Abstract
Pomegranate peel, as the major part of pomegranate fruit, contains several important bioactive chemical compounds that are unstable and must be protected. In this study, ethanolic pomegranate peel extract (EPPE) was encapsulated with gelatin by electrospinning. First, based on the results, the lowest fiber diameter was obtained at 20% wt/v gelatin in 30:70 (water/acetic acid) ratio and operating conditions (applied voltage 20 kV, distance 10 cm, and flow rate 0/1 mL/h). To investigate the possibility of encapsulation of EPPE in optimum gelatin fibers, two ratios of EPPE/gelatin (25:75, 50:50) were used. 50:50 ratio was selected as the best ratio because of its encapsulation efficiency for further analyses, including the simulated gastrointestinal digestion, Fourier transform infrared (FTIR) spectroscopy, and X-ray diffraction. The in vitro digestion showed that the fibers could protect the extract.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, pomegranate fruit’s numerous properties (Punica granatum L.) are no longer concealed to anyone (Bertolo et al., 2020). Pomegranate compounds are found even in inedible parts such as pomegranate peel (Nair et al., 2018). Due to the high volume of cultivation worldwide and the production of high amounts of pomegranate peel as a waste, the extraction of bioactive compounds and their incorporation into food additives or supplements can create excellent value for pomegranate peel. The bioactive compounds obtained from pomegranate peel have antioxidant, antifungal, and antimicrobial properties (Malviya et al., 2014).
These bioactive compounds mainly include tannins, alkaloids, flavonoids, and organic acids. Properties such as cancer treatment and prevention, cardiovascular disease, diabetes, and UV protection have been reported (Qu et al., 2010; Zhang et al., 2011; Akhtar et al., 2015). Punicalagins, punicalins, gallic acid, and ellagic acid are the four well-known compounds in pomegranate peel extract with various properties (Lu et al., 2007).
However, environmental factors during storage, such as oxidation and the digestive system of the body, much interfere with the absorption of these compounds, and even some digestive enzymes metabolize these compounds (Johanningsmeier & Harris, 2011). Encapsulating pomegranate peel extract is an easy and effective way to prevent the destruction of sensitive compounds and help control these compounds’ release into the human body (Surendhiran et al., 2020). Encapsulation of the extract with a suitable shell (protein, carbohydrate, or lipid) retards oxidation by preventing direct connection of oxygen with the extract and also protects the extract from digestive enzymes (Zou et al., 2014).
Electrospinning is a simple, one-step, and effective method for encapsulating bioactive compounds with high encapsulation efficiency (Aceituno-Medina et al., 2015a; Moomand & Lim, 2014; Seethu et al., 2020). This method charges the polymer solution containing bioactive material by creating a strong electromagnetic field between the device’s positive and negative poles and directs it from one pole to the opposite pole. The polymer solution is transformed into thin fibers at the nano-/microscale as it moves toward the opposite pole, and by evaporation of the solvent of the polymer solution, the fibers are collected on the collector (Panthi et al., 2015; Pérez-Masiá et al., 2014). Electrospinning is known as a non-thermal method and, unlike methods such as spray dryers, prevents the destruction of heat-sensitive compounds (Robert et al., 2010). A freeze-drying system has been used in order to avoid the destruction of anthocyanins of pomegranate skin extract, and acceptable results have been obtained. However, because of the lengthy process of freezing drying technique and the expensive installations, this method is not cost-effective (Marín et al., 2018; Azarpazhooh et al., 2019).
Several polymeric compounds such as chitosan, polyethylene oxide (Surendhiran et al., 2020), polyvinyl alcohol (He et al., 2019), and gelatin (Bertolo et al., 2020) have been reported to encapsulate pomegranate peel extract. Gelatin has been introduced as a suitable polymer for encapsulating pomegranate peel extract by creating a suitable emulsifier property. On the other hand, this polymer has the necessary properties, such as electrical conduction and surface tension, to form the jet in the electrospinning process. In this study, nanofibers from different gelatin polymer solutions were produced by electrospinning technique, and different proportions of pomegranate peel extract were added to the optimum sample. The release of pomegranate peel extract from the gelatin nanofibers was simulated in the gastrointestinal tract study to pass the pomegranate peel extract through the gastrointestinal tract and transfer it to the clone. Delivering pomegranate peel extract to the clone exerts its anti-cancer properties by providing prebiotic properties for the microbial flora (Akhtar et al., 2015).
Materials and Methods
Materials
Gelatin (type A, pork skin) with moisture content be lower than 13% was purchased from Bio Basic (Bio Basic Inc., Canada). Glacial acid acetic (>99%), sodium carbonate, nutrient broth, and nutrient agar medium culture were supplied from Merck (Darmstadt, Germany). Folin Ciocalteu (FC) reagent, gallic acid, and DPPH (2,2-diphenyl-1-picrylhydrazyl) were purchased from Sigma (St Louis, MO, USA). Ethanol, methanol, and sodium bicarbonate were bought from Dr. Mojallali (Dr. Mojallali Industrial Chemical Complex Co, Iran). The other chemicals are supplied from Bio Basic and have the analytical grade (Bio Basic Inc., Canada).
Preparation of Ethanolic Pomegranate Peel Extracts
Pomegranate peels (PPs), as a by-product of fruit juice companies, were obtained from local producers (Karaj, Alborz, Iran). After separation of burnt and inappropriate parts, the collected peels were cleaned and washed with distilled water and then dried in an oven (at 35 ± 5°C) for 7 days (to avoid heat damage and to reach a moisture content less than 15%). The peels were powdered in a laboratory grinder to reach 50 mesh, and then dried samples were packed and stored in dark dishes at −18°C until extraction. Ten grams of finely powdered peels was mixed with 200 mL of ethanol/water (50/50% v/v) to obtain ethanol pomegranate peel extract (EPPE). Then the mixtures were stirred for 2h at 40°C. The extract was centrifuged, and the supernatant was filtered using a membrane (pore size 0.45 um). The extract was then concentrated to obtain 16.8% of solid matter in a rotary vacuum evaporator (IKA® RV 05-ST). Samples were stored at 8°C until usage.
Determination of Total Polyphenol Content
The total polyphenol content (TPC) of the ethanolic pomegranate peel extracts and electrospun (Fig. 1) samples was determined by the Folin Ciocalteu (FC) method, which was defined by Tabaraki et al. (2012). Fifty microliters of each sample was mixed with 1.8 mL of the freshly prepared FC reagent (10 times diluted with distilled water), and after 5 min at room temperature, 1.2 mL of Na2CO3 (7.5% w/v) was added to the previous mixture and stand for 60 min at room temperature. Finally, the absorbance was read by UV-Visible spectrophotometer (Cecil Instruments Ltd., Cambridge, UK) at 765 nm. Seven different concentrations of gallic acid solutions (0–100 mg/L) were used to create a linear regression equation (R2=0.994).
Determination of Antioxidant Activity by the Radical Scavenging DPPH Method
The spectrophotometric method’s antioxidant activity of EPPE was evaluated by measuring the free radical scavenging capacity by the DPPH according to the method reported by Tabaraki et al. (2012) with some changes (Tabaraki et al., 2012). 0.5 mL of the sample was wholly mixed with 3 mL of 0.1 mM of DPPH solution (3.943 mg DPPH was dissolved with 100 mL 95% ethanol). The mixture was shaken strenuously and placed for 30 min in the dark at room temperature (25 ± 2°C). The blank sample was ethanol with a DPPH solution. The absorbance was recorded against blank at 517 nm. Equation (1) was utilized to calculate the percentage of inhibition of the DPPH radical:
Determination of MIC and MBC
The extract’s antimicrobial activity was evaluated to determine the minimum concentration of added pomegranate peel extract to gelatin solution for the inactivation of microorganisms. For this test, two bacterial strains, such as E. coli (0157:H7) (ATCC35218) and S. aureus (PTCC1431), were used. Bacterial strains were provided by the Department of Food Science, Technology and Engineering at the University of Tehran, Iran. In order to measure both minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), six concentrations of EPPE were used in the broth dilution susceptibility (BDS) test. 1 mL of standard microbial suspension (106 Cfu/mL) was added to 1 mL of different dilutions (70–2500 ppm) of antimicrobial extract that diluted with nutrient broth culture (Merck, Darmstadt, Germany) and incubated at 37±1°C for 24h. A microtube containing a culture medium without microbial suspension and extract was used as the negative control, and a microtube containing culture medium with microbial suspension without extract as the positive control was prepared for visual comparison. Microbial growth was determined by measuring the absorbance at 625 nm by an optical spectrometer and measuring the number of colonies grown on nutrient agar medium (Merck, Darmstadt, Germany).
Preparation of Electrospinning Solutions
To prepare the spinning able solution according to the pretests, the gelatin was dissolved in acetic acid/double-distilled water (70/30% v/v) at a concentration of 20% w/v. The solution was stirred at 40°C for 2 h, and after mixing the solution thoroughly, the EPPE was added at two different concentrations (25, 50% v/v) and stirred at room temperature to make solutions clear.
Characterization of Solutions Properties
The electrical conductivities and the surface tension of feed solutions were measured in triplicate for each sample by a conductometer (Jenway 4510, England) and a tensiometer (Nanometric, Iran) respectively at room temperature (25 ± 2°C). The viscosity of samples was measured in triplicate for each sample by a rotating viscometer (Brookfield DV-III, LV Viscometer, USA) using the spindle 18 at 25 ± 2°C. The shear rate range was between 0.1 and 100 s−1 within 5-s intervals.
Electrospinning of Solutions
The equipment used for the electrospinning process of this study was composed of a high voltage power supply (Pars Nano-ris Co., Esfahan, Iran), a syringe pump (Medifusion, Ms 2200, Korea), a drum collector enveloped with aluminum foil, and a plastic syringe with needle (inner diameter 0.8 mm) that placed in the syringe pump which stands horizontally to the drum collector. The applied voltage was 20kV, the distance between the tip of the needle and the collector was 10 cm, and the flow rate was adjusted to 0.1 mL/h. In this study, electrospinning was applied to the gelatin solution and to encapsulate the EPPE with gelatin.
Characterization of Electrospun Samples
Scanning Electron Microscopy
The fiber morphology was investigated by scanning electron microscope (SEM; Tescan Vega II LMU, Czech Republic) at 30KV. The images were taken at 5000 and 30,000 magnifications. The distribution of gelatin fiber diameters was defined by measuring 50 fibers using ImageJ software, and the results are determined as the mean diameter ± standard deviation.
Fourier Transform Infrared Analysis
FTIR analysis of electrospun samples and EPPE was performed by a Bruker model TENSOR 27 spectrometer (Bruker, Germany). The spectra were collected at room temperature (25 ± 2°C) in the range 500–4000 cm−1 at a resolution of 4 cm−1.
X-ray Diffraction Analysis
X-ray diffraction (XRD) of samples were carried out by X-ray diffractometer (STOE, STADI P. Germany) in the angular range (2θ) of 0–50 at a scanning rate of 0.01 min−1 with Cu kα radiation (λ =0.154 nm). Irradiation was performed at a voltage of 40 kV and a current of 30 mA.
Encapsulation Efficiency
The encapsulation efficiencies of electrospun samples were determined in terms of total phenolic by spectrophotometric analyses according to the method established by Sutaphanit and Chitprasert, with some modifications (Sutaphanit & Chitprasert, 2014). First, the extract was diluted with ethanol 50% at different concentrations, and obtained solutions were scanned at the range of 200–600 nm using a UV-Visible spectrophotometer (Cecil Instruments Ltd., Cambridge, UK), and absorption maximum was observed at 259.6 nm. The absorbance values at this wavelength for various concentrations of the EPPE solutions were recorded and plotted. The data were fit to a linear regression equation (R2=0.9975).
Then 5.5 mg of gelatin fibers with 25% and 50% extract ratios was added to 20 mL of ethanol 50% and then mixed using a vortex for 1 min and sonicated for 20 min. The suspensions were filtered via a syringe filter (pore size= 0.45 μm), and their absorbance was read using a UV-Visible spectrometer (Cecil Instruments Ltd., Cambridge, UK) at 259.6 nm. To evaluate the encapsulation efficiency (EE), Eq. (2) was used:
In Vitro Digestion
To evaluate the bioavailability of encapsulated pomegranate peel extract, in vitro digestion model was performed in laboratory conditions according to the method of Isik et al. (2018) with some changes. For gastric conditions, 10 mg of the sample was dissolved in 5 mL of distilled water, and then its pH reached 2 by adding HCl (6 M) under constant stirring conditions. Then 0.6 mL pepsin solution (160 mg/mL in 0.1 M HCl) was added, and the final volume of the solution was fixed to 10 mL with distilled water. This solution was incubated in the shaker machine for 2 h at 37°C. After 2 h, the total amount of released polyphenols in the solution was measured according to the method described in Section 2.3.
After gastric digestion, 2 mL of the solution was collected, and its pH increased to 5 using NaHCO3 (0.045 M). To simulate intestinal conditions, 2.4 mL of pancreatic–bile solution (pancreatic 4 mg/mL, bile salt 25 mg/mL in 0.1 M NaHCO3) was added into the solution. Then the pH of the solution was increased to 7 by gradually adding NaHCO3 (0.005 M). The solution was incubated at 37°C for 2 h. After 2 h, the total amount of polyphenols present in the solution was measured. In vitro gastrointestinal digestion was performed with two replications for pomegranate peel extract and pomegranate peel extract in gelatin solution and gelatin fibers containing pomegranate peel extract.
It is noteworthy that to measure the amount of TPC present in the fibers and gelatinized mixture before digestion (t=0), according to the method described in Section 2.9.4, samples were added to ethanol 50% (v/v), and after vortexing and sonication, the TPC was evaluated.
Statistical Analysis
Each experiment was carried out in triplicate except in vitro digestion that was performed with two replications. The results were reported as means ± standard deviation (SD), and significance was considered at p<0/05. IBM SPSS Statistics 26 software was employed for statistical analysis. Response surface methodology was applied to optimize the diameter of gelatin fibers by using Design Expert 11.
Results and Discussion
Determination of Total Polyphenol and Antioxidant Capacity of EPPE
Spectrophotometric methods determined TPC and antioxidant activity of EPPE. The phenolic content of EPPE at a concentration of 1 mg/mL was 452 mg gallic acid/L that slightly lower than previous studies. For example, Emam-Djomeh et al. (2015) reported 572 mg gallic acid/L concentration for phenolic content of methanolic pomegranate peel extracts (Emam-Djomeh et al., 2015). The difference between these results can be due to different extraction and analysis methods applied. Besides genetic sources, weather conditions and agricultural processes influence the polyphenolic content and antioxidant capability of fruits (Çam et al., 2009).
The antioxidant activity of EPPE was measured by the DPPH method, which was 92% at the concentration of 192 ppm. The DPPH of pomegranate peel in other studies was reported to range from 45 to 94% (Tabaraki et al., 2012; Derakhshan et al., 2018; Tehranifar et al., 2011). The variance between the results can be explained by differences in cultivars and applied methods, as described above.
Antimicrobial Activity of Pomegranate Peel Extract
The addition of pomegranate peel extract to the polymer solution may affect viscosity and surface tension and affect the electrospun’s diameter and morphology. Therefore, it is necessary to determine the minimum concentration of the extract to deactivate the target microorganisms. Table 1 shows the antimicrobial effects of different pomegranate peel extract concentrations on Staphylococcus aureus and Escherichia coli microorganisms. The results showed that S. aureus was more susceptible to pomegranate peel extract than Escherichia coli (Table 1). The MIC and MBC values for Staphylococcus aureus were 156.25 and 312.5 ppm, respectively, whereas for Escherichia coli, these concentrations were 312.5 and 625 ppm, respectively. It is noted that no growth was observed for the negative control sample, which confirms the sterile condition of the experiments.
The higher complexity of the gram-negative bacterial membrane bilayer than the glycoprotein/teichoic acid monolayer membrane of gram-positive bacteria may be due to their resistance to plant extracts. The antimicrobial extract’s solubility in the lipid phase of the membrane may be related to the observed resistance. However, it would not be a complete description of the difference in gram-positive and gram-negative cells (Emam-Djomeh et al., 2015).
Electrospinning and Properties of Solutions
In the electrospinning process and nanofibers’ morphology, effective parameters such as electrical conductivity, viscosity, and surface tension of the feed solution were noted. Therefore before any further experiments, these properties must be evaluated (Manea et al., 2018). The electrospinning process is fundamentally based on the transmission of electrical charges from the electrode to the droplet of feed solution at the tip of the needle, so the specified level of electrical conductivity is required for nanofibers formation. As a result, solutions with the lowest conductivity cannot be turned into fibers. Electrical conductivity is under the influence of polymer and solvent type, the polymer’s concentration, and the temperature. The results in Table 2 showed that the electroconductivity of the gelatin solution increased with increasing concentration. The conductivity of gelatin solution with 20% w/v gelatin (1.383 ± 0.04 mS/cm) was higher than the gelatin solution with 15% w/v gelatin (1.042 ± 0.03 mS/cm), and they were statistically different (p<0.05). The result conforms to the literature. A study on gelatin type A and B’s electrospinning showed that the gelatin solution’s conductivities gradually increased when the polymer solution concentration increased (Ratanavaraporn et al., 2010). In general, increasing the polymer concentration with increasing effective groups in conducting electric charges increases the polymer solution’s electrical conductivity. This increase in polymer concentration is effective until reaching a maximum concentration, and by further increasing the concentration, the decrease in electrical conductivity is caused by a decrease in the ions’ mobility. This phenomenon can be seen in the 35% w/v gelatin solution (Table 2). The addition of the EPPE at the concentration of 25% v/v resulted in a decrease in the gelatin solution’s electrical conductivity, which is probably directly related to the gelatin solution’s viscosity. The addition of 50% w/v EPPE to gelatin solution increased the conductivity of the gelatin solution, which is compatible with the reports of Aceituno-Medina et al. (2015a) and Isik et al. (2018) (Isik et al., 2018; Aceituno-Medina et al., 2015b). Perhaps it was due to the presence of phenolic compounds in EPPE and an increase in viscosity of gelatin solution that conformance with the studies by (Tavassoli-Kafrani et al., 2017).
Rheological characteristics, especially viscosity, affect the process of fiber formation. In order to make the electrospinning process conceivable, the viscosity must be in a relatively limited range. The solutions with very high viscosity values cannot pass through the injection needle, whereas the solutions with very low viscosity values do not produce fiber, and polymer droplets are created (Manea et al., 2018). There is a direct relationship between the concentration of the gelatin solution and its viscosity (Table 2), and there was a significant difference between solutions (p<0.05). Studies by Ratanavaraporn et al. (2010) have shown that, in general, higher viscosity means the formation of fibers with a large diameter. The addition of EPPE at different concentrations has different results in viscosity. It is probably under the influence of the degree of the polymer molecule chain entanglement within the EPPE. As seen in Tavassoli's studies (Tavassoli-Kafrani et al., 2017), phenolic compounds, depending on their molecular weight, can decrease and increase gelatin’s viscosity solution. The addition of the EPPE at the concentration of 25% v/v leads to a decrease in the polymeric solution’s apparent viscosity, probably due to the lower viscosity of the EPPE (234.8±1.5 mPa.s) or the high osmotic pressure of the EPPE. Elevated osmotic pressure due to the presence of numerous phenolic and inorganic compounds can lead to the removal of water from the gelatin structure, which reduces the apparent viscosity of the polymer solution. The addition of the extract at a concentration of 50% v/v caused an increase in the polymer solution’s apparent viscosity due to hydrocolloid compounds and carbohydrate residues in the EPPE (1026 cm−1 peak in Fig. 2). Water extracted from the three-dimensional gelatin structure was rapidly absorbed by these hydrocolloid compounds and increased viscosity.
Surface tension is another important parameter for the electrospinning process that depends on the polymer’s type and concentration. To create nanofiber morphology by electrospinning, the electrical force has to overcome the surface tension. Simultaneously, the polymer solution’s high superficial tension is formed by droplets instead of the nanofibers (Manea et al., 2018). Compared with electrical conductivity and viscosity results, surface tensions decreased with increasing gelatin concentration (Table 2). The decrease in surface tension with gelatin concentration is consistent with the paper of Okutan et al. (2014). The addition of the extract at concentrations of 25 and 50% v/v caused an increase and a decrease in the polymeric solution’s surface tension, respectively. These changes are probably directly related to changes in the gelatin solution’s viscosity due to the addition of the EPPE (Table 2).
Preliminary studies were performed to obtain a suitable range of electrospinning parameters. According to the investigation of the effect of four independent variables, including operating voltage, the polymer solution’s flow rate, needle-to-collector distance, and gelatin concentration on the dependent variable (fiber diameter), the optimum condition of the electrospinning process was determined. Scanning electron microscopy (SEM) results at different gelatin concentrations at ×5000 magnification (Fig. 1) show that gelatin has a fibrous structure at concentrations above 20% w/v. As mentioned earlier, by increasing the polymer solution concentration, increasing viscosity, and reducing surface tension, the fiber diameter increased (Table 2). When the range of concentration was 15% and slightly lower, the combination of beads and slim fibrous structures was found (Fig. 1a) (Torkamani et al., 2018). This feed solution’s viscosity was very low, and it was not sufficient to suppress jet instability and prevent it from breaking. The concentration of gelatin solution is between 20 and 40% wt. The fibers are formed smoothly and without beads, according to Fig. 1 (Ratanavaraporn et al., 2010). In this range, the fibers’ size depends on the concentration (Table 2); the higher concentration of the solution produced the fiber with a larger diameter.
As mentioned in Table 2, the addition of pomegranate peel extract changes the properties of the gelatin solution, including viscosity and surface tension and electrical conductivity. So a certain amount of it should be added to the gelatin. According to the initial tests, the solution becomes viscous and loses electrospinning ability if the amount is too high (more than 50% v/v). The morphology of optimum gelatin fibers and the optimum gelatin fibers containing 25% and 50% v/v EPPE samples is shown in Fig. 1. It appears that the fibrous structure of gelatin nanofibers was not affected by EPPE. As seen in Fig. 1, by increasing the extract, fiber diameter has increased. The reason may be related to the increase in the viscosity and electrical conductivity because of the increasing the extract content (Table 2).
Encapsulation Efficiency
The values of encapsulation efficiency were evaluated to give a concept about the quality of encapsulation. At the concentration of 25% and 50%, v/v extracts loaded in gelatin fibers, the encapsulation efficiency values were 71.82±0.015 and 94.1±0. 135 respectively. These results indicated that during the electrospinning process, above 70% of the extract could be encapsulated into gelatin fibers, and according to the results, the ratio (50:50) was more efficient and was chosen as the best ratio for the other experiments.
Characterization of Electrospun Samples
In order to determined gelatin structural modification along with the electrospinning process, FTIR and XRD analyses were accomplished on the electrospun samples (gelatin fibers and gelatin fibers with EPPE) and lyophilized EPPE.
FTIR
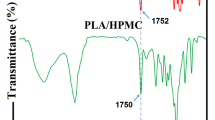
The FTIR spectrum of electrospun samples and lyophilized EPPE is shown in Fig. 2. According to data from previous literature, the band between 1600 and 1700 cm−1 corresponds to amide I (CO stretching), and it was the most useful peak for FTIR analysis of the secondary structure of the protein. The peak around 1540 cm−1 can be assigned to amide II (the CN stretching and NH bending). The amide III bond (CH stretching and NH bending) has been associated with the triple helical structure found between 1200 and 1350 cm−1 (Muyonga et al., 2004). The peaks between 1000 and 1100 cm−1 are attributed to C-O vibration due to collagen carbohydrate residues. As shown in Fig. 2, the main gelatin bonds were in the wavenumbers 1644 and 1533, and the peak at 1087 cm−1 can be related to carbohydrate remaining.
In the infrared spectrum of EPPE (Fig. 2), the peak of about 3290 cm−1 can be allocated to the stretching vibration bond of hydroxylic groups (carboxylic acid, phenol, or alcohols), and the band indicated at about 2934 cm−1 is assigned to the stretching vibration bond of aliphatic C-H groups especially due to C-CH and C-CH2 bonds. The peak at 1717 cm−1 displays C=O groups of carboxylic acid and COO groups of ketone and aldehyde. The band at 1603 cm−1 exhibits the stretching vibration bond of C=O and C=C. Besides, one of the major bands of the EPPE FT-IR spectrum corresponding to 1026 cm−1 can be related to CO stretching bonding and OH deformation in alcohols.
It can be seen that the addition of the EPPE shifted the gelatin fiber peak from 1087 to 1068 cm−1 wavenumber, and also absorption intensity was increased. Due to the sharpness of the 1026 cm−1 peak in the EPPE, this shift and absorption intensity were probably occurred due to the overlap of the two peaks 1026 cm−1 in EPPE with 1087 cm−1 in gelatin fiber. Due to the high similarity of the two spectra of gelatin fiber and gelatin fiber-containing EPPE, encapsulation may have occurred physically.
XRD
X-ray diffraction pattern of the samples of optimized gelatin fibers and gelatin fibers containing 50% v/v EPPE in Fig. 3 contains two diffraction reflections, one at about 8 and the other at 20. According to the Panzavolta studies (2011), the first peak corresponds to a periodicity of about 1.1 nm and is related to the diameter of the triple helix; the second peak corresponds to a periodicity of about 0.45 nm and has been allocated to the distance between adjacent polypeptide strands (Panzavolta et al., 2011). As shown in Fig. 3, the gelatin fibers’ pattern containing pomegranate peel extract at both peaks was less intense than that of the non-extracted gelatin fibers.
Bragg’s equation (λ = 2d sin θ) is used to calculate the distance between chains in which d represents the distance between the planes of the atomic networks, λ is the X-ray wavelength used (0.154 nm), and θ denotes the Bragg diffraction angle (the angle at which the reflected beams have the most amplitude) (Yan et al., 2011). The amount of d in the optimal gelatin fiber sample is 0.454, and the amount of d gelatin fibers containing EPPE is 0.424. It seems that phenolic compounds present in EPPE, such as caffeic acid and gallic acid, can enter the space between polypeptide strands and enhance intermolecular interaction and reduce the distance between them cross-links.
In Vitro Digestion
To determine the bioaccessibility of ethanolic pomegranate peel extract (EPPE) in gelatinized solution and encapsulated in gelatin fibers, a simulated gastrointestinal digestion test with two replications was used, and TPC of samples was measured by spectrophotometric (Table 3).
In EPPE, total polyphenol content (TPC) decreased significantly after crossing the intestinal stage. This decrease can be attributed to the high pH in the intestinal stage, which can damage the extract’s polyphenols. Similar reductions of the total polyphenol content and antioxidant activities have also been observed in other studies. For example, in a study by Toydemir et al. (2013), 20% of the initial amount of TPC of the sour cherry extract was reduced. Moreover, the same decrease of the total phenolic compound was observed in other in vitro digestion studies of sour cherry, pomegranate, and blackberries (Toydemir et al., 2013; Toydemir et al., 2012; Sengul et al., 2014; Isik et al., 2018). A significant decrease in TPC after digestion was observed in the pomegranate peel extract in the gelatinized solution sample.
On the other hand, the amount of TPC was increased in the electrospun sample containing pomegranate peel extract. One reason could be an inhibitory effect on the correct measurement of TPC before in vitro digestion by nano-/microencapsulation. The other reason could be the interactions between proteins and polyphenols. The lower content of polyphenols in undigested samples may be due to the formation of a complex protein and polyphenols present in the extract. While after digestion, due to high pH and digestive enzymes such as pancreatin and bile salt, the complex can be broken, and the amount of polyphenols may be measured more elevated than the initial value.
Improved bioavailability of TPC by electrospinning can be explained by reducing the size of bioactive materials. Increasing the surface-to-volume ratio might be raised the level of contact of the surface groups in gelatin and thus increased the antioxidant properties (Tavassoli-Kafrani et al., 2017). The delivery of any bioactive compounds to different body organs is directly affected by the particles’ size. As a result, reducing the nanoscale capsules’ size provides an ample opportunity for long-term storage in the gastrointestinal tract due to improved adhesion to the mucosal surface of the intestinal epithelium (Anandharamakrishnan, 2014). Therefore, it has been shown that size is a critical parameter for the absorption of compounds. Besides, nanofibers have a higher surface-to-volume ratio and have better solubility. Therefore, the nanoscale size enables better biological access and controlled release (Mozafari et al., 2008). It is observed that the antioxidant activity of nanofibers containing extract at the end of gastric and intestinal digestion had higher antioxidant activity than other samples. These results are probably due to the decrease in the size of the gelatin fibers. Increasing the surface-to-volume ratio increased digestive enzymes’ activity on the gelatin protein, thus increasing antioxidant activity by producing bioactive peptides. This increase has also been observed in other studies. The amount of total polyphenols and antioxidant activity in encapsulated sour cherry concentrated increased after simulated digestion. The bioavailability and total polyphenol content of dairy products and enriched eggs with grape extract increased after in vitro digestion (Pineda-Vadillo et al., 2016; Isik et al., 2018).
Conclusion
In this study, we studied the influence of different parameters such as gelatin concentration, electrical conductivity, surface tension, and viscosity on electrospun samples’ morphology. At 15% (w/v), the gelatin solution produced fibers with beads, whereas regular smooth nanofiber formations occurred at 20% (w/v) gelatin concentration. Moreover, as an agrobiological by-product, pomegranate peel is a rich source of valuable compounds such as phenolic, which are well-known for powerful antioxidant and antimicrobial activities. The results presented in this study indicate that the electrospinning process was applied successfully to encapsulate EPPE. Moreover, it provided high encapsulation efficiency and better protection. Simulated gastrointestinal digestion of encapsulated EPPE in gelatin fibers showed that encapsulation with the electrospinning method could improve the bioavailability of bioactive compounds in EPPE. Furthermore, these gelatin nanofibers containing EPPE might be appropriate for various applications in the field of biomaterials and the pharmaceutical or food industry. According to the results of this study, it is suggested to investigate gelatin nanofibers’ ability in the coating of other bioactive compounds by the core-shell method.
References
Aceituno-Medina, M., Mendoza, S., Lagaron, J. M., & López-Rubio, A. (2015a). Photoprotection of folic acid upon encapsulation in food-grade amaranth (Amaranthus hypochondriacus L.) protein isolate–Pullulan electrospun fibers. LWT-Food Science and Technology, 62(2), 970–975
Aceituno-Medina, M., Mendoza, S., Rodríguez, B. A., Lagaron, J. M., & López-Rubio, A. (2015b). Improved antioxidant capacity of quercetin and ferulic acid during in-vitro digestion through encapsulation within food-grade electrospun fibers. Journal of Functional Foods, 12, 332–341
Akhtar, S., Ismail, T., Fraternale, D., & Sestili, P. (2015). Pomegranate peel and peel extracts: Chemistry and food features. Food Chemistry, 174, 417–425
Anandharamakrishnan, C. (2014). Techniques for nanoencapsulation of food ingredients. Springer
Azarpazhooh, E., Sharayei, P., Zomorodi, S., & Ramaswamy, H. S. (2019). Physicochemical and phytochemical characterization and storage stability of freeze-dried encapsulated pomegranate peel anthocyanin and in vitro evaluation of its antioxidant activity. Food and bioprocess technology, 12(2), 199–210
Bertolo, M. R. V., Martins, V. C. A., Horn, M. M., Brenelli, L. B., & Plepis, A. M. G. (2020). Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydrate polymers, 228, 115386
Çam, M., Hışıl, Y., & Durmaz, G. (2009). Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chemistry, 112(3), 721–726
Derakhshan, Z., Ferrante, M., Tadi, M., Ansari, F., Heydari, A., Hosseini, M. S., Conti, G. O., & Sadrabad, E. K. (2018). Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food and Chemical Toxicology, 114, 108–111
Emam-Djomeh, Z., Moghaddam, A., & Yasini Ardakani, S. A. (2015). Antimicrobial activity of pomegranate (Punica granatum L.) peel extract, physical, mechanical, barrier and antimicrobial properties of pomegranate peel extract-incorporated sodium caseinate film and application in packaging for ground beef. Packaging Technology and Science, 28(10), 869–881
He, L., Lan, W., Ahmed, S., Qin, W., & Liu, Y. (2019). Electrospun polyvinyl alcohol film containing pomegranate peel extract and sodium dehydroacetate for use as food packaging. Food Packaging and Shelf Life, 22, 100390
Isik, B. S., Altay, F., & Capanoglu, E. (2018). The uniaxial and coaxial encapsulations of sour cherry (Prunus cerasus L.) concentrate by electrospinning and their in vitro bioaccessibility. Food Chemistry, 265, 260–273
Johanningsmeier, S. D., & Harris, G. K. (2011). Pomegranate as a functional food and nutraceutical source. Annual review of food science and technology, 2(1), 181–201
Lu, J., Wei, Y., & Yuan, Q. (2007). Preparative separation of punicalagin from pomegranate husk by high-speed countercurrent chromatography. Journal of Chromatography B, 857(1), 175–179
Malviya, S., Jha, A., & Hettiarachchy, N. (2014). Antioxidant and antibacterial potential of pomegranate peel extracts. Journal of food science and technology, 51(12), 4132–4137
Manea, L. R., Bertea, A.-P., Nechita, E., & Popescu, C. V. (2018). Mathematical Modeling of the relation between electrospun nanofibers characteristics and the process parameters. Electrospinning Method Used to Create Functional Nanocomposites Films, 91
Marín, D., Alemán, A., Sánchez-Faure, A., Montero, P., & Gómez-Guillén, M. (2018). Freeze-dried phosphatidylcholine liposomes encapsulating various antioxidant extracts from natural waste as functional ingredients in surimi gels. Food Chemistry, 245, 525–535
Moomand, K., & Lim, L.-T. (2014). Oxidative stability of encapsulated fish oil in electrospun zein fibres. Food Research International, 62, 523–532
Mozafari, M. R., Khosravi-Darani, K., Borazan, G. G., Cui, J., Pardakhty, A., & Yurdugul, S. (2008). Encapsulation of food ingredients using nanoliposome technology. International Journal of Food Properties, 11(4), 833–844
Muyonga, J., Cole, C., & Duodu, K. (2004). Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocolloids, 18(4), 581–592
Nair, M. S., Saxena, A., & Kaur, C. (2018). Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chemistry, 240, 245–252
Okutan, N., Terzi, P., & Altay, F. (2014). Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids, 39, 19–26
Panthi, G., Park, M., Kim, H.-Y., & Park, S.-J. (2015). Electrospun polymeric nanofibers encapsulated with nanostructured materials and their applications: A review. Journal of Industrial and Engineering Chemistry, 24, 1–13
Panzavolta, S., Gioffrè, M., Focarete, M. L., Gualandi, C., Foroni, L., & Bigi, A. (2011). Electrospun gelatin nanofibers: Optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomaterialia, 7(4), 1702–1709
Pérez-Masiá, R., Lagaron, J. M., & López-Rubio, A. (2014). Development and optimization of novel encapsulation structures of interest in functional foods through electrospraying. Food and Bioprocess Technology, 7(11), 3236–3245
Pineda-Vadillo, C., Nau, F., Dubiard, C. G., Cheynier, V., Meudec, E., Sanz-Buenhombre, M., Guadarrama, A., Tóth, T., Csavajda, É., & Hingyi, H. (2016). In vitro digestion of dairy and egg products enriched with grape extracts: Effect of the food matrix on polyphenol bioaccessibility and antioxidant activity. Food Research International, 88, 284–292
Qu, W., Pan, Z., & Ma, H. (2010). Extraction modeling and activities of antioxidants from pomegranate marc. Journal of Food Engineering, 99(1), 16–23
Ratanavaraporn, J., Rangkupan, R., Jeeratawatchai, H., Kanokpanont, S., & Damrongsakkul, S. (2010). Influences of physical and chemical cross-linking techniques on electrospun type A and B gelatin fiber mats. International journal of biological macromolecules, 47(4), 431–438
Robert, P., Gorena, T., Romero, N., Sepulveda, E., Chavez, J., & Saenz, C. (2010). Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. International Journal of Food Science & Technology, 45(7), 1386–1394
Seethu, B. G., Pushpadass, H. A., Emerald, F. M. E., Nath, B. S., Naik, N. L., & Subramanian, K. S. (2020). Electrohydrodynamic encapsulation of resveratrol using food-grade nanofibres: Process optimization, characterization and fortification. Food and Bioprocess Technology, 13(2), 341–354
Sengul, H., Surek, E., & Nilufer-Erdil, D. (2014). Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (Punica granatum) phenolics and anthocyanins using an in-vitro gastrointestinal digestion model. Food Research International, 62, 1069–1079
Surendhiran, D., Li, C., Cui, H., & Lin, L. (2020). Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packaging and Shelf Life, 23, 100439
Sutaphanit, P., & Chitprasert, P. (2014). Optimisation of microencapsulation of holy basil essential oil in gelatin by response surface methodology. Food Chemistry, 150, 313–320
Tabaraki, R., Heidarizadi, E., & Benvidi, A. (2012). Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Separation and Purification Technology, 98, 16–23
Tavassoli-Kafrani, E., Goli, S. A. H., & Fathi, M. (2017). Fabrication and characterization of electrospun gelatin nanofibers cross-linked with oxidized phenolic compounds. International journal of biological macromolecules, 103, 1062–1068
Tehranifar, A., Selahvarzi, Y., Kharrazi, M., & Bakhsh, V. J. (2011). High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Industrial Crops and Products, 34(3), 1523–1527
Torkamani, A. E., Syahariza, Z. A., Norziah, M. H., Mahmood, W. A. K., & Juliano, P. (2018). Production and characterization of gelatin spherical particles formed via electrospraying and encapsulated with polyphenolic antioxidants from Momordica charantia. Food and Bioprocess Technology, 11(11), 1943–1954
Toydemir, G., Capanoglu, E., Boyacioglu, D., Beekwilder, J., de Vos, R., & Hall, R. (2012). Sour cherry (Prunus cerasus L.) anthocyanins: Effects of juice processing on phenolic compounds and bioavailability. X International Symposium on Vaccinium and Other Superfruits, 1017, 387–398
Toydemir, G., Capanoglu, E., Kamiloglu, S., Boyacioglu, D., De Vos, R. C., Hall, R. D., & Beekwilder, J. (2013). Changes in sour cherry (Prunus cerasus L.) antioxidants during nectar processing and in vitro gastrointestinal digestion. Journal of Functional Foods, 5(3), 1402–1413
Yan, M., Li, B., Zhao, X., & Yi, J. (2011). Physicochemical properties of gelatin gels from walleye pollock (Theragra chalcogramma) skin cross-linked by gallic acid and rutin. Food Hydrocolloids, 25(5), 907–914
Zhang, L., Fu, Q., & Zhang, Y. (2011). Composition of anthocyanins in pomegranate flowers and their antioxidant activity. Food Chemistry, 127(4), 1444–1449
Zou, L.-q., Peng, S.-f., Liu, W., Gan, L., Liu, W.-l., Liang, R.-h., Liu, C.-m., Niu, J., Cao, Y.-l., & Liu, Z. (2014). Improved in vitro digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Research International, 64, 492–499
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saadat, S., Emam-Djomeh, Z. & Askari, G. Antibacterial and Antioxidant Gelatin Nanofiber Scaffold Containing Ethanol Extract of Pomegranate Peel: Design, Characterization and In Vitro Assay. Food Bioprocess Technol 14, 935–944 (2021). https://doi.org/10.1007/s11947-021-02616-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02616-z