” Polym. Int. 45, 133 (1998)
Lee TY, Roper TM, Jonsson ES, Kudyakov I, Viswanathan K, Nason C, Guymon CA, Hoyle CE, “The Kinetics of Vinyl Acrylate Photopolymerization” Polymer 44, 2859 (2003)
El-Ghayoury A, Boukaftane C, de Ruiter B, van der Linde R, “Ultraviolet-Ultraviolet Dual-Cure Process Based On Acrylate Oxetane Monomers” J. Polym. Sci. Part A: Polym. Chem. 41, 469 (2002)
Mascioni M, Sands JM, Palmese GR, “Real Time In situ Spectroscopic Characterization of Radiation Induced Cationic Polymerization of Glycidyl Ethers” Nucl. Inst. Met. Phys. Res. B 208, 353 (2003)
Davidson S, Technology and Applications of UV and EB Curing SITA Technology Limited, London (1999) p 133
Winter HH, Chambon F, “Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point” J. Rheol. 30, 367 (1986)
Chambon F, Winter HH, “Linear Viscoelasticity at the Gel Point of a Crosslinking PDMS with Imbalanced Stoichiometry” J. Rheol. 31, 683 (1987)
Tung CYM, Dynes PJ, “Relationship Between Viscoelastic Properties and Gelation in Thermosetting Systems” J. Appl. Polym. Sci. 27, 569 (1982)
Ross-Murphy SB, “Structure and Rheology of Gelatin Gels: Recent Progress” Polymer 33, 2622 (1992)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castell, P., Wouters, M., Fischer, H. et al. Kinetic studies of a UV-curable powder coating using photo-DSC, real-time FTIR and rheology. J Coat Technol Res 4, 411–423 (2007). https://doi.org/10.1007/s11998-007-9056-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-007-9056-6