Abstract
Hot air–assisted radio frequency (HARF) heating was used to dry and blanch inshell hazelnuts with high (19%) and low (11%) moisture content (MC) simultaneously. The effects of target temperature (70, 80, and 90 °C) and holding time (0 or 5 min at target temperature) on heating uniformity and inactivation of polyphenol oxidase (PPO) and peroxidase (POD) were investigated. The dielectric properties and penetration depth of the ground kernels, shells, and inshell hazelnuts were measured at 3 MCs (6–22%), 2 frequencies (13.56 and 27.12 MHz), and 7 temperatures (25–95 °C). Hazelnut kernels showed higher dielectric constant and loss factor than shells and inshell hazelnuts. Target temperature did not affect heating uniformity for inshell hazelnuts. A 5-min holding time at the target temperature resulted in better heating uniformity and lower POD and PPO activities (27–35% and 40–45% reduction, respectively). This study demonstrated the feasibility of simultaneous rapid drying and blanching of inshell hazelnuts using HARF technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radio frequency (RF) heating is a promising thermal treatment technology providing fast, deep, and volumetric heating within the sample. In our previous studies, hot air–assisted radio frequency (HARF) heating was investigated for drying Oregon hazelnuts and identifying HARF conditions (such as electrode gap, hot air temperature and velocity, and sample thickness) to achieve a high drying efficiency as well as better quality retention (low lipid oxidation and shell cracking ratio, high retention of phenolic content, etc.) in the inshell hazelnuts (Wang et al., 2020a, b, 2021).
During hazelnut storage, enzymatic activity in kernels can cause a series of quality deteriorations such as discoloration, off-flavor and odor, loss of nutrients, and texture change. For instance, polyphenol oxidase (PPO) as a copper-containing enzyme might lead to browning discoloration, and peroxidase (POD) as a heme iron-containing enzyme might cause nutrition loss, color change, and off-flavor development in given food items (Burnette, 1977; López et al., 1997; Zhang et al., 2018b). Since these two enzymes have been linked to the quality deterioration of hazelnuts during storage, blanching to inactivate their activity is a critical step for ensuring quality and prolonging the storability of hazelnuts (Bonvehi & Rosua, 1996). To the best of our knowledge, there is no federal regulation specifying a limit for the percent reduction of enzymes to be achieved in tree nuts; however, the higher enzyme reduction decreases the degree of quality degradation arising from the enzyme-initiated oxidation. Hence, it is important to study the thermal inactivation of enzymes for ensuring quality and storability of hazelnuts.
Conventional blanching uses steam, hot water, or hot acid/salt solutions (Zhang et al., 2018a). Due to the significant amount of wastewater generated in conventional blanching, alternative technologies are necessary. A few previous studies reported the RF application for blanching, but were limited to the fresh fruits and vegetables including carrot cubes (Gong et al., 2019), leafy vegetables (Ponne et al., 1994), apple slices (Zhang et al., 2020), and potato cuboids (Zhang et al., 2018a). Inshell hazelnuts are significantly different from fresh fruits and vegetables when considering blanching due to their complex structure, low moisture content (MC) and thermal conductivity, different chemical composition, and a hard shell around its kernel. In our previous studies (Wang et al., 2021), the effect of the RF heating on reduced PPO and POD activity was discovered, in which the inshell hazelnuts dried in the HARF oven showed significantly lower PPO and higher retention of phenolic contents compared to the ones heated in the hot air oven. In light of these findings, it was thus hypothesized that simultaneous drying and blanching could be achieved for inshell hazelnuts using HARF technology owing to its volumetric and rapid heating characteristics. However, there is a lack of information on how HARF drying/blanching temperature and holding time as well as initial MC may affect enzyme inactivation in hazelnuts. Therefore, it is necessary to systematically investigate the effect of RF energy on enzyme inactivation in hazelnuts while achieving the primary goal of drying.
Dielectric properties (DPs) of a material determine the interaction of the material with the alternating electromagnetic field (EM), i.e., the heating rate. The DPs of food materials are influenced by MC, temperature, density, chemical composition, and frequency. In RF drying, since the temperature and MC of the food material change continuously, the information of DPs for giving materials at different MCs and temperatures is essential for predicting the heating rate and achieving uniform RF drying (Zhou and Wang, 2019). DPs of the food material can be also used to estimate the penetration depth of EM waves into the material, i.e., the RF heating uniformity (Mao and Wang, 2021). To the best of our knowledge, no study has reported the DPs and penetration depth of the inshell hazelnut, hazelnut kernel, and shell in the RF region.
The overall goal of this study was to investigate the feasibility of using RF technology to simultaneously dry and blanch inshell hazelnuts. The specific objectives were (1) to study the DPs (dielectric constant and loss factor) and penetration depth of the ground hazelnut shell, kernel, and inshell hazelnuts at different MCs, temperatures, and frequencies, (2) to evaluate the HARF heating uniformity at different temperatures (70, 80, and 90 °C) with 0- and 5-min holding time at the target temperature, and (3) to identify the optimal temperature and time combination for achieving the drying and inactivation of PPO and POD at 70, 80, and 90 °C. The results from this study would lead to expanding the potential commercial application of RF technology to simultaneous drying and blanching of inshell hazelnuts.
Materials and Methods
Materials and Chemical Reagents
Machine-harvested Oregon Jefferson hazelnuts (Corylus avellana L.) were provided by the Oregon Hazelnut Marketing Board in October 2021. Washed and cleaned nuts were primarily packed in a woven sack and stored at 2.2 °C and 60% RH before RF heating within 8 months. Chemical reagents were obtained from different manufacturers: hexane and formaldehyde from Macron Fine Chemicals (Avantor Performance Materials, Radnor, PA, USA); hydrogen peroxide from Millipore Sigma (Billerica, MA, USA); polyvinylpyrrolidone K 30 and o-dianisidine from TCI America (Portland, OR, USA.); catechol from EMD Millipore (Billerica, MA, USA); and zinc acetate and potassium ferrocyanide from Sigma-Aldrich (St. Louis, MO, USA).
Experimental Design
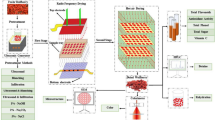
In the first part of the study for investigating the effect of MC, frequency, and temperature on the penetration depth, dielectric constant, and dielectric loss factor of the ground kernel, shell, and inshell hazelnuts, a completely randomized design with 3 MCs (~ 21, 11, and 6% for the ground kernels; 21, 17, and 7% for the ground hazelnut shells; and 22, 15, and 7% for the ground inshell hazelnuts), 2 frequencies (13.56 and 27.12 MHz), and 7 temperatures (25, 60, 70, 80, 85, 90, and 95 °C) was applied. In the second part of the study for evaluating the effect of initial MC, treatment temperature, and holding time on the HARF heating uniformity, the final MC, and PPO and PPO activity of the inshell hazelnuts, a completely randomized design with 2 initial MCwb (~ 11 and 19%) of inshell nuts, 3 treatment temperatures (70, 80, and 90 °C), and 2 holding times (0 and 5 min) was applied. The scheme of the first and second parts of the study is shown in Fig. 1 a and b, respectively.
Sample Preparation and MC Measurement
In our previous study, the optimum hot-air drying conditions for three Oregon hazelnut cultivars (Barcelona, Yamhill, and Jefferson) were determined as 43 °C and 40% RH to retain the nut quality with reduced enzyme activity and lipid oxidation (Wang et al., 2018). These predetermined hot-air drying conditions were used to dry the hazelnuts used for the dielectric properties and penetration depth measurements and for comparing the quality of hot air–dried and HARF-dried hazelnuts.
To prepare the hazelnut samples for dielectric properties and penetration depth measurements, inshell hazelnuts with an initial MC of 22% were first dried at 43 °C and 40% RH using a forced-air dryer (Shelf-Pak Series, Model: CHU-150E, Enviro-Pak, Clackamas, OR, USA) with horizontal airflow of 1.5 m/s. The inshell nuts were placed on a dryer tray in single layer and shaken frequently (every 3 to 6 h) to achieve uniform heating. The dried inshell nuts were then cracked manually, and inshell nuts, kernels, and shells were ground using a waring blender (Model: 1001, Waring Products Co, Winsted, Conn, USA), respectively, and sieved with a mesh sieve having 1.18 mm opening (USA Standard Testing Sieve, No. 16; Mentor, OH).
MCs of inshell hazelnuts and hazelnut kernels were analyzed upon delivery. MC was measured using the oven drying method at 105 °C for 24 h (AOAC, 2000) in a 1.66 kW forced air oven (VWR Model: 89,511–412, VWR International, Radnor, PA, USA). Four randomly selected inshell hazelnuts were collected for each MC analysis and measurement was done in triplicate. The final MCs of the ground kernels, shells, and inshell nuts were measured using the same method as stated above.
Dielectric Property Measurement
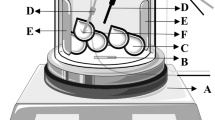
The parallel plate method was applied in order to measure the dielectric constant (ε′) and loss factor (ε″) of the ground hazelnut samples due to its high measurement accuracy and ease of sample preparation and setup. The parallel capacitance (Cp) and resistance (Rp) of the samples were measured using an inductance capacitance and resistance (LCR) meter (4285A, Agilent Technologies, Palo Alto, CA) and a liquid test fixture (16,452, Agilent Technologies, Palo Alto, CA) following the procedures from Dag et al. (2019).
For conducting the test, the appropriate amount of sample was calculated, based on the following equation:
where \(\rho\) is the density of the sample (g/cm3), m is the total mass of the sample (g), and v is the volume of the test fixture (cm3). The volume of the test fixture was based on the thickness of the spacer (3 mm) used in the experiments.
For each test, the loaded test fixture with the hazelnut sample was closed tightly and heated in the oven for approximately 60 min to reach the maximum temperature target of 95 °C. A thermocouple (421,509, EXTECH, Taiwan) was used to monitor the sample’s temperature during heating. At 25, 60, 70, 80, 85, 90, and 95 °C, the parallel capacitance (Cp) and resistance (Rp) of the samples were measured by the LCR instrument at 13.56 and 27.12 MHz. With the data obtained, the dielectric constant and loss factor were calculated using equation (2) and (3), respectively (Agilent Technologies, 2000):
where D is the gap between electrodes of the test fixture (m), Cp is parallel capacitance (F), Rp is the parallel resistance (Ω), ƒ is the frequency (Hz), ε0 is the permittivity of vacuum (8.854 × 10−12 F m−1), and A is the electrode area (m2).
Penetration Depth (dp) Determination
To determine the proper thickness of food for uniform dielectric heating, the penetration depth was determined as the depth at which the applied power decreases by 1/e (e = 2.718) (Wang et al., 2003). Penetration depth was calculated by the following formula (Ozturk et al., 2016):
where c is the speed of light in a vacuum (2.998 × 108 m/s), f is the frequency (Hz), ε′ and ε″ are relative dielectric constant and loss factor, respectively, and tanδ is defined as (ε″/ε′).
HARF Heating of Inshell Hazelnuts
A 10-kW, 27.12 MHz RF system (GJG-10B-27II-JY, Hebei Huas Jiyuan High-Frequency Equipment Co., Ltd., Guangdong, China) with a top electrode size of 60 cm × 40 cm (L × W) was utilized for this study. The machine was equipped with a hot-air system using a 2-kW electrical heater and a blower fan. The electrode gap was adjustable between 5 and 30 cm. For each RF run, Jefferson inshell nuts (1200 g) were loaded in a rectangular container made of Teflon with a perforated bottom and sides (inner L × W × H: 25.5 cm × 15.5 cm × 7.5 cm) with 0.5 cm wall thickness. The container was placed at the center of the bottom electrode. The vertical position of the container was set by the adjustable legs attached to the container. The air gap (the distance between the parallel electrode and the sample container surface) both at the top and bottom was set as 1.5 cm. The electrode gap of 11 cm was selected based on the preliminary studies giving the lowest temperature differences within the hazelnut kernels at five different locations. The hot air temperature was recorded in the range of 40–50 °C measured by a fiber optic temperature sensor (Model: HQ-FTS-PBA0B-0600-X, Herch Opto Electronic Technology Co., Ltd., Shaanxi, China) connected to the fluorescence fiber temperature monitoring system (Model: HQ-12, Herch Opto Electronic Technology Co., Ltd., Shaanxi, China).
Five fiber optic temperature sensors were inserted into pre-drilled holes located in the core of the hazelnut kernels and were placed in (1) the center on the top layer, (2) the center on the middle layer, (3) the center on the bottom layer, (4) the center of the long edge on the top layer, and (5) the center of the long edge on the bottom layer, respectively, for recording kernel temperatures (Fig. 2). The temperature sensors were fixed into the hazelnut cores using labeling tapes. The temperatures of hazelnut kernels at each position were recorded every 3 s and the heating profiles were drawn using the temperature data recorded during HARF heating. Samples were heated to 70, 80, and 90 °C, and held either for 5 min at the corresponding temperature or not. The heating rate (°C/min) was calculated by dividing the temperature difference by the treatment time. Heating time was calculated as the time needed for the cold spot to reach the target temperature starting from 30 °C. The thermal images of the HARF heated inshell hazelnuts on the top layer were captured by a digital infrared camera calibrated by the manufacturer (Teledyne FLIR, Model: E6-XT, Wilsonville, OR, USA) with 43,200 (240 × 180) pixels and a wide temperature range from − 20 to 550 °C. Thermal images were analyzed on the FLIR Tools software.
Schematic view of a HARF unit, b the sample container, and the positions of nuts in the sample container where five individual fiber optic probes were inserted (point 1, the center on the top layer; point 2, the center on the middle layer; point 3, the center on the bottom layer; point 4, the center of the long edge on the top layer; point 5, the center of the long edge on the bottom layer)
To compare hot-air drying with HARF drying, the inshell hazelnuts with an initial MCwb of ~ 19% were also dried at 43 °C and 40% RH using a forced-air dryer (Shelf-Pak Series, Model: CHU-150E, Enviro-Pak, Clackamas, OR, USA) with horizontal airflow of 1.5 m/s to compare the drying and blanching effect of HARF heating with hot-air heating.
The MC (wet basis) after drying was calculated as (Wang et al., 2020a, b):
where m1 and MC1 are the weight and the MC of inshell hazelnuts before drying, and m2 and MC2 are the weight and the MC of inshell hazelnuts after drying, respectively.
Uniformity Index
The uniformity index is defined as the ratio of the change in the standard deviation to the change in mean surface temperature after HARF heating and is widely used in RF studies to evaluate the heating uniformity (Wang et al., 2005). The lower the uniformity index value, the better the RF heating uniformity, meaning that the sample temperature distribution has a lower standard deviation difference throughout the layer. The uniformity index was calculated as (Wang et al., 2005, 2021):
where \({\mu }_{0}\) and \(\mu\) are the initial and final mean of the hazelnut surface temperature (°C), and \({\sigma }_{0}\) and \(\sigma\) are the initial and final standard deviation of the hazelnut surface temperatures (°C), respectively. It should be noted that the uniformity index was calculated from the thermal images captured on the top surface and reported as an indicator of the heating uniformity for the entire sample volume.
Enzyme Activity
The inshell hazelnuts were cracked manually into kernels and shells, and the kernels were ground using a waring blender with the addition of liquid nitrogen to prevent the possible temperature increase during grinding. Hazelnut kernel oil was extracted by shaking the kernel powder in hexane (1:10, w/v) for 2 h at room temperature. The mixture of kernel powder and hexane was then filtered through Whatman #1 paper. The residue was dried and then further clarified by mixing 3 g of defatted hazelnut kernel with 15 mL of DI water and 15 mL of 0.05 M pH 5.8 sodium phosphate buffer containing 2.25 g of polyvinylpyrrolidone K 30. The samples were shaken in an ice bath for 1 h and centrifuged at 8000 g and 4 °C for 10 min. The supernatant was then filtered through Whatman #1 paper. Five drops of Carrez I (zinc acetate solution) and Carrez II (potassium ferrocyanide solution) were added to precipitate the proteins. The samples were then centrifuged at 8000 g at 4 °C for 10 min and the supernatant was collected for measuring POD and PPO activities.
For POD activity, 1 mL of formaldehyde (35%, v/v), 1 mL of hydrogen peroxide (1%, v/v), and 1 mL of o-dianisidine ethanol solution (0.5 g/L) were mixed. One milliliter of enzyme extract sample was added to initiate the reaction. The absorbance at 430 nm in 0 and 5 min was recorded. For PPO activity, 1.5 mL of 0.2 mol/L catechol solution (in 0.05 M, pH 6.8 sodium phosphate buffer) was mixed with 1.48 mL of DI water. Twenty microliters of enzyme extract was then added to initiate the reaction. The absorbance at 410 nm for 0 and 2 min was recorded. Both POD and PPO activities were expressed as μkatal/L. One katal for POD activity was defined as the amount of the POD converted by one mol of o-dianisidine per second. One katal for PPO activity was defined as the amount of the PPO converted by one mol of catechol per second (López et al., 1997).
Statistical Analysis
The dielectric property measurements were performed in triplicate and the RF heating experiments and quality analysis were conducted in duplicate. The data were reported in mean value and standard deviation. The difference among treatments for individual analysis was determined by a one-way and two-way ANOVA and means were separated using Tukey’s multiple comparison test with SAS v9.2 (The SAS Institute, Cary, NC, USA) at a significance level of 0.05.
Results and Discussion
Dielectric Properties of the Ground Hazelnut Kernels, Shells, and Inshell Hazelnuts
The dielectric constant of the ground hazelnut kernel, shell, and inshell as a function of temperature with MC ranging from 6 to 22%, and at 13.56 and 27.12 MHz frequencies, is presented in Fig. 3. All samples showed a decrease in dielectric constant with increasing frequency and an increase with increasing temperature (up to 85 °C) and MC. At higher MC and temperature, the decrease in the dielectric constant was greater as the frequency increased. For example, by increasing the frequency from 13.56 to 27.12 MHz, the dielectric constant of hazelnut kernels with the MC of 11 and 21% decreased to 15.77 and 15.5% at 80 °C, and 9.5 and 14.45% at 25 °C, respectively. The similar trends have been observed in peanut, pistachio, hazelnut, and almonds (Li et al., 2017; Ling et al., 2015; Zhang et al., 2016; Zhu et al., 2014). The reason for this decrease in dielectric constant is due to the reduction of ionic conduction, which occurs when the molecule begins to lag behind the electric field oscillation by increasing frequency (Dawy et al., 1998).
Figure 3 illustrates that the dielectric constant increases with an increase in MC mainly due to an increase in the dielectric polarization (Zhu et al., 2014). This increase was greater for hazelnut kernels than for hazelnut shells. For example, at 13.56 MHz and 25 °C, the dielectric constants of hazelnut kernel at 6 and 21% MC were 2.99 and 11.69, respectively. However, for the hazelnut shell, the dielectric constants for samples with 7 and 21% MC at the same temperature and frequency were 2.67 and 6.44, respectively. A possible explanation for this difference can be due to the difference in their physical characteristics and chemical compositions. At a similar MC, since the kernel primarily consists of fat, the water content is more in the form of free water, which has a greater impact on the dielectric properties than bound water.
The dielectric properties of hazelnuts were positively influenced by temperature increases between 25 and 85 °C, whereas negative effects were observed at temperatures over 85 °C. When the temperature was increased up to 85 °C, ionic mobility was improved by decreasing viscosity, while when the temperature was increased above 85 °C, dielectric properties were reduced due to vaporizing free water. At higher MCs, the dielectric constant increased faster by temperature. For instance, increasing the temperature from 25 to 80 °C for hazelnut kernel with 6 and 21% MC resulted in 30 and 57% increase in dielectric constant at 27.12 MHz, respectively.
Figure 4 presents the loss factor for ground hazelnut kernels, shells, and inshells. The loss factor of samples with MC below 7% was not shown, since the values were too low and the LCR instrument was unable to provide stable data. Similar to the dielectric constant, the loss factor of samples increased with increasing temperature and MC and decreased with increasing frequency. For example, the loss factor of the ground kernel at 25 °C with 11% MC at 27.12 MHz was 1.38, but it increased to 104.62 at 80 °C with 21% MC at 13.56 MHz. This increase can be attributed to higher ionic conductivity at lower frequencies and higher MC (Zhu et al., 2014). A comparison of the loss factor between the shell and kernel revealed that the kernel had a greater loss factor than the shell. For example, the loss factors of kernel and shell at 21% MC were 104.62 and 8.32 at 80 °C and 13.56 MHz, respectively. As the shell primarily consists of lignin, the increasing temperature did not affect its physical characteristics. In contrast, increasing temperature causes fat-rich kernels to become less viscous and more mobile, thus increasing their ionic conductivity (Tang et al., 2002).
Penetration Depth
Figure 5 illustrates the penetration depths for hazelnut samples. For samples with MC below 11%, penetration depths could not be calculated due to unstable values of the loss factor. Penetration depth decreased sharply as the MC and temperature increased. The maximum penetration depth was 14.95 m for ground shells with 17% MC at 27.12 MHz and 25 °C, which had the lowest dielectric constant and loss factor among the tested samples. The lowest penetration depth was 0.189 m for ground kernels with 21% MC at 27.12 MHz and 90 °C. The similar effects of frequency, temperature, and MC were reported for nuts, dried fruit, and vegetables, and milk powders (Alfaifi et al., 2013; Dag et al., 2019; Gao et al., 2012; Ozturk et al., 2016).
At 25 °C, MC had a high effect on penetration depth, but as the temperature rose, the effect of MC on penetration depth decreased. This trend can be explained by the effects of MC and temperature on the dielectric properties, as well as the reverse relationship between dielectric properties and penetration depth.
HARF Heating Performance and Uniformity
Temperature profiles at five locations of the sample tray and hot air temperature in the oven cavity during HARF heating to 70, 80, and 90 °C are shown in Fig. 6 a, b, and c, respectively. A cold spot was found at the center point of the bottom layer for all HARF treatments which might be caused by the path of hot air circulation in the HARF cavity, because hot air was blown horizontally from the bottom edges of the oven cavity (Fig. 2) and circulated from the bottom to the top. Since the bottom part of the container was just above where hot air was blown, the hazelnuts on the bottom part might not get exposed to hot air as much as the top layer of the container. Moreover, water vapor evaporated from the inshell hazelnuts at the bottom layer may not escape as the top layer. The accumulated water vapor at the middle and bottom layers may affect the temperature profiles of the hazelnuts at these locations. To prevent water vapor accumulation, mixing during HARF heating is suggested for removing water vapor steadily from the sample surface.
The effect of holding on the maximum, minimum, and average temperatures of nuts with initial MC of 19% and 11% is reported in Table 1. For all target temperatures (70, 80, and 90 °C), no significant difference was seen for Tmax, Tmin, and Tave (P > 0.05) between 0 and 5 min holding time. In general, it is expected that holding the sample at the target temperature after HARF heating allows heat conduction of heated samples, which results in higher final temperatures on the sample surface. This different result may be explained by the cooling effect of hot air in the later stage of the drying. As observed in Fig. 6, the temperature of the HARF oven cavity was around 40–50 °C, lower than hazelnut temperatures. Heat loss from hazelnuts to the environment and conduction among the hazelnuts could take place simultaneously during holding time, resulting in no temperature change on the sample surface. Overall, the hazelnuts with an initial 11% MC had slightly lower maximum, minimum, and average temperatures than those with 19% MC although the difference was not significant (P > 0.05) for most of the samples, meaning that the initial MC did not affect the temperature distribution on the sample top layer. The maximum and average temperature of nuts with 19% MC heated to 70 °C was significantly lower than those heated to 80 and 90 °C (P < 0.05) while the effect of temperature on the maximum, minimum, and average temperature was not significant for the hazelnuts with initial 11% MC (P > 0.05). This trend might be associated with the treatment time and heating rate. The nuts with an initial 19% MC heated to 70 °C had the shortest treatment time (i.e., the highest heating rate) which might limit the heat conduction throughout the sample, thus resulting in a significantly lower final temperature (P < 0.05) on the top layer.
All samples heated in the HARF oven had a similar uniformity index ranging from 0.11 to 0.14 (P > 0.05) regardless of the initial MC, target temperatures, and holding time (Table 1). Similar uniformity index values were obtained for inshell hazelnut in our previous study (), in which the uniformity index was between 0.08 and 0.20 depending on the hot air temperature circulation and electrode gap. In another study (Wang et al., 2021), the uniformity index ranged from 0.134 to 0.165. Two phenomena can be the potential reasons for non-uniform temperature distribution in HARF heated samples. Inherently, the refraction and reflection of the electric field at the edges and corners of the sample container cause a higher volumetric power density, resulting in overheating on the edges and corners. Furthermore, the irregularity in hazelnut shape, size, and composition leads to different HARF heating rates (). Since the intrinsic properties of hazelnut samples could not be altered, heating uniformity should be improved. Previous studies have tried to mix samples, adjust hot air velocity and temperature, and modify sample container shape and sample size to improve heating uniformity. However, further studies are necessary to develop an effective HARF drying and blanching system and optimize the process parameters to improve the heating uniformity with the help of structural change in the RF oven design as well as computer simulation (Li et al., 2018; Wang et al., 2006, 2020a, b, 2021).
Although a high heating rate is associated with the high throughout, it can cause non-uniform heating as a result of the rapid and runaway heating (Jiao et al., 2012). Therefore, the heating rate should be optimized for the sample of interest according to heating uniformity evaluation. In this study, the HARF treatment with a high heating rate might be preferred since all HARF treatments resulted in a similar heating uniformity index. Regardless of the initial MC, the target temperature did not affect the heating rate (P > 0.05). The heating rates of nuts with an initial MC of 11% with 5-min holding were slightly lower than those without holding due to a fairly constant sample temperature (± 1 °C) during the holding period.
Thermal images on the top surface of the inshell hazelnuts heated in the HARF oven are exhibited in Fig. 7. In general, the edge and corner heating was observed. At the very outer edges, the lower temperature was also seen, which may be related to the inevitable heat loss occurring during the time of transferring the sample from the RF oven to the infrared camera stand. The edge and corner heating is a common problem in RF heating due to the fact that the electromagnetic energy is focused on the edges and corners of the sample. Since DPs are higher at the elevated temperature (Figs. 3 and 4), the portion of the sample at the higher temperature constantly absorbed more electromagnetic energy, resulting in an even more severe thermal runaway (Zhao et al., 2000). Thermograms also revealed that some individual hazelnuts had a lower surface temperature probably due to the irregular shape, size, or composition of the nuts. The hazelnuts with a higher MC (~ 19%) reached to higher surface temperature on the top surface compared to the ones with a lower MC (~ 11%). This result may be explained by the higher DPs values of hazelnuts with a higher MC as a result of available water. The higher MC hazelnuts could convert more electromagnetic energy into heat during HARF heating. A relatively more uniform temperature distribution was seen for the hazelnuts held at the target temperature for 5 min. Although the samples are heated volumetrically in the HARF oven, the heat conduction still takes place within the individual hazelnut. Holding the nuts for an additional 5 min allowed heat conduction within the samples, resulting in more uniform temperature distribution.
For comparing the drying efficiency of HARF with hot air, inshell hazelnuts with the initial MC (19%) were dried using both technologies, and the final MC and treatment time are shown in Table 2. Hot-air dryer at 43 °C and 40% RH took 2880 min (48 h) for inshell hazelnut to reach a MC of 11.30%. However, it only took 12.87 and 15.06 min to reach similar MCs of 12.67 and 12.46% using HARF heating to 80 and 90 °C, respectively. Hot-air drying relies on the thermal conductivity of samples, with the low thermal conductivity of the inshell hazelnuts associated with the void between shell and kernel; it would take a long time to dry the nuts (Özilgen and Özdemir, 2001). Different from the hot-air dryer, RF heating occurs volumetrically in the sample as a result of the back-and-forth movement of the ions and rotation of the dipolar molecules when an alternating electric field is applied to the sample (Marra et al., 2014).
It was observed that holding hazelnuts at the target temperature after HARF heating did not affect the final MC significantly (P > 0.05). For instance, the final MCs of inshell hazelnuts were 8.17 and 8.64% when heated to 70 °C in the HARF oven with 0- and 5-min holding, respectively. In the early stage of HARF drying, the net energy from both RF and hot air circulation in the oven increased the hazelnut temperature rapidly, and the energy loss due to water evaporation was relatively low causing a high heating rate. In this stage, mostly free water in hazelnut was removed. In the later stage of drying, the heating rate decreased as a result of the lower DPs at the reduced MC and heat loss caused by the water evaporation. In this stage, the remaining water in dried hazelnuts was mainly in the bound form, thus requiring a longer time and/or higher temperature to remove ().
Enzyme Inactivation
The POD and PPO activities of the inshell hazelnuts with an initial 11% MC heated in the HARF oven to 70, 80, and 90 °C with 0- and 5-min holding are demonstrated in Fig. 8 a and b, respectively. The POD and PPO activities of the control sample (without thermal treatment) were 0.21 and 49.57 μkatal/L, respectively, whereas POD and PPO activities of hazelnuts heated to 70, 80, and 90 °C were significantly reduced (P < 0.05) to 0.16, 0.18, and 0.16 and 38.10, 38.59, and 33.15 μkatal/L, respectively (Fig. 8 a and b). The percent reduction in POD and PPO activities ranged from 15 to 35% and 24 to 45% for HARF heated inshell hazelnuts depending on the initial MC, target temperature, and holding time.
The target temperature did not affect either POD or PPO activity (P > 0.05), but holding the hazelnuts at the corresponding target temperature for 5 min led to a significant decrease in both POD and PPO activities (P < 0.05) (Fig. 8). The insignificant effect of the target temperature on enzyme activity can be explained by the temperature results stated in the “HARF Heating Performance and Uniformity” section. The maximum, minimum, and average temperatures on the top layer of the hazelnuts with an initial 11% MC were not significantly affected by the target temperature (P > 0.05). Therefore, the thermal effect of HARF heating resulted in similar POD and PPO activities when the hazelnuts were heated to 70, 80, and 90 °C.
It is well known that heating and/or holding time leads to further enzyme inactivation as a result of the long heat exposure of the sample. However, a long thermal treatment might cause severe quality deterioration and nutritional loss (Wang et al., 2018). For this reason, thermal blanching treatment with a high heating rate is desired to inactivate the enzymes that may cause quality deterioration, while preserving the nutritional and quality value of the end product simultaneously. The significant effect of the holding time (P < 0.05) can be interpreted that the degree of the enzyme inactivation may be further modified by extending the holding time at the corresponding temperature. The findings from this study indicate that the HARF heating is a promising dry and blanching technology for inshell hazelnuts, but future studies investigating the effect of HARF blanching on hazelnut quality are necessary to draw a systematic conclusion.
The effects of HARF and hot-air heating on POD and PPO inactivation of inshell hazelnut with an initial 19% MC are reported in Fig. 9 a and b. No significant difference was detected for POD and PPO activities between hot-air and HARF drying (P > 0.05) while HARF and hot-air heated inshell hazelnuts had lower POD and PPO activities than control. The reduction in PPO activity was found significant (P < 0.05) except for the HARF heated hazelnut to 70 °C, whereas POD activity reduction was not significant for both HARF and hot air–dried hazelnuts (P > 0.05). Similar observations were found by ) reporting a significant reduction in PPO activity and no significant change in POD activity for inshell hazelnuts heated by HARF. POD was known as a more heat-resistant enzyme and requires a higher temperature and/or longer treatment time to be inactivated while PPO was considered a relatively more heat unstable enzyme (López et al., 1997). The fact that PPO and POD activities of the inshell hazelnuts dried by hot air and HARF were not significantly different from each other can be interpreted that the enzyme inactivation took place mainly due to the thermal effect of HARF technology. However, more systematic studies are needed to investigate the enzyme inactivation mechanisms of the HARF technology. Overall, it can be concluded that the same PPO reduction can be obtained by using HARF technology in only 7–15 min depending on the target temperature, which was significantly shorter than the hot-air drying time of 2880 min.
Conclusion
This study generated new information on the dielectric properties (dielectric constant, loss factor, and penetration depth) of the ground Oregon Jefferson hazelnut kernels, shells, and inshell nuts. The higher dielectric constant and loss factor were found in ground hazelnut kernels compared to the ground shells and inshell hazelnuts. Dielectric properties of the ground hazelnut samples decreased along with increasing frequency, but increased with increasing temperature and MC. The penetration depth of the hazelnut samples decreased with increasing temperature and MC. Target temperature of heated samples (70, 80, and 90 °C) and initial sample MC (19 or 11%) did not affect the HARF heating uniformity index. Holding HARF heated inshell hazelnuts at the target temperature for an additional 5 min further decreased polyphenol oxidase and peroxidase activities and improved the heating uniformity. This study revealed that HARF heating can simultaneously dry and blanch inshell hazelnuts with significantly shortened time, 7–15 min using HARF vs. 2880 min using hot-air drying to achieve similar MC and enzyme inactivation. Studies to investigate the quality and storability of inshell hazelnuts dried and blanched using HARF technology in comparison with those processed commercially are underway to generate information that is necessary for potential commercial application of the HARF technology.
Data Availability
Data will be provided upon request.
References
Agilent Technologies. (2000). Agilent 16452 Liquid Test Fixture Operation Manual. Palo Alto, CA.
Alfaifi, B., Wang, S., Tang, J., Rasco, B., Sablani, S., & Jiao, Y. (2013). Radio frequency disinfestation treatments for dried fruit: Dielectric properties. LWT - Food Science and Technology, 50(2), 746–754. https://doi.org/10.1016/j.lwt.2012.07.012
Bonvehi, J. S., & Rosua, N. S. (1996). Enzymatic activities in the varieties of hazelnuts (Corylus avellana L.) grown in Tarragona, Spain. Food Chemistry, 56(1), 39–44. https://doi.org/10.1016/0308-8146(95)00151-4
Burnette, F. S. (1977). Peroxidase and its relationship to food flavor and quality: A review. Journal of Food Science, 42(1), 1–6. https://doi.org/10.1111/j.1365-2621.1977.tb01204.x
Dag, D., Singh, R. K., & Kong, F. (2019). Dielectric properties, effect of geometry, and quality changes of whole, nonfat milk powder and their mixtures associated with radio frequency heating. Journal of Food Engineering, 261(February), 40–50. https://doi.org/10.1016/j.jfoodeng.2019.04.017
Dawy, M., Shabaka, A. A., & Nada, A. M. A. (1998). Mulecular structure and dielectile properties of some treated ligulus. Polymer Degradation and Stability, 62(3), 455–462. https://doi.org/10.1016/S0141-3910(98)00026-3
Gao, M., Tang, J., Johnson, J. A., & Wang, S. (2012). Dielectric properties of ground almond shells in the development of radio frequency and microwave pasteurization. Journal of Food Engineering, 112(4), 282–287. https://doi.org/10.1016/j.jfoodeng.2012.05.011
Gong, C., Zhao, Y., Zhang, H., Yue, J., Miao, Y., & Jiao, S. (2019). Investigation of radio frequency heating as a dry-blanching method for carrot cubes. Journal of Food Engineering, 245, 53–56. https://doi.org/10.1016/j.jfoodeng.2018.10.004
Jiao, S., Johnson, J. A., Tang, J., & Wang, S. (2012). Industrial-scale radio frequency treatments for insect control in lentils. Journal of Stored Products Research, 48, 143–148. https://doi.org/10.1016/j.jspr.2011.12.001
Li, R., Zhang, S., Kou, X., Ling, B., & Wang, S. (2017). Dielectric properties of almond kernels associated with radio frequency and microwave pasteurization. Scientific Reports, 7(42452), 1–10. https://doi.org/10.1038/srep42452
Li, Y., Li, F., Tang, J., Zhang, R., Wang, Y., Koral, T., & Jiao, Y. (2018). Radio frequency tempering uniformity investigation of frozen beef with various shapes and sizes. Innovative Food Science and Emerging Technologies, 48(February), 42–55. https://doi.org/10.1016/j.ifset.2018.05.008
Ling, B., Guo, W., Hou, L., Li, R., & Wang, S. (2015). Dielectric properties of pistachio kernels as influenced by frequency, temperature, moisture and salt content. Food and Bioprocess Technology, 8(2), 420–430. https://doi.org/10.1007/s11947-014-1413-8
López, A., Piqué, M. T., Ferrán, A., Romero, A., Boatella, J., & García, J. (1997). Influence of drying conditions on the hazelnut quality: II. Enzymatic Activity. Drying Technology, 15(3–4), 979–988. https://doi.org/10.1080/07373939708917272
Mao, Y., & Wang, S. (2021). Recent developments in radio frequency drying for food and agricultural products using a multi-stage strategy: a review. Critical Reviews in Food Science and Nutrition, 1–18. https://doi.org/10.1080/10408398.2021.1978925
Marra, F., Bedane, T. F., Uyar, R., Erdogdu, F., & Lyng, J. G. (2014). Application of radiowave frequency in food processing. Conventional and Advanced Food Processing Technologies, 501–514. https://doi.org/10.1002/9781118406281.ch20
Özilgen, M., & Özdemir, M. (2001). A review on grain and nut deterioration and design of the dryers for safe storage with special reference to Turkish hazelnuts. Critical Reviews in Food Science and Nutrition, 41(2), 95–132. https://doi.org/10.1080/20014091091779
Ozturk, S., Kong, F., Trabelsi, S., & Singh, R. K. (2016). Dielectric properties of dried vegetable powders and their temperature profile during radio frequency heating. Journal of Food Engineering, 169(May), 91–100. https://doi.org/10.1016/j.jfoodeng.2015.08.008
Ponne, C. T., Baysal, T., & Yuksel, D. (1994). Blanching leafy vegetables with electromagnetic energy. Journal of Food Science, 59(5), 1037–1041. https://doi.org/10.1111/j.1365-2621.1994.tb08184.x
Tang, J., Hao, F., & Lau, M. (2002). Microwave heating in food processing. In Advances in Bioprocessing Engineering (pp. 1–44). https://doi.org/10.1142/9789812706584_0001
Wang, S., Tang, J., Sun, T., Mitcham, E. J., Koral, T., & Birla, S. L. (2006). Considerations in design of commercial radio frequency treatments for postharvest pest control in in-shell walnuts. Journal of Food Engineering, 77, 304–312. https://doi.org/10.1016/j.jfoodeng.2005.06.037
Wang, S., Yue, J., Tang, J., & Chen, B. (2005). Mathematical modelling of heating uniformity for in-shell walnuts subjected to radio frequency treatments with intermittent stirrings. Postharvest Biology and Technology, 35(1), 97–107. https://doi.org/10.1016/j.postharvbio.2004.05.024
Wang, W., Wang, W., Jung, J., Yang, R., Tang, J., & Zhao, Y. (2020a). Investigation of hot-air assisted radio frequency (HARF) dielectric heating for improving drying efficiency and ensuring quality of dried hazelnuts (Corylus avellana L.). Food and Bioproducts Processing, 120, 179–190. https://doi.org/10.1016/j.fbp.2020.01.006
Wang, W, Wang, W., Wang, Y., Yang, R., Tang, J., & Zhao, Y. (2020b). Hot-air assisted continuous radio frequency heating for improving drying efficiency and retaining quality of inshell hazelnuts (Corylus avellana L. cv. Barcelona). Journal of Food Engineering, 279, 109956. https://doi.org/10.1016/j.jfoodeng.2020b.109956
Wang, W., Jung, J., McGorrin, R. J., Traber, M. G., Leonard, S. W., Cherian, G., & Zhao, Y. (2018). Investigation of drying conditions on bioactive compounds, lipid oxidation, and enzyme activity of Oregon hazelnuts (Corylus avellana L.). LWT - Food Science and Technology, 90(2018), 526–534. https://doi.org/10.1016/j.lwt.2018.01.002
Wang, W., Tang, J., & Zhao, Y. (2021). Investigation of hot-air assisted continuous radio frequency drying for improving drying efficiency and reducing shell cracks of inshell hazelnuts: The relationship between cracking level and nut quality. Food and Bioproducts Processing, 125, 46–56. https://doi.org/10.1016/j.fbp.2020.10.013
Wang, Y., Wig, T. D., Tang, J., & Hallberg, L. M. (2003). Dielectric properties of foods relevant to RF and microwave pasteurization and sterilization. Journal of Food Engineering, 57(3), 257–268. https://doi.org/10.1016/S0260-8774(02)00306-0
Zhang, S., Zhou, L., Ling, B., & Wang, S. (2016). Dielectric properties of peanut kernels associated with microwave and radio frequency drying. Biosystems Engineering, 145, 108–117. https://doi.org/10.1016/j.biosystemseng.2016.03.002
Zhang, X., Shi, Q., Gao, T., Zhang, Z., Guo, C., Fu, H., & Wang, Y. (2020). Developing radio frequency blanching process of apple slice. Journal of Food Engineering, 273, 109832. https://doi.org/10.1016/j.jfoodeng.2019.109832
Zhang, Z., Guo, C., Gao, T., Fu, H., Chen, Q., & Wang, Y. (2018a). Pilot-scale radiofrequency blanching of potato cuboids: Heating uniformity. Journal of the Science of Food and Agriculture, 98, 312–320. https://doi.org/10.1002/jsfa.8473
Zhang, Z., Wang, J., Zhang, X., Shi, Q., Xin, L., Fu, H., & Wang, Y. (2018b). Effects of radio frequency assisted blanching on polyphenol oxidase, weight loss, texture, color and microstructure of potato. Food Chemistry, 248(2018), 173–182. https://doi.org/10.1016/j.foodchem.2017.12.065
Zhao, Y., Flugstad, B., Kolbe, E., Park, J. W., & Wells, J. H. (2000). Using capacitive (radio frequency) dielectric heating in food processing and preservation-A review. Journal of Food Process Engineering, 23(860), 25–55. https://doi.org/10.1111/j.1745-4530.2000.tb00502.x
Zhou, X., & Wang, S. (2019). Recent developments in radio frequency drying of food and agricultural products: A review. Drying Technology, 37(3), 271–286. https://doi.org/10.1080/07373937.2018.1452255
Zhu, X., Guo, W., & Wang, S. (2014). Dielectric properties of ground hazelnuts at different frequencies, temperatures, and moisture contents. Transactions of the ASABE, 57(1), 161–168. https://doi.org/10.13031/trans.57.10407
Funding
This work was financially supported by the USDA Technical Assistance for Specialty Crops program (TASC 2021–07) through the Oregon Hazelnut Marketing Board in the USA.
Author information
Authors and Affiliations
Contributions
Damla Dag: conceptualization, investigation, methodology, data curation, writing—original draft. Azin Farmanfarmaee: data curation, writing—original draft. Fanbin Kong: writing—review and editing. Jooyeoun Jung: conceptualization, supervision, writing—review and editing. Robert J. McGorrin: writing—review and editing. Yanyun Zhao: secured funding, conceptualization, supervision, project administration, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dag, D., Farmanfarmaee, A., Kong, F. et al. Feasibility of Simultaneous Drying and Blanching Inshell Hazelnuts (Corylus avellana L.) Using Hot Air–Assisted Radio Frequency (HARF) Heating. Food Bioprocess Technol 16, 404–419 (2023). https://doi.org/10.1007/s11947-022-02946-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02946-6