Abstract
The aim of this study was to compare the ability of endogenous Lactobacillus paracasei ATR6 to grow in unsupplemented bovine cheese whey (CW) and ricotta whey (RW), and to evaluate the efficiency of these media as encapsulating agents for the spray-drying (SD) of this bacterium. Growth assays indicated that both CW and RW are adequate culture media since L. paracasei ATR6 grew satisfactorily (~ 4 log cycles) in both compared to the control medium (skimmed milk). Concerning their potential as encapsulating agents, results showed that survival rates after SD were similar for CW and RW (> 78%), as were the yields (> 47%) of this process. CW allowed the formation of smaller capsules (5.31 ± 1.27 μm) than RW (15.21 ± 7.24 μm), with lower moisture: 8.60% and 13.52%, respectively. Powders were stored at 25 °C for 60 days, during which CW and RW maintained satisfactory bacterial viability. Water activity increased, yet remained below 0.3. Moisture content of CW and RW powders was relatively stable throughout the 60 days; however, values obtained in this study are considered to lie outside the adequate range (4–7%) for product stability. We did not observe color changes in CW and RW microcapsules during storage. Due to its lower protein content, RW offered less protection, which led to higher loss in acidification ability of the strain. CW and RW are suitable culture media and encapsulating agents for the growth of L. paracasei ATR6; however, the former offered better protection, which enabled the maintenance of biological activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) are microorganisms commonly used in the food industry as fermentation agents for dairy, meat, and vegetable products (Jensen et al. 2012). In this context, LAB can be employed either as starters (SLAB), with the function of rapidly converting lactose into lactic acid, thus promoting acidification of the medium, or non-starters (NSLAB), involved in the production of aromatic compounds (Motta and Gomes 2015). These microorganisms, whether they are starters or non-starters, tend to help enhance taste, texture, nutritional value, shelf life, and safety of food products (Souza and Dias 2017). The indiscriminate use of commercial LAB cultures, however, leads to a flattening of sensory characteristics in a variety of products, making them no longer distinguishable by production technology or geographical origin (Guarcello et al. 2016). As an alternative, endogenous LAB represent a market differentiation since they are used to maintain the traditional characteristics of regional products (Oliszewski et al. 2013; Speranza et al. 2015). In addition, some LAB strains are classified as probiotic, which means that they can provide health benefits to the host when supplied in adequate amounts (FAO/WHO 2002).
The major LAB genera are Lactococcus, Lactobacillus, Leuconostoc, Pediococcus, Enterococcus, and Streptococcus (Motta and Gomes 2015). Lactobacilli are versatile and can be employed as either starter, non-starter, or probiotic cultures (Angmo et al. 2016; Li and Han 2018; Marco et al. 2017). Studies report Lactobacillus spp. as tolerant to high temperatures, to the presence of bile salts, and to extreme pH levels (Ferrando et al. 2016; Rushdy and Gomaa 2013). This genus is also dominant in the microbiome of many traditional fermented milk products (Domingos-Lopes et al. 2017; Farimani et al. 2016; Picon et al. 2016). Specifically regarding Lactobacillus paracasei, there is an array of studies using this strain as starter (Bartkiene et al. 2018; Donkor et al. 2005), non-starter (Burns et al. 2012; Maragkoudakis et al. 2006), and probiotic (Brignone et al. 2017; Terpou et al. 2018) cultures for food applications, which shows the broad use of this LAB species.
In general, LAB have fastidious nutritional requirements. Aside from the presence of a carbon source and minerals (such as manganese and iron), reports show that the growth of these microorganisms increases in the presence of several amino acids, including l-glycine, l-alanine, and the branched-chain amino acids (BCAAs), composed of l-valine, l-leucine, and l-isoleucine (Aller et al. 2014; Terrade and Orduña 2009). These amino acids are present in cheese whey (CW), a primary by-product of the dairy industry. This material contains approximately 60% of milk contents, especially lactose, minerals, and proteins (Dragone et al. 2011; Guimarães et al. 2010). In the industry, CW can be used in the production of ricotta, which releases ricotta whey (RW), primarily composed of lactose (Carvalho et al. 2013). CW and RW contain high organic loads (1/150 compared to loads from domestic wastewater), and could represent an environmental threat if discarded inappropriately (Gallego-Schmid and Tarpani 2019; Pega et al. 2018; Sansonetti et al. 2009). Following that reasoning, these by-products from the dairy industry can be exploited as culture media for the growth of LAB.
LAB cultures must be in the form of dried powders for industrial applications. Spray-drying (SD) is a method that enables the formation of a capsule for the protection of bioactive ingredients, including microorganisms (Maciel et al. 2014). Technologies such as SD have been used for several LAB, with the best cost–benefit ratio among the best known methods, e.g., freeze-drying and membrane filtration (Ambros et al. 2016; Huang et al. 2017b; Pérez-Chabela et al. 2013). Microorganisms are sensitive to high temperatures used in SD; for that reason, encapsulating agents provide a coating that protects them from immediate death, especially via the stabilization of cell membranes (Bustos and Bórquez 2013). In this context, the presence of carbohydrates and proteins is essential for the formation of a structured capsule (Đorđević et al. 2014). Since dairy by-products are rich in these nutrients, they can be employed as encapsulation agents for SD of LAB (Bustos and Bórquez 2013; Eckert et al. 2017; Huang et al. 2017b).
In order to develop LAB culture, the first step is to choose an adequate strain for the desired product. Endogenous LAB tend to reinforce typicality and originality, which may lead to the development of unique products, e.g., with Protected Denomination of Origin (Farimani et al. 2016; Guarcello et al. 2016; Tsafrakidou et al. 2016). In fact, endogenous lactobacilli such as L. paracasei were evaluated as part of a starter culture in Spanish PDO cheese manufacture (González et al. 2015). The second step would be bacteria multiplication to obtain biomass; in order to meet their nutritional demands, CW has been reported to be a suitable growth medium for LAB (Burns et al. 2008; Pescuma et al. 2012; Soriano-Perez et al. 2012). However, there is little literature addressing the use of RW for that purpose (Lavari et al. 2014; Maragkoudakis et al. 2010; Secchi et al. 2012). The third step consists of defining the best drying method since bacterial cultures must be presented in an adequate form, which allows the survival of microorganisms and facilitates handling and storage of the material. As previously mentioned, CW has been reported as encapsulating agent for SD of LAB; however, to the best of our knowledge, studies about the use of RW for this purpose are nonexistent.
Therefore, the aim of this study was to evaluate the ability of a Brazilian endogenous strain, L. paracasei ATR6, to grow in bovine CW and RW, and to compare the protection effects of these by-products in SD of this strain. For that, L. paracasei ATR6 was cultivated in flasks, with agitated and static incubation, using skimmed milk (SM) as control. Subsequently, growth was advanced using a bioreactor, after which CW and RW batches were immediately taken to the spray-dryer. Thus, we propose a two-step process that does not employ supplements either for the growth or for the drying of L. paracasei ATR6. The powders were evaluated throughout a storage period of 60 days, regarding cell viability, moisture content, water activity, and color. After drying, the acidification potential of the encapsulated L. paracasei ATR6 was evaluated and compared to that of free microorganisms.
Materials and Methods
Microorganisms and Media
The L. paracasei strain ATR6 used in this study is endogenous from the region of Cachoeira Paulista, São Paulo, Brazil, and was isolated from milk samples using the following procedure: 25 mL of raw milk was serially diluted in peptone water (0.1% (w/v)). Dilutions were plated in M17 and in Rogosa agar, in duplicates. M17 plates were incubated aerobically at 30 °C for 48 h, while Rogosa plates were incubated anaerobically at 37 °C for 72 h. After choosing the plate that allowed the best isolation, 10 to 15 colonies were transferred from each plate (one M17 plate and one Rogosa agar plate) to de Man, Rogosa, and Sharpe (MRS) agar plates, and incubated at 37 °C for 24 to 48 h. One colony of each sample was resuspended in MRS broth, incubated under the same conditions until growth was visually observed, and stored at − 20 °C in MRS broth and glycerol (20% v/v). Skimmed milk (SM) was purchased from Elebat Alimentos S.A. (Teutônia, RS, Brazil) and was prepared as recommended by the manufacturer, with a 10% (w/v) reconstitution in distilled water. This medium was sterilized using fluent vapor at 100 °C for 15 min. CW powder was purchased from BRF S.A. (Ijuí, RS, Brazil) and was also reconstituted in distilled water, in order to attain 5% (w/v) of lactose. RW was kindly provided by a local dairy factory (Cotrilac Ltda., Anta Gorda, RS, Brazil), and was transported under refrigeration and maintained at − 20 °C until its use. RW was used in its natural form. Both CW and RW were pasteurized in a water bath at 65 °C for 30 min. All reagents used in this study were of analytical grade, and synthetic culture media were purchased from Merck®. SM, CW, and RW used in this study are bovine.
Physicochemical Characterization of Milk and Dairy By-Products
Physicochemical parameters of SM, CW, and RW were analyzed according to AOAC International (2012). Total solids were evaluated using an oven dryer at 105 °C (method no. 990.20), ash was evaluated using a muffle furnace at 550 °C (method no. 968.08), fat was evaluated using the Mojonnier method (no. 2000.18), and protein was evaluated using the Kjeldahl method (no. 991.20). Lactose content was evaluated using the dinitrosalicylic acid (DNS) method (Miller 1959). CW and RW colors were measured using a Konica Minolta CM-5 colorimeter and a color scale to measure the parameters L*, a*, and b*.
Growth of L. paracasei ATR6 in Flasks
L. paracasei strain ATR6 was isolated from milk samples in Rogosa agar, and the pure culture was then stored in MRS broth, with 20% (v/v) glycerol at − 20 °C. The frozen sample was resuspended in MRS broth and subsequently incubated at 37 °C for 24 h. Liquid culture was then transferred to MRS agar plates, which were incubated under the same conditions. Plates were kept in a refrigerator at 10 °C for up to 1 month and were used as needed. Several colonies from MRS agar plates were resuspended in 140 mL of MRS broth, incubated at 37 °C for 48 h. This culture was standardized to optical density at 600 nm (OD600) of 2.0 ± 0.1, and then divided in 20-mL aliquots that were centrifuged (Universal 320R, Hettich Zentrifugen®) at 3850×g and 4 °C for 20 min. The supernatant was discarded and cells were resuspended in 20 mL of SM, CW, or RW. These aliquots were then inoculated in 180 mL (in 1-L Erlenmeyer flasks) of the correspondent medium, representing an inoculum of 10% (v/v). Flasks of SM, CW, and RW were incubated at 37 °C for 48 h in a static incubator (EthikTechnology®) and orbital agitation incubator, called shaker (MA830, Marconi®) at 250 rpm. This generated duplicate samples for each incubation regime: SMI, CWI, and RWI for static samples, and SMS, CWS, and RWS for shaker samples.
In order to evaluate the ability of L. paracasei ATR6 to grow in both CW and RW compared to SM, cells were counted using the drop plate technique, in which 10 μL of the diluted cultivated broths were plated in MRS agar and incubated at 37 °C for 48 h. Values of pH were measured using a pH-meter (DM-22; Digimed®). Lactose consumption was evaluated using the DNS method (Miller 1959), following observations with spectrophotometer (i3; SpectraMax®) at 470 nm. Samples for all the analysis were taken at the following times: 0, 12, 24, 36, and 48 h. This experiment was conducted in triplicates.
Growth of L. paracasei ATR6 in Bioreactor
L. paracasei ATR6 cultivation was analyzed separately in CW and in RW in the bioreactor. Similarly to the standardization process for growth in flasks, 24–48-h L. paracasei ATR6 cultures with OD600 = 2.0 ± 0.1 were inoculated (10% (v/v)) in both media, reaching a final working volume of 1300 mL in a 2-L bioreactor (BioStat® B; Sartorius Stedim). Cultivations were carried out at 37 °C and 150 rpm for 72 h, in which sampling was performed at the following times: 0, 2, 4, 8, 12, 16, 20, 24, 32, 40, 48, 60, and 72 h. The methods for cell counting and lactose consumption were the same as the ones used in Section 2.3. Values of pH throughout cultivation were measured using an input electrode (EasyFerm Plus VP 225, Hamilton®) from the bioreactor system. Batches were performed in triplicates. Maximum growth rate (μmax) for the cultivation of L. paracasei ATR6 in both CW and RW was calculated by linear fitting the exponential phase of the growth curve and obtaining the angular coefficient of the equation (slope), according to de Souza et al. (2009).
Spray-Drying of L. paracasei ATR6
Spray-drying parameters were based on previously published studies (Eckert et al. 2017). A laboratory-scale spray-dryer (MSD 0.5; Labmaq) was fed (0.25 L/h) with CW and RW solutions containing L. paracasei ATR6, working with air inlet temperature of 90 °C and outlet temperature of 75 °C. Drying airflow was established as 1.10 m3/min, and the diameter of the spray nozzle employed in the encapsulation process was 1.0 mm. Dried powder samples were collected from the base of the cyclone in sterile bottles. Spray-drying processes were carried out in triplicates.
In order to evaluate L. paracasei ATR6 survival after spray-drying, 0.1 g of microcapsules were diluted in 0.9 mL of peptone water (0.1% (w/v)). The solutions were stirred in vortex for 10 min until complete release of the capsules. Survival rate (%) after the process is calculated by Eq. 1, where N is the number of viable cells (log CFU/g of dry matter) in the microcapsules and N0 is the number of viable cells (log CFU/g of dry matter) in the feed solutions (before drying) (Picot and Lacroix 2004; Pinto et al. 2015).
Spray-drying yield (SDY) was calculated by dividing the weight of solids in the dried powder (Wp) immediately after drying and the weight of solids in the solutions (Ws) fed in the dryer, as shown in Eq. 2 (Bustos and Bórquez 2013).
Microcapsule Characterization
Immediately after drying, samples were fixed with double-sided carbon tape, coated with gold on a sputter coater (Q150R ES, Quorum Technologies), and viewed using scanning electron microscopy (SEM) (LS 10; Carl Zeiss) in high-vacuum mode to examine surface morphology and to estimate the size of the microcapsules. Software ImageJ was used for measuring mean size. Cell viability during storage was measured as mentioned in Section 2.3 and is expressed in log CFU per gram of dry matter. Moisture content was evaluated according to AOAC method no. 990.20, using an oven dryer at 105 °C (AOAC 2012). Water activity (aw) was measured using an AquaLab water activity instrument (AquaLab Lite; Decagon Devices). Color was analyzed using a Konica Minolta CM-5 colorimeter and a color scale to measure parameters L*, a*, and b*. In all these experiments (except for SEM), samples were collected at 0, 15, 30, 45, and 60 days of storage; powders were stored in sterile flasks at 25 °C, protected from light. Assays were conducted in triplicates.
Acidification Potential of Encapsulated L. paracasei ATR6
The acidification potential of the dried powders (at 0, 30, and 60 days) was evaluated by incubation in SM, and by comparing with free (non-encapsulated) L. paracasei ATR6 cells. For the bacteria encapsulated with CW and RW, the viability of 0.1 g of each powder was evaluated as described in Section 2.3. After that, a given mass of powder was resuspended in 10 mL of SM and homogenized in vortex for 10 min, in order to achieve a final population of approximately 106 CFU/mL. Free cultures were also standardized, by diluting samples originally containing approximately 109 CFU/mL (achieved at 24 h of incubation in MRS broth), followed by centrifugation (3850×g, 20 min at 4 °C) and resuspension in SM, to obtain the same bacterial population as the encapsulates; approximately 106 CFU/mL of milk.
For encapsulated and free L. paracasei ATR6, 90 mL of SM was inoculated (10% (v/v)) with 10 mL of these standardized cultures and incubated statically at 37 °C for 14 h, in which pH variation was measured using a pH-meter (DM-22; Digimed®) at 0, 2, 4, 6, 8, 10, 12, and 14 h. In addition, the following kinetic parameters of acidification were evaluated, according to Eckert et al. (2018): Vmax (maximum rate of acidification, in units of pH per minute (upH/min)); tVmax (time in hours to reach Vmax); pHmax (pH value corresponding to the Vmax); ΔpHmax (pH variation between beginning and end of incubation and pHmax).
Data Analysis
Assays with three or more samples were evaluated using an analysis of variance (ANOVA) followed by Tukey’s test with a significance of 95% (p ≤ 0.05). Assays with two samples were analyzed using Student’s t test, also with p ≤ 0.05. Analyses were performed using the BioEstat 5.3 software. Graphs were generated using Origin Lab 8.0. MEV images were edited using CorelPHOTO-PAINT® 2019 and CorelDRAW® 2019.
Results and Discussion
Physicochemical Composition of Milk and Dairy By-Products
The composition (g/L) of the dairy media is shown in Table 1. Total solid and protein contents are different (p ≤ 0.05) among the three media, and SM is the medium that contains the highest values of these parameters. Lactose concentration showed no statistical difference between SM and CW, being slightly lower in RW. SM and CW have similar (p ≤ 0.05) ash and fat contents. Regarding milk composition, values obtained in this study are similar to those reported by the standard references of the United States Department of Agriculture (USDA 2018). As to CW, physicochemical composition is also in accordance with what has been published by USDA and other authors (Eckert et al. 2017; USDA 2018). RW composition is also similar to that obtained in other studies (Lavari et al. 2014; Sansonetti et al. 2009). CW and RW color analyses resulted in different values for each sample. CW values were 65.10, − 0.96, and 28.72, for parameters L*, a*, and b*, respectively. On the other hand, RW values were 84.56, − 0.32, and 16.92. This means they both are prone to light coloration (because L* values are positive), although RW white tones are brighter. Both of the materials had negative a* values, which indicates they tend to have green tones. Similarly, b* values were both positive, meaning both samples also tended to have yellow coloration. This corroborates the findings by Carvalho et al. (2013), who state that whey is a greenish-yellow by-product.
Growth of L. paracasei ATR6 in Flasks
Table 2 shows L. paracasei ATR6 growth results (in log CFU/mL) in the three media tested. No statistical difference (p ≤ 0.05) was found between them, which indicates that CW and RW are equally suitable for the growth of this bacterium, compared to SM. Lavari et al. (2014) reported similar increase in L. paracasei population between CW and RW, obtaining a growth of approximately 1.5 log cycles after 18 h of static incubation in these media, which is slightly lower than the values observed in the present study. Soriano-Perez et al. (2012) studied agitated incubation (in shaker at 200 rpm) of Lactobacillus helveticus in CW and observed a similar increase in this bacterium population (1.1 log cycles). In fact, agitation during incubation seems to be strain dependent since some authors (Ibrahim et al. 2010) have found that increased agitation (between 50 and 250 rpm in shaker) negatively affected the growth of Lactococcus lactis, while others (Gupta et al. 2011) have reported an opposite reaction for Lactobacillus plantarum (growing at an agitation speed of 0, 50, and 100 rpm), which had a higher growth rate under agitated incubation. In the present study, the maximum log increase occurred between 4.70 and 5.59 log CFU/mL, with no statistical differences between media or incubation systems.
Furthermore, regarding lactose consumption (Fig. 1), statistical analysis showed that the total decrease in the concentration of this sugar was similar (p ≤ 0.05) between SMS, SMI, and CWI, with results of 10.09 ± 2.86, 10.48 ± 0.54, and 12.49 ± 0.66 g of lactose per liter, respectively, after 48 h of cultivation. Higher values were obtained for incubation in CWS (14.39 ± 1.75 g/L) and RWS (14.62 ± 3.11 g/L), in the same period of 48 h. L. paracasei ATR6 had its maximum value of lactose consumption (18.70 ± 0.77 g/L) in RWI. Statistical analysis reveals no difference among the three samples (CWS, RWS, and RWI). These results show that the incubation system (agitated or static) only caused a difference in CW since lactose consumption in the other media was similar (p ≤ 0.05) between static and shaker samples. Therefore, static incubation can be considered more efficient since it does not require agitation energy and allows bacteria to multiply in the same manner, as explained above. Additionally, although growth was satisfactory in the three media, L. paracasei ATR6 was unable to consume all the available lactose in any of them, which has also been reported by other authors (Bernárdez et al. 2008; Secchi et al. 2012). This can be explained by increased concentration of lactic acid, which inhibits growth, and consequently, LAB metabolism (Soriano-Perez et al. 2012).
This inhibition can be correlated to the total variation in pH throughout incubation time. Final values of all media lay between 3.62 and 3.97, and the lowest decrease occurred in RW samples, while CW and SM had similar ∆pH (p ≤ 0.05). Hence, L. paracasei ATR6 had the same growth in RW as in CW and SM with lower lactic acid production, even though it consumed the same mean amounts of lactose. Furthermore, incubation system (whether agitated or not) showed no differences in pH variation. In addition, the initial pH of RW was lower than that of CW and SM, which is due to the use of organic acids in ricotta manufacture (Carvalho et al. 2013). Nevertheless, growth in both CW and RW was proven to be satisfactory; that is why these media were chosen to continue the study with bioreactors.
Growth of L. paracasei ATR6 in Bioreactor
Figure 2 shows L. paracasei ATR6 growth, lactose consumption, and pH variation in CW and RW, cultivated in bioreactor. The maximum increase in bacterial population found in CW was 4.25 ± 0.84 log CFU/mL (48 h), which corroborates reports by other authors who found a 4 log increase in L. reuteri (Jantzen et al. 2013) and L. paracasei (Bartkiene et al. 2018) in CW. In RW, on the other hand, the maximum population increase was 5.88 ± 0.46 log CFU/mL (48 h). Statistical analyses indicate that there is a difference (p ≤ 0.05) between these values, which shows that L. paracasei ATR6 populations had higher ability to increase in RW than in CW in this experiment. Growth rates, however, were considered similar in CW (μmax = 0.71 ± 0.35/h) and RW (μmax = 0.63 ± 0.11/h), meaning that this bacterium showed the same ability to duplicate itself in CW and in RW.
Total lactose consumption in CW was 13.76 ± 1.78 g/L; in RW, lactose metabolism was slightly higher (14.48 ± 1.75 g/L), but with no statistical difference (p ≤ 0.05). As occurred with the flask experiment, L. paracasei ATR6 consumed similar amounts of lactose in RW, which indicates this strain has a similar ability to grow in either CW or RW. However, the sugar available was not fully consumed in either media, which again happened in the bioreactor and is explained by lactic acid inhibition. Additionally, Lactobacillus genus of LAB is described as having the ability of degrading only hexoses for ATP release, especially via the Embden–Meyerhof–Parnas (EMP) pathway (Schleifer and Ludwig 1995). Therefore, lactose itself has been reported as an adequate carbon source for the growth of lactobacilli (Dong et al. 2014).
In the present study, L. paracasei ATR6 had a higher growth profile in RW (5.88 ± 0.46 log CFU/mL) than in CW while consuming the same average concentration of lactose (approximately 14 g/L). Bacteria need ATP to grow, and the EMP pathway (glycolysis) is reported to be the only path for LAB to obtain this energy (Buron-Moles et al. 2019; Pessione 2012; Schleifer and Ludwig 1995). However, CW is a culture medium that enables resistance to several stressful conditions such as heat, pH, and presence of bile salts, induced by the accumulation of intracellular compounds (Huang et al. 2018; Huang et al. 2016). In addition, the BCAA’s l-isoleucine (Ile), l-leucine (Leu), and l-valine (Val), present in CW, were found to be essential for the growth of not only lactobacilli but also other LAB genera (Aller et al. 2014; Terrade and Orduña 2009). Bearing this in mind, our results indicate that L. paracasei ATR6 in CW may be using the energy from lactose degradation to assimilate other metabolic pathways involved in tolerance mechanisms. On the other hand, the energy from glycolysis in RW may have been entirely used for growth, which explains the better ability of the bacteria to grow in this medium than in CW. Indeed, several other papers employ the use of these dairy by-products without any supplementation for the growth of LAB, and their results, like ours, indicate that these are suitable media for this purpose (Ardanareswari et al. 2017; Burns et al. 2008; Lavari et al. 2014; Pescuma et al. 2012; Secchi et al. 2012; Soriano-Perez et al. 2012).
Considering the potential for large-scale production of LAB cultures, the use of unsupplemented medium is highly recommended since these supplements (glucose, maltodextrin, yeast extract, casein peptone) can be very expensive. Soriano-Perez et al. (2012), for example, showed that the use of yeast extract does not enhance the growth of L. helveticus. On the other hand, Burns et al. (2008) found that a small supplementation (0.3%) with the same ingredient enables several LAB to grow in a similar way to how they perform in commercial medium (MRS). The results obtained in the present study are higher than those obtained by these two authors (Soriano-Perez et al. 2012; Burns et al. 2008), although we used longer fermentation time. Additionally, in another study (Rama et al. 2019), we found that the need for supplementation is possibly genus- or even strain-dependent since similar strains perform differently in either the presence or absence of extra carbon/nitrogen sources. For that reason, we strongly suggest testing individual strains for their nutritional demands.
Furthermore, total variations in pH were 3.03 ± 0.05 and 2.27 ± 0.08 for CW and RW, respectively. These values are statistically different (p ≤ 0.05). Similar to what happened in flasks, L. paracasei had less acidification ability in RW. Hence, less lactic acid in the medium led to a similar growth profile between CW and RW. Optimum initial pH for the growth of lactobacilli is reported to lie between 5.4 and 6.4, which includes the CW and RW used in this study. However, this genus has a high tolerance to pH variations, being able to grow in extreme values such as 2 and 11 (Popescu et al. 2014). Therefore, authors (Ai et al. 2017; Silva et al. 2005) report, for instance, that the growth of Lactobacillus spp. in an uncontrolled pH environment produces higher yields of biomass and enables the expression of proteins related to heat-shock resistance, which are very useful in terms of post-growth processes such as SD and/or lyophilization. The cultivation experiments carried out in this study did not employ pH control since the aim was to obtain a protocol for biomass production that involved minimal use of reagents and/or supplements that could increase the costs and complexity of the process.
Spray-Drying of L. paracasei ATR6
Samples at a stationary phase were directly submitted to SD, without going through previous steps of harvesting and resuspension in encapsulation material. Jantzen et al. (2013) report that it is more suitable to spray-dry bacteria in a stationary phase since lower proliferation rate leads to less microbial activity and increased stress tolerance. SD results are shown in Table 3. Survival rates of L. paracasei ATR6 were similar (p ≤ 0.05) in CW and RW. The importance of this result relies on the fact that, according to a study recently published (Rama et al. 2019), there are no reports on the use of RW for the encapsulation of LAB using SD.
Ilha et al. (2015) encapsulated L. paracasei with CW and found survival rates of 93%, higher than those obtained in the present study for any of the protective materials evaluated. This may be explained by the fact that the outlet temperature was lower (55 °C) than the one used in our experiment (75 °C); this temperature was chosen due to the experience of other researchers that used the same equipment and encapsulating agent, as well as a similar microorganism (Eckert et al. 2017). However, a lower range of temperatures (55–65 °C) was also employed for SD of Lactobacillus acidophilus LA-5, with survival rates (~ 76%) more similar to our results for both CW and RW (Maciel et al. 2014), corroborating once again the biotechnological potential of these two dairy by-products.
Finally, there was no significant difference (p ≤ 0.05) in the SDY of CW and RW microcapsules, which was close to 50% in both cases. These results are higher than those found by other authors (Bustos and Bórquez 2013) who encapsulated a Lactobacillus strain with CW as encapsulating agent and obtained a maximum yield of 35%. According to Bustos and Bórquez (2013), SDY is directly affected by spray nozzle size and atomizing pressure, which are equipment characteristics. Since the spray-dryer used in this study is different from the one used in the study mentioned, process yields are expected to be different.
Microcapsule Characterization
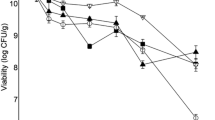
Table 4 shows the evolution of microcapsule characteristics during the storage period of 60 days at 25 °C. This temperature was chosen to evaluate the stability of dried powders without the need for refrigeration, which is a characteristic of spray-dried materials (Peighambardoust et al. 2011). Initial viability in storage was different in the two encapsulating agents; however, log reduction after 60 days was found to be similar (p ≤ 0.05) for CW (1.69 ± 0.09 log CFU/g of dry matter) and RW (1.17 ± 0.43 log CFU/g of dry matter). During this time, L. paracasei ATR6 encapsulated with CW lost significant (p ≤ 0.05) viability only after 45 days, while RW enabled the microorganism to remain stable throughout storage. Other authors (Eckert et al. 2017) who encapsulated L. plantarum ATCC8014 using CW as wall material have found 100% survival in microcapsules stored at 20 °C for 56 days. In the present study, however, we stored powders at 25 °C. Huang et al. (2017a), who used the same temperature, reported a gradual decrease in viability of Lactobacillus casei after 7 days. This indicates that the robustness of LAB under SD probably depends on which species or even which strain is undergoing the process. In fact, reports on the use of CW as encapsulating agent for SD of LAB are almost restricted to lactobacillus species. This genus has shown high tolerance to the temperatures used with this technology, being able to survive for long storage periods (Bustos and Bórquez 2013; Maciel et al. 2014). Lactobacilli encapsulated with CW have also shown high activity when inserted in milk or in milk-based products, as well as after going through simulated gastrointestinal tract (Aragón-Rojas et al. 2018; De Castro-Cislaghi et al. 2012; Eckert et al. 2017; Ilha et al. 2015).
The use of supplements for SD of LAB does not seem offer better protection during powder storage, at least regarding cell viability. A good example is shown by Bustos and Bórquez (2013), who encapsulated an endogenous L. plantarum with CW and tested several supplements, such as maltodextrin, skimmed milk, Arabic gum, and pectin. In this case, CW was the substance that provided the best results in strain stability, losing the lowest viability in 70 days of storage at 4 °C: nearly 1 log cycle. In comparison, Eckert et al. (2017) also encapsulated a strain of L. plantarum in unsupplemented cheese whey and, as mentioned above, observed no loss in viability after 56 days of storage at 20 °C, which is a condition that allows more instability due to the lack of refrigeration. This reinforces the argument that supplements are not necessarily correlated to better outcomes in storage, and they do not seem to have influence in growth and survival rates immediately after SD.
Furthermore, aw of encapsulated L. paracasei ATR6 at 0 days was approximately 0.2 for any of the materials used as protective agents. There was a significant (p ≤ 0.05) increase in aw with CW on the 30th day, and after that, values remained stable. We observed several variations throughout storage time in RW; however, aw of these microcapsules was similar at 0 and 60 days. In addition, both encapsulating agents caused values to remain below 0.3, which according to some authors (De Castro-Cislaghi et al. 2012; Tonon et al. 2009) is an adequate range for dried powders; bacteria are able to survive within this range, but the small amount of free water prevents the occurrence of several biochemical reactions that could damage capsule structure. For this reason, viability of L. paracasei ATR6 was satisfactorily maintained throughout the 60 days of storage.
Initially, the moisture content of CW microcapsules was significantly (p ≤ 0.05) lower than that of RW microcapsules. In fact, values of L. paracasei ATR6 encapsulated with CW remained stable throughout storage, while RW enabled a few variations, as moisture gradually decreased over time, until (after day 30) it became similar (p ≤ 0.05) to the content of CW microcapsules. Nonetheless, the value range obtained in our study is considered to be outside the adequate moisture content for this type of material, which is supposed to lie between 4% and 7% (Ananta et al. 2005). Some authors even state that a suitable moisture content for whey powders is supposed to be around 3% (Schuck 2011). Our high values may be related to the fact that, in the drying process of milk-based materials, high temperatures cause a phenomenon called caking, which happens due to water absorption by the hygroscopic amorphous lactose, which is converted into α-lactose monohydrate crystals (Fox et al. 2015). Caking also leads to binding of particles, which can be seen in Fig. 3.
Scanning electron micrographs (SEM) of CW (a) and RW (c) microcapsules with ×1000 magnification and 20 μm scale; and CW (b) and RW (d) microcapsules with ×500 magnification and also 20 μm scale. Fig. A1 is a single CW microcapsule with ×15,000 magnification and 2-μm scale, for proper comparison of surface and morphology
This same figure shows microcapsule size and morphology. Mean particle size was different between CW (5.31 ± 1.27 μm) and RW (15.21 ± 7.24 μm). CW microcapsules had sizes similar to those obtained by Eckert et al. (2017), while the size of RW microcapsules was similar to the ones found by Soukoulis et al. (2014). In SD, particle size is modulated by parameters such as spray nozzle type and atomizing pressure, as well as composition of the emulsions submitted to drying (Peighambardoust et al. 2011). In addition, Đorđević et al. (2014) report that SD is a technology that enables the formation of particle size in the range of millimeters to nanometers, which includes the sizes observed in our experiments. In Fig. 3, it is also possible to see that CW capsules have a smoother surface than RW capsules. Khem et al. (2016) reported that it is essential that the encapsulation agent contains a certain amount of proteins since they will accumulate on capsule surface and avoid adhesion to the spray-drier wall. This may explain why CW microcapsules have a more homogeneous morphology. In addition, defects on capsule surface lead to higher instability during storage and/or food processing (Gong et al. 2014).
Some of these instabilities are expressed in color changes. Color changes in spray-dried powders are caused by an array of biochemical reactions, such as lactose crystallization and migration of free fat to microcapsule surface (Chudy et al. 2015; Ho et al. 2019). In the present study, however, color parameters for the two encapsulating gents remained relatively stable throughout storage. L* values were high for CW and RW, indicating that both powders tend to have light (white) coloration; these values remained stable for the two encapsulating agents, except for RW microcapsules on the 60th day, when a significant (p ≤ 0.05) increase in L* color was observed. Negative a* values were obtained for all the samples, meaning they both tend to have a green coloration, and b* values were all positive, indicating a tendency toward yellow coloration. However, RW powder is shown to be greener, while CW shows a stronger yellow tone. This was expected since CW is reported to be a greenish-yellow by-product (Carvalho et al. 2013), which is confirmed by the color parameters observed in Section 3.1. After 60 days of storage, there was no statistical difference (p ≤ 0.05) between a* and b* values in either of the encapsulating agents, showing once again the versatility of dairy by-products as sources of biotechnological applications.
Acidification Potential of Encapsulated L. paracasei ATR6
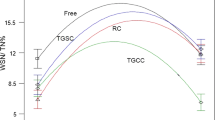
The biological activity of LAB is defined as the ability of this microorganism to acidify a certain medium (Peighambardoust et al. 2011). When selecting a bacterium to be used for cheese production, for example, this medium is milk, and the microorganism must achieve a pH of 5.3 after 6 h (Beresford et al. 2001). Acidification potential of free and encapsulated L. paracasei ATR6 at 0, 30, and 60 days of storage is shown in Fig. 4. At 0 days, encapsulated bacteria were able to vary pH by 1.46 ± 0.04 and 1.40 ± 0.19 units in CW and RW microcapsules, respectively. There is no statistical difference between these values. On day 30, encapsulated bacteria decreased pH by 1.75 ± 0.07 (CW) and 0.82 ± 0.28 (RW) units, and on day 60, these values dropped to 1.07 ± 0.33 and 0.59 ± 0.06 pH units in CW and RW, respectively. Considering the 30th and 60th days, ∆pH values are statistically different (p ≤ 0.05) between the two encapsulating agents, indicating that CW allowed for the best acidification activity of L. paracasei ATR6 during storage. Nonetheless, free cells were able to decrease pH by 1.72 ± 0.01 units after 14 h, which is higher (p ≤ 0.05) than the values obtained for bacteria encapsulated with either materials tested in this study.
Acidification kinetic parameters are shown in Table 5. At 0 days, RW samples showed a better relative profile in the acidification assay, even though, as mentioned above, the final pH variation was similar for both encapsulating agents. However, we observed a reduction in Vmax and increase in ∆pHmax with increased storage time, meaning that L. paracasei ATR6 gradually lost its ability to acidify milk, regardless of the material used for encapsulation. Considering tVmax values, the bacteria encapsulated with RW needed more time to reach its maximum acidification rate. CW also enabled higher pHmax and ∆pHmax values during storage. In spite of that, free bacteria also showed better acidification kinetic profile, which means that SD negatively affects the biological activity of this LAB.
On the other hand, Ferreira et al. (2017) showed that SD enabled a better performance in reducing pH of SM in L. plantarum undergoing freeze- and spray-drying using CW as encapsulating/cryoprotecting agent. Spray-dried L. plantarum was also more resistant to acidic environments and to the presence of bile salts compared to freeze-dried LAB (Ferreira et al. 2017). Hence, the very process of drying, which is not exclusive to a particular technology, is harmful to bacteria. Therefore, it is of the utmost importance to study the use of different materials and their protective effects for LAB undergoing drying protocols.
In this regard, we observed that L. paracasei ATR6 encapsulated with CW maintained its acidification properties at a higher level compared to RW. This may be explained by the protection that proteins offer for cell membranes in the SD process. In fact, lactose also helps to minimize damages by interacting with the polar section of phospholipids in bacterial cell membranes (Maciel et al. 2014). However, the temperature used in SD unfolds protein structures, and denatured proteins contain free carboxylic and amino groups available for reactions, which increases hydrophobic forces, as well as hydrogen and sulfide bonds, resulting in aggregation, coagulation, and even precipitation. This creates a microcapsule capable of protecting LAB from harsh conditions (Khem et al. 2016). CW contains higher amounts of proteins than RW (see Section 3.1). Without these proteins, unprotected cells will suffer injuries such as the loss of cell functions caused by changes in protein and enzyme structures, which result from the heat employed in SD (De Prisco et al. 2017; Gong et al. 2014). For that reason, CW-encapsulated L. paracasei ATR6 had a higher protection and, therefore, showed better activity when acidifying milk. Moreover, aside from using materials as protective agents, the study and optimization of SD parameters is necessary to overcome the negative effects of this drying technology on LAB.
In addition, neither free nor encapsulated L. paracasei were able to decrease pH to 5.3 in 6 h (Beresford et al. 2001). Nonetheless, this bacterium is not considered a starter strain. In cheese production, this species is more commonly used as non-starter, meaning it helps form aromatic compounds, and is not primarily involved in acidification (and consequently, in the coagulation of milk proteins) (Guarrasi et al. 2017; Tsafrakidou et al. 2016). It might also be used as probiotic since this species has already been described for this purpose (Caggia et al. 2015; Pino et al. 2017). Therefore, although SD negatively affects the ability of this bacterium to acidify milk, it is safe to state that its employment as a non-starter or probiotic culture may not represent a problem for the food industry. As a matter of fact, the encapsulation of probiotic LAB using SD technology and CW as encapsulating agent has been reported to increase the survival of these microorganisms under the harsh conditions of food processing and also through simulated gastrointestinal tract (Eckert et al. 2017; Huang et al. 2017a; De Castro-Cislaghi et al. 2012). However, further studies are required on SD of starter LAB employing different materials as encapsulating agents.
Conclusions
In the present study, we used two by-products from the dairy industry for the growth of an endogenous strain of L. paracasei. This bacterium had similar ability to multiply itself in both media, compared to incubation in skimmed milk. In bioreactor, CW and RW continued to allow for good cell growth, which translated in similar lactose consumption throughout cultivation, although L. paracasei ATR6 had lower ability to decrease pH in RW. Thus, CW and RW can be considered suitable culture media for the growth of this LAB. As to SD of this bacterial species, both by-products enabled similar protection and yields throughout the process. During storage, both materials enabled the bacteria to remain satisfactorily viable, while the microcapsules showed stability in terms of aw, moisture content, and color, with few variations, which reinforces the biotechnological potential of these materials. It is thus possible to conclude that CW is a suitable encapsulating agent for SD of LAB. On the other hand, RW allowed for L. paracasei ATR6 to suffer more cell injuries than CW, which resulted in a lower ability to acidify milk. In summary, we proposed a two-step process that involves the growth in unsupplemented dairy by-products and the direct SD of the cultivation medium. The proposed protocol proved to be satisfactory; by diminishing the number of steps, we simplified the process and enabled a possible cost reduction.
References
Ai, Z., Lv, X., Huang, S., Liu, G., Sun, X., Chen, H., et al. (2017). The effect of controlled and uncontrolled pH cultures on the growth of Lactobacillus delbrueckii subsp. bulgaricus. LWT - Food Science and Technology, 77, 269–275. https://doi.org/10.1016/j.lwt.2016.11.057.
Aller, K., Adamberg, K., Timarova, V., Seiman, A., Feštšenko, D., & Vilu, R. (2014). Nutritional requirements and media development for Lactococcus lactis IL1403. Applied Microbiology and Biotechnology, 98(13), 5871–5881. https://doi.org/10.1007/s00253-014-5641-7.
Ambros, S., Bauer, S. A. W., Shylkina, L., Foerst, P., & Kulozik, U. (2016). Microwave-vacuum drying of lactic acid bacteria: influence of process parameters on survival and acidification activity. Food and Bioprocess Technology, 9(11), 1901–1911. https://doi.org/10.1007/s11947-016-1768-0.
Ananta, E., Volkert, M., & Knorr, D. (2005). Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. International Dairy Journal, 15(4), 399–409. https://doi.org/10.1016/j.idairyj.2004.08.004.
Angmo, K., Kumari, A., Savitri, & Bhalla, T. C. (2016). Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT - Food Science and Technology, 66, 428–435. https://doi.org/10.1016/J.LWT.2015.10.057.
AOAC International. (2012). Official Methods of Analysis of AOAC International. https://www.worldcat.org/title/official-methods-of-analysis-of-aoac-international/oclc/817542290. .
Aragón-Rojas, S., Quintanilla-Carvajal, M. X., & Hernández-Sánchez, H. (2018). Multifunctional role of the whey culture medium in the spray drying microencapsulation of lactic acid bacteria. Food Technology and Biotechnology, 56(3), 381–397. https://doi.org/10.17113/ftb.56.03.18.5285.
Ardanareswari, K., Utami, T., & Rahayu, S. (2017). Effect of heat adaptation and pH adjustment on the survival of spray-dried Lactobacillus paracasei SNP2. British Food Journal, 119(10), 2267–2276. https://doi.org/10.1108/BFJ-10-2016-0519.
Bartkiene, E., Zavistanaviciute, P., Lele, V., Ruzauskas, M., Bartkevics, V., Bernatoniene, J., et al. (2018). Lactobacillus plantarum LUHS135 and paracasei LUHS244 as functional starter cultures for the food fermentation industry: characterisation, mycotoxin-reducing properties, optimisation of biomass growth and sustainable encapsulation by using dairy by-produc. LWT - Food Science and Technology, 93, 649–658. https://doi.org/10.1016/j.lwt.2018.04.017.
Beresford, T. P., Fitzsimons, N. A., Brennan, N. L., & Cogan, T. M. (2001). Recent advances in cheese microbiology. International Dairy Journal, 11(4–7), 259–274. https://doi.org/10.1016/S0958-6946(01)00056-5.
Bernárdez, P. F., Amado, I. R., Castro, L. P., & Guerra, N. P. (2008). Production of a potentially probiotic culture of Lactobacillus casei subsp. casei CECT 4043 in whey. International Dairy Journal, 18(10–11), 1057–1065. https://doi.org/10.1016/J.IDAIRYJ.2008.05.004.
Brignone, D., Radmann, P., Behr, J., & Vogel, R. F. (2017). Boosting the growth of the probiotic strain Lactobacillus paracasei ssp. paracasei F19. Archives of Microbiology, 199(6), 853–862. https://doi.org/10.1007/s00203-017-1352-7.
Burns, P., Vinderola, G., Molinari, F., & Reinheimer, J. (2008). Suitability of whey and buttermilk for the growth and frozen storage of probiotic lactobacilli. International Journal of Dairy Technology, 61(2), 156–164. https://doi.org/10.1111/j.1471-0307.2008.00393.x.
Burns, P., Cuffia, F., Milesi, M., Vinderola, G., Meinardi, C., Sabbag, N., & Hynes, E. (2012). Technological and probiotic role of adjunct cultures of non-starter lactobacilli in soft cheeses. Food Microbiology, 30(1), 45–50. https://doi.org/10.1016/J.FM.2011.09.015.
Buron-Moles, G., Chailyan, A., Dolejs, I., Forster, J., & Mikš, M. H. (2019). Uncovering carbohydrate metabolism through a genotype–phenotype association study of 56 lactic acid bacteria genomes. Applied Microbiology and Biotechnology, 103(7), 3135–3152. https://doi.org/10.1007/s00253-019-09701-6.
Bustos, P., & Bórquez, R. (2013). Influence of osmotic stress and encapsulating materials on the stability of autochthonous Lactobacillus plantarum after spray drying. Drying Technology, 31(1), 57–66. https://doi.org/10.1080/07373937.2012.717325.
Caggia, C., De Angelis, M., Pitino, I., Pino, A., & Randazzo, C. L. (2015). Probiotic features of Lactobacillus strains isolated from Ragusano and pecorino Siciliano cheeses. Food Microbiology, 50, 109–117. https://doi.org/10.1016/J.FM.2015.03.010.
Carvalho, F., Prazeres, A. R., & Rivas, J. (2013). Cheese whey wastewater: characterization and treatment. Science of the Total Environment, 445–446, 385–396. https://doi.org/10.1016/J.SCITOTENV.2012.12.038.
Chudy, S., Pikul, J., & Rudzińska, M. (2015). Effects of storage on lipid oxidation in milk and egg mixed powder. Journal of Food and Nutrition Research, 54(1), 31–40.
De Castro-Cislaghi, F. P., Silva, C. D. R. E., Fritzen-Freire, C. B., Lorenz, J. G., & Sant’Anna, E. S. (2012). Bifidobacterium Bb-12 microencapsulated by spray drying with whey: survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. Journal of Food Engineering, 113(2), 186–193. https://doi.org/10.1016/j.jfoodeng.2012.06.006.
De Prisco, A., van Valenberg, H. J. F., Fogliano, V., & Mauriello, G. (2017). Microencapsulated starter culture during yoghurt manufacturing, effect on technological features. Food and Bioprocess Technology, 10(10), 1767–1777. https://doi.org/10.1007/s11947-017-1946-8.
de Souza, C. F. V., Rodrigues, R. C., & Ayub, M. A. Z. (2009). Effects of oxygen volumetric mass transfer coefficient on transglutaminase production by Bacillus circulans BL32. Biotechnology and Bioprocess Engineering, 14(5), 571–576. https://doi.org/10.1007/s12257-008-0076-6.
Domingos-Lopes, M. F. P., Stanton, C., Ross, P. R., Dapkevicius, M. L. E., & Silva, C. C. G. (2017). Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiology, 63, 178–190. https://doi.org/10.1016/J.FM.2016.11.014.
Dong, Z., Gu, L., Zhang, J., Wang, M., Du, G., Chen, J., & Li, H. (2014). Optimisation for high cell density cultivation of Lactobacillus salivarius BBE 09-18 with response surface methodology. International Dairy Journal, 34(2), 230–236. https://doi.org/10.1016/j.idairyj.2013.07.015.
Donkor, O. N., Henriksson, A., Vasiljevic, T., & Shah, N. P. (2005). Probiotic strains as starter cultures improve angiotensin-converting enzyme inhibitory activity in soy yogurt. Journal of Food Science, 70(8), m375–m381. https://doi.org/10.1111/j.1365-2621.2005.tb11522.x.
Đorđević, V., Balanč, B., Belščak-Cvitanović, A., Lević, S., Trifković, K., Kalušević, A., et al. (2014). Trends in encapsulation technologies for delivery of food bioactive compounds. Food Engineering Reviews, 7(4), 452–490. https://doi.org/10.1007/s12393-014-9106-7.
Dragone, G., Mussatto, S. I., Almeida e Silva, J. B., & Teixeira, J. A. (2011). Optimal fermentation conditions for maximizing the ethanol production by Kluyveromyces fragilis from cheese whey powder. Biomass and Bioenergy, 35(5), 1977–1982. https://doi.org/10.1016/J.BIOMBIOE.2011.01.045.
Eckert, C., Serpa, V. G., Felipe dos Santos, A. C., Marinês da Costa, S., Dalpubel, V., Lehn, D. N., & Volken de Souza, C. F. (2017). Microencapsulation of Lactobacillus plantarum ATCC 8014 through spray drying and using dairy whey as wall materials. LWT - Food Science and Technology, 82, 176–183. https://doi.org/10.1016/j.lwt.2017.04.045.
Eckert, C., Agnol, W. D., Dallé, D., Serpa, V. G., Maciel, M. J., Lehn, D. N., & Volken de Souza, C. F. (2018). Development of alginate-pectin microparticles with dairy whey using vibration technology: effects of matrix composition on the protection of Lactobacillus spp. from adverse conditions. Food Research International, 113, 65–73. https://doi.org/10.1016/j.foodres.2018.07.001.
FAO/WHO. (2002). Guidelines for the evaluation of probiotics in food. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. doi:https://doi.org/10.1111/j.1469-0691.2012.03873.
Farimani, R. H., Najafi, M. B. H., Bazzaz, B. S. F., Edalatian, M. R., Bahrami, A. R., Flórez, A. B., & Mayo, B. (2016). Identification, typing and functional characterization of dominant lactic acid bacteria strains from Iranian traditional yoghurt. European Food Research and Technology, 242(4), 517–526. https://doi.org/10.1007/s00217-015-2562-3.
Ferrando, V., Quiberoni, A., Reinheimer, J., & Suárez, V. (2016). Functional properties of Lactobacillus plantarum strains: a study in vitro of heat stress influence. Food Microbiology, 54, 154–161. https://doi.org/10.1016/J.FM.2015.10.003.
Ferreira, A. A., Huang, S., Perrone, I. T., Schuck, P., Jan, G., & Carvalho, A. F. (2017). Tracking Amazonian cheese microbial diversity: development of an original, sustainable, and robust starter by freeze drying/spray drying. Journal of Dairy Science, 100(9), 6997–7006. https://doi.org/10.3168/jds.2016-12418.
Fox, P. F., Uniacke-Lowe, T., McSweeney, P. L. H., & O’Mahony, J. A. (2015). Heat-induced changes in milk. In Dairy chemistry and biochemistry (pp. 345–375). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-14892-2_9.
Gallego-Schmid, A., & Tarpani, R. R. Z. (2019). Life cycle assessment of wastewater treatment in developing countries: a review. Water Research, 153, 63–79. https://doi.org/10.1016/J.WATRES.2019.01.010.
Gong, P., Zhang, L., Han, X., Shigwedha, N., Song, W., Yi, H., et al. (2014). Injury mechanisms of lactic acid bacteria starter cultures during spray drying: a review. Drying Technology, 32(7), 793–800. https://doi.org/10.1080/07373937.2013.860458.
González, L., Cuadrillero, A. F., Castro, J. M., Bernardo, A., & Tornadijo, M. E. (2015). Selection of lactic acid bacteria isolated from San Simón da Costa cheese (PDO) in order to develop an autochthonous starter culture. Advances in Microbiology, 05(11), 748–759. https://doi.org/10.4236/aim.2015.511079.
Guarcello, R., Carpino, S., Gaglio, R., Pino, A., Rapisarda, T., Caggia, C., Marino, G., Randazzo, C. L., Settanni, L., & Todaro, M. (2016). A large factory-scale application of selected autochthonous lactic acid bacteria for PDO pecorino Siciliano cheese production. Food Microbiology, 59, 66–75. https://doi.org/10.1016/J.FM.2016.05.011.
Guarrasi, V., Sannino, C., Moschetti, M., Bonanno, A., Di Grigoli, A., & Settanni, L. (2017). The individual contribution of starter and non-starter lactic acid bacteria to the volatile organic compound composition of Caciocavallo Palermitano cheese. International Journal of Food Microbiology, 259, 35–42. https://doi.org/10.1016/j.ijfoodmicro.2017.07.022.
Guimarães, P. M. R., Teixeira, J. A., & Domingues, L. (2010). Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnology Advances, 28(3), 375–384. https://doi.org/10.1016/J.BIOTECHADV.2010.02.002.
Gupta, S., Abu-Ghannam, N., & Scannell, A. G. M. (2011). Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food and Bioproducts Processing, 89(4), 346–355. https://doi.org/10.1016/j.fbp.2010.10.001.
Ho, T. M., Chan, S., Yago, A. J. E., Shravya, R., Bhandari, B. R., & Bansal, N. (2019). Changes in physicochemical properties of spray-dried camel milk powder over accelerated storage. Food Chemistry, 295, 224–233. https://doi.org/10.1016/J.FOODCHEM.2019.05.122.
Huang, S., Rabah, H., Jardin, J., Briard-Bion, V., Parayre, S., Maillard, M. B., le Loir, Y., Chen, X. D., Schuck, P., Jeantet, R., & Jan, G. (2016). Hyperconcentrated sweet whey, a new culture medium that enhances Propionibacterium freudenreichii stress tolerance. Applied and Environmental Microbiology, 82(15), 4641–4651. https://doi.org/10.1128/AEM.00748-16.
Huang, S., Méjean, S., Rabah, H., Dolivet, A., Le Loir, Y., Chen, X. D., et al. (2017a). Double use of concentrated sweet whey for growth and spray drying of probiotics: towards maximal viability in pilot scale spray dryer. Journal of Food Engineering, 196, 11–17. https://doi.org/10.1016/j.jfoodeng.2016.10.017.
Huang, S., Vignolles, M.-L., Chen, X. D., Le Loir, Y., Jan, G., Schuck, P., & Jeantet, R. (2017b). Spray drying of probiotics and other food-grade bacteria: a review. Trends in Food Science & Technology, 63, 1–17. https://doi.org/10.1016/J.TIFS.2017.02.007.
Huang, S., Gaucher, F., Cauty, C., Jardin, J., Le Loir, Y., Jeantet, R., et al. (2018). Growth in hyper-concentrated sweet whey triggers multi stress tolerance and spray drying survival in Lactobacillus casei BL23: from the molecular basis to new perspectives for sustainable probiotic production. Frontiers in Microbiology, 9. doi:https://doi.org/10.3389/fmicb.2018.02548.
Ibrahim, S. B., Aini, N., Rahman, A., Mohamad, R., & Abdul, R. (2010). Effects of agitation speed, temperature, carbon and nitrogen sources on the growth of recombinant Lactococcus lactis NZ9000 carrying domain 1 of aerolysin gene. African Journal of Biotechnology, 9(33), 5392–5398.
Ilha, E. C., da Silva, T., Lorenz, J. G., de Oliveira Rocha, G., & Sant’Anna, E. S. (2015). Lactobacillus paracasei isolated from grape sourdough: acid, bile, salt, and heat tolerance after spray drying with skim milk and cheese whey. European Food Research and Technology, 240(5), 977–984. https://doi.org/10.1007/s00217-014-2402-x.
Jantzen, M., Göpel, A., & Beermann, C. (2013). Direct spray drying and microencapsulation of probiotic Lactobacillus reuteri from slurry fermentation with whey. Journal of Applied Microbiology, 115(4), 1029–1036. https://doi.org/10.1111/jam.12293.
Jensen, H., Grimmer, S., Naterstad, K., & Axelsson, L. (2012). In vitro testing of commercial and potential probiotic lactic acid bacteria. International Journal of Food Microbiology, 153(1–2), 216–222. https://doi.org/10.1016/j.ijfoodmicro.2011.11.020.
Khem, S., Small, D. M., & May, B. K. (2016). The behaviour of whey protein isolate in protecting Lactobacillus plantarum. Food Chemistry, 190, 717–723. https://doi.org/10.1016/j.foodchem.2015.06.020.
Lavari, L., Páez, R., Cuatrin, A., Reinheimer, J., & Vinderola, G. (2014). Use of cheese whey for biomass production and spray drying of probiotic lactobacilli. Journal of Dairy Research, 81(03), 267–274. https://doi.org/10.1017/S0022029914000156.
Li, L., & Han, N. S. (2018). Application of lactic acid bacteria for food biotechnology. In Emerging areas in bioengineering (pp. 375–398). Weinheim: Wiley-VCH Verlag GmbH & Co.. https://doi.org/10.1002/9783527803293.ch22.
Maciel, G. M., Chaves, K. S., Grosso, C. R. F., & Gigante, M. L. (2014). Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. Journal of Dairy Science, 97(4), 1991–1998. https://doi.org/10.3168/jds.2013-7463.
Maragkoudakis, P. A., Miaris, C., Rojez, P., Manalis, N., Magkanari, F., Kalantzopoulos, G., & Tsakalidou, E. (2006). Production of traditional Greek yoghurt using Lactobacillus strains with probiotic potential as starter adjuncts. International Dairy Journal, 16(1), 52–60. https://doi.org/10.1016/J.IDAIRYJ.2004.12.013.
Maragkoudakis, P., Nardi, T., Bovo, B., Corich, V., & Giacomini, A. (2010). Valorisation of a milk industry by-product as substrate for microbial growth. Journal of Biotechnology, 150, 340–340. https://doi.org/10.1016/j.jbiotec.2010.09.361.
Marco, M. L., Heeney, D., Binda, S., Cifelli, C. J., Cotter, P. D., Foligné, B., et al. (2017). Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology, 44, 94–102. https://doi.org/10.1016/j.copbio.2016.11.010.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. https://doi.org/10.1021/ac60147a030.
Motta, A. D. S., & Gomes, M. D. S. M. (2015). Technological and functional properties of lactic acid bacteria: the importance of these microorganisms for food. Revista do Instituto de Laticínios Cândido Tostes, 70(3), 172. doi:https://doi.org/10.14295/2238-6416.v70i3.403
Oliszewski, R., Wolf, I. V., Bergamini, C. V, Candioti, M., & Perotti, M. C. (2013). Influence of autochthonous adjunct cultures on ripening parameters of Argentinean goat’s milk cheeses. Journal of the Science of Food and Agriculture, 93(11), 2730–2742. doi:https://doi.org/10.1002/jsfa.6092.
Pega, J., Denoya, G. I., Castells, M. L., Sarquis, S., Aranibar, G. F., Vaudagna, S. R., & Nanni, M. (2018). Effect of high-pressure processing on quality and microbiological properties of a fermented beverage manufactured from sweet whey throughout refrigerated storage. Food and Bioprocess Technology, 11(6), 1101–1110. https://doi.org/10.1007/s11947-018-2078-5.
Peighambardoust, S. H., Golshan Tafti, A., & Hesari, J. (2011). Application of spray drying for preservation of lactic acid starter cultures: a review. Trends in Food Science and Technology, 22(5), 215–224. https://doi.org/10.1016/j.tifs.2011.01.009.
Pérez-Chabela, M. L., Lara-Labastida, R., Rodriguez-Huezo, E., & Totosaus, A. (2013). Effect of spray drying encapsulation of thermotolerant lactic acid bacteria on meat batters properties. Food and Bioprocess Technology, 6(6), 1505–1515. https://doi.org/10.1007/s11947-012-0865-y.
Pescuma, M., Hébert, E. M., de Valdez, G. F., Mozzi, F., & Bru, E. (2012). Diversity in growth and protein degradation by dairy relevant lactic acid bacteria species in reconstituted whey. Journal of Dairy Research, 79(02), 201–208. https://doi.org/10.1017/s0022029912000040.
Pessione, E. (2012). Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Frontiers in Cellular and Infection Microbiology, 2, 86p. https://doi.org/10.3389/fcimb.2012.00086.
Picon, A., Garde, S., & Ávila, M. (2016). Microbiota dynamics and lactic acid bacteria biodiversity in raw goat milk cheeses. International Dairy Journal, 58, 14–22. https://doi.org/10.1016/J.IDAIRYJ.2015.09.010.
Picot, A., & Lacroix, C. (2004). Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. International Dairy Journal, 14(6), 505–515. https://doi.org/10.1016/J.IDAIRYJ.2003.10.008.
Pino, A., Van Hoorde, K., Pitino, I., Russo, N., Carpino, S., Caggia, C., & Randazzo, C. L. (2017). Survival of potential probiotic lactobacilli used as adjunct cultures on pecorino Siciliano cheese ripening and passage through the gastrointestinal tract of healthy volunteers. International Journal of Food Microbiology, 252, 42–52. https://doi.org/10.1016/j.ijfoodmicro.2017.04.012.
Pinto, S. S., Verruck, S., Vieira, C. R. W. W., Prudêncio, E. S., Amante, E. R., & Amboni, R. (2015). Influence of microencapsulation with sweet whey and prebiotics on the survival of Bifidobacterium BB-12 under simulated gastrointestinal conditions and heat treatments. LWT - Food Science and Technology, 64(2), 1004–1009. https://www.sciencedirect.com/science/article/pii/S0023643815300372?via%3Dihub. Accessed 4 September 2018.
Popescu, A. A. D., Savu, C., Dobrea, M., Gájáilä, I., Cotor, G., Geogescu, M., et al. (2014). The influence of PH on the growth of some Lactobacillus strains with different origins. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Veterinary Medicine, 71(1), 270–271. https://doi.org/10.15835/BUASVMCN-VM:71:1:10149.
Rama, G. R., Kuhn, D., Beux, S., Maciel, M. J., & Volken de Souza, C. F. (2019). Potential applications of dairy whey for the production of lactic acid bacteria cultures. International Dairy Journal. https://doi.org/10.1016/j.idairyj.2019.06.012.
Rushdy, A. A., & Gomaa, E. Z. (2013). Antimicrobial compounds produced by probiotic Lactobacillus brevis isolated from dairy products. Annals of Microbiology, 63(1), 81–90. https://doi.org/10.1007/s13213-012-0447-2.
Sansonetti, S., Curcio, S., Calabrò, V., & Iorio, G. (2009). Bio-ethanol production by fermentation of ricotta cheese whey as an effective alternative non-vegetable source. Biomass and Bioenergy, 33(12), 1687–1692. https://doi.org/10.1016/j.biombioe.2009.09.002.
Schleifer, K. H., & Ludwig, W. (1995). Phylogeny of the genus Lactobacillus and related genera. Systematic and Applied Microbiology, 18(4), 461–467. https://doi.org/10.1016/S0723-2020(11)80404-2.
Schuck, P. (2011). Dehydrated dairy products—milk powder: types and manufacture. Encyclopedia of Dairy Sciences, 108–116. doi:https://doi.org/10.1016/B978-0-12-374407-4.00121-7.
Secchi, N., Giunta, D., Pretti, L., García, M. R., Roggio, T., Mannazzu, I., & Catzeddu, P. (2012). Bioconversion of ovine scotta into lactic acid with pure and mixed cultures of lactic acid bacteria. Journal of Industrial Microbiology & Biotechnology, 39(1), 175–181. https://doi.org/10.1007/s10295-011-1013-9.
Silva, J., Carvalho, A. S., Ferreira, R., Vitorino, R., Amado, F., Domingues, P., Teixeira, P., & Gibbs, P. A. (2005). Effect of the pH of growth on the survival of Lactobacillus delbrueckii subsp. bulgaricus to stress conditions during spray-drying. Journal of Applied Microbiology, 98(3), 775–782. https://doi.org/10.1111/j.1365-2672.2004.02516.x.
Soriano-Perez, S., Flores-Velez, L., Alonso-Davila, P., Cervantes-Cruz, G., & Arriaga, S. (2012). Production of lactic acid from cheese whey by batch cultures of Lactobacillus helveticus. Annals of Microbiology, 62(1), 313–317. https://doi.org/10.1007/s13213-011-0264-z.
Soukoulis, C., Behboudi-Jobbehdar, S., Yonekura, L., Parmenter, C., & Fisk, I. (2014). Impact of milk protein type on the viability and storage stability of microencapsulated Lactobacillus acidophilus NCIMB 701748 using spray drying. Food and Bioprocess Technology, 7(5), 1255–1268. https://doi.org/10.1007/s11947-013-1120-x.
Souza, J. V., & Dias, F. S. (2017). Protective, technological, and functional properties of select autochthonous lactic acid bacteria from goat dairy products. Current Opinion in Food Science, 13, 1–9. https://doi.org/10.1016/j.cofs.2017.01.003.
Speranza, B., Bevilacqua, A., Corbo, M. R., Altieri, C., & Sinigaglia, M. (2015). Selection of autochthonous strains as promising starter cultures for Fior di Latte, a traditional cheese of southern Italy. Journal of the Science of Food and Agriculture, 95(1), 88–97. https://doi.org/10.1002/jsfa.6686.
Terpou, A., Bosnea, L., Kanellaki, M., Plessas, S., Bekatorou, A., Bezirtzoglou, E., & Koutinas, A. A. (2018). Growth capacity of a novel potential probiotic Lactobacillus paracasei K5 strain incorporated in industrial white brined cheese as an adjunct culture. Journal of Food Science, 83(3), 723–731. https://doi.org/10.1111/1750-3841.14079.
Terrade, N., & Orduña, R. (2009). Determination of the essential nutrient requirements of wine-related bacteria from the genera Oenococcus and Lactobacillus. International Journal of Food Microbiology, 133(1–2), 8–13. https://doi.org/10.1016/j.ijfoodmicro.2009.03.020.
Tonon, R. V., Brabet, C., Pallet, D., Brat, P., & Hubinger, M. D. (2009). Physicochemical and morphological characterisation of açai (Euterpe oleraceae Mart.) powder produced with different carrier agents. International Journal of Food Science and Technology, 44(10), 1950–1958. https://doi.org/10.1111/j.1365-2621.2009.02012.x.
Tsafrakidou, P., Bozoudi, D., Pavlidou, S., Kotzamanidis, C., Hatzikamari, M., Zdragas, A., & Litopoulou-Tzanetaki, E. (2016). Technological, phenotypic and genotypic characterization of lactobacilli from Graviera Kritis PDO Greek cheese, manufactured at two traditional dairies. LWT - Food Science and Technology, 68, 681–689. https://doi.org/10.1016/J.LWT.2016.01.002.
USDA. (2018). Nutritional Nutrient Database for Standard Reference. https://ndb.nal.usda.gov/ndb/foods/show/01175?fgcd=&manu=&format=&count=&max=25&offset=&sort=default&order=asc&qlookup=milk+fluid+1%25+fat&ds=&qt=&qp=&qa=&qn=&q=&ing=. .
Acknowledgments
We are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing scholarship (grant no. 311655/2017-3). We also thank Milk, Education, and Quality (MEQ), Tecnovates, and Universidade do Vale do Taquari—Univates for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rabaioli Rama, G., Kuhn, D., Beux, S. et al. Cheese Whey and Ricotta Whey for the Growth and Encapsulation of Endogenous Lactic Acid Bacteria. Food Bioprocess Technol 13, 308–322 (2020). https://doi.org/10.1007/s11947-019-02395-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02395-8