Abstract
Modified atmosphere packaging (MAP) systems have been shown as beneficial to prolong postharvest life of pomegranate arils. Current application of such system is limited due to a lack of appropriate packaging films which are able to control in-package moisture condensation. Therefore, the aim of this study was to design a MAP system to balance the optimum gas composition with packaging film permeability as well as optimum in-package relative humidity (RH). The following packaging materials design were used: (i) 100% cellulose-based film NatureFlex (NF), (ii) bi-axial-oriented polypropylene (BOPP)-based film PropaFilm (PF), (iii) NF-PF (66:33%) film, and (iv) PF-NF (33:66%) film. The effects of package design on quality attributes of pomegranate arils stored at 10 °C (91 ± 2% RH) for 9 days were investigated. Package design had significant (p ≤ 0.05) impact in changing the in-package RH and gas composition. The 100% cellulose-based (NF) package created the lowest RH (60–66%) with highest reduction in O2 concentration, highest total soluble solids (TSS), hardness, colour change, and high bacterial count. However, the 100% BOPP-based (PF) film resulted in highest in-package water vapour condensation and mould growth at the end of storage. The optimized packages using PF-NF and NF-PF films, respectively, maintained quality of pomegranate arils and minimized water vapour condensation. The results indicated that PF packaging film fitted with NF film window was the best to maintain the quality of pomegranate arils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modified atmosphere packaging (MAP) during postharvest preservation of horticultural commodities has been recognized as one of the important technologies to maintain quality, extend shelf life, and reduce losses of fresh fruit and vegetables (Caleb et al. 2012; Charles et al. 2006; Gomes et al. 2010; Mangaraj et al. 2009). Low O2 and enriched CO2 concentrations have been reported as beneficial to reduce respiration rate (RR) and prevent quality changes of pomegranate arils during postharvest handling (Artés 1993; Banda et al. 2015; Belay et al. 2017). Pomegranate arils provide a convenient means to foster the fruit consumption because they are ready to eat and have reputed health-promoting effect related to the anti-mutagenicity, anti-inflammatory, anti-cancer, and anti-oxidative potentials (Caleb et al. 2012; Opara et al. 2009). However, pomegranate arils are susceptible to quality loss after extracting from the fruit. The perishable nature of the arils requires the development of appropriate methods such as MAP for postharvest preservation of quality. Consequently, a modified atmosphere of 3 to 5 kPa O2 and 10 to 15 kPa CO2 was considered optimum for ‘Mollar of Elche’ pomegranate aril packaging (Gil et al. 1996a, b; López-Rubira et al. 2005). However, the successful application of MAP not only requires selection of optimum gas but also correct gas permeability and water vapour transmission rate (WVTR) of packaging film to minimize moisture loss and prevent condensation (Somboonkaew and Terry 2010).
Condensation inside the package of fresh fruit and vegetables represents a treat of product quality and safety (Bovi et al. 2016; Hussein et al. 2015). Excess of water vapour condensation on the surface will cause an unpleasant package appearance, increase water activity, and consequently create ideal conditions for microbial growth (Song et al. 2002). Therefore, for fresh fruit and vegetables, these mass exchanges must be restricted in order to guarantee the quality and safety. Suitable water vapour and gas permeations are needed to maintain fresh produce physiological activities (Del-Valle et al. 2004). Controlling in-package humidity via modified atmosphere and humidity (MAHP) has been identified as an important parameter in order to avoid condensation and accelerated degradation of packed fresh produce (Caleb et al. 2016).
Several types of polymeric films have been applied package pomegranate arils such as oriented polypropylene (OPP) and bi-axial-oriented polypropylene (BOPP) film for cv. ‘Mollar de Elche’ reported by Gil et al. (1996a, b) and López-Rubira et al. (2005), respectively. However, the study provided limited information on the effects of packaging film and packaging film selection. Caleb et al. (2013a) reported effects of polylid polymeric packaging film on ‘Acco’ and ‘Herskawitz’ pomegranate arils. The authors suggested the need for a polymeric film with high CO2 permeability but provided no information on in-package RH. In another study, Banda et al. (2015) investigated the effect of low- and high-barrier bi-axial-oriented polyester (BOP) polymeric film for Wonderful pomegranate arils. This study reported the necessity of further optimization of packaging material and gas composition. Therefore, the objective of this study was focused on designing a modified atmosphere and humidity packaging for minimally processed pomegranate arils cv. ‘Wonderful’, based on integrated designing approach. The study considered the product and packaging film characteristics to design the optimized package and investigated the effects of such packaging design on the quality attributes.
Materials and Methods
Plant Material and Sample Preparation
Pomegranate fruit cv. ‘Wonderful’ was obtained at commercial maturity stage based on characteristic deep red skin and arils with mature kernel, total soluble solids (TSS, %), and titratable acidity (TA, mg L−1) (Mphahlele et al. 2016). At harvest, the TSS and TA contents were 17.5% and 1100 mg L−1, respectively. The fruits were obtained from Sonlia Pack House, Wellington, Western Cape (33° 38′ 23″ S, 19° 00′ 40″ E), South Africa. Fruits were air freighted in well-ventilated boxes and transported to Department of Horticultural Engineering, Leibniz Institute for Agricultural Engineering and Bioeconomy (ATB), Germany. On arrival, the fruits were stored in a cold storage chamber (5 ± 0.5 °C) for 24 h until processing to extract the arils. Damaged fruits were removed and the outer skins of selected healthy fruit were surface disinfected using 70% ethanol (Belay et al. 2017). Arils were extracted manually by carefully removing the husk under cool temperature. Extracted arils were collected in a tray and mixed to assure uniformity prior to packaging.
Packaging Design and Active Modified Atmosphere Storage
In order to identify suitable MA for pomegranate arils, a packaging material designing approach was adopted from Caleb et al. (2016). Pomegranate arils (150 g) were placed into polypropylene plastic tray (102 × 85 × 55 mm3) and flow wrapped (360 cm2). Selected polymeric films were BOPP film PropaFilm™ (Innovia films, UK) and cellulose-based NatureFlex™ NVS23 (Innovia films, UK), with distinct permeability to gases and water vapour (Table 1). The films were fitted with a fixed ratio window designed based on desirable in-package gas composition and RH (Table 2). Films were attached to the window using double-sided hermetic sealing tapes to ensure complete airtightness edges of the attached films. Prior to sealing, each package was gas flushed with 4.67 kPa O2 + 12.67 kPa CO2 + 82.67 kPa N2. Packages were heat sealed manually using Kopp HZ sealer (Verpackungsysteme, Reichenbach, Germany) and stored at 10 °C (which reflects average retail market display condition) for 9 days. Three packages were taken per treatment on each sampling days (0, 3, 6, and 9) for analysis.
Prior to opening the packages on each sampling day, O2 and CO2 concentrations inside the package were measured using gas analyser (Chechmate 3, PBI Dansensor, Ringstead, Denmark). An additional two packages per treatment were set up with humidity sensors (FHA 646R, Ahlborn, Holzkirchen, Germany) for continuous monitoring of RH inside the packages during the storage period. The performance of each packaging system was measured for the amount of water vapour transmitted through the package and amount of condensation inside the package (Caleb et al. 2016). Water vapour transmission was gravimetrically determined by measuring change in total mass of the packaged product at the end of storage period. The amount of condensation was measured after pomegranate arils were carefully removed from the package.
Physiological Response and Packaging Design
In-packaging Atmosphere and Respiration Rate
The approach for this study was based on the mass balance, accounting for the physiological activity of pomegranate arils, where the transient behaviour of \( {Y}_{O_2} \) and \( {Y}_{CO_2} \) can be obtained by using mass balance Eqs. (1) and (2), respectively:
where\( \frac{\partial {Y}_{O_2}}{\partial t} \) and \( \frac{\partial {Y}_{CO_2}}{\partial t} \) are change in concentration of O2 and CO2 through time, \( {P}_{O_2} \)is permeability to O2, P atm is atmospheric pressure, A p is surface area of the package, L is thickness of the packaging film, W is weight of the arils and V is free volume, \( {Y}_{O_{2i}} \)and \( {Y}_{CO_{2i}} \)are initial O2 and CO2 concentration respectively, and \( {Y}_{O_{2f}} \) and \( {Y}_{CO_{2f}} \)are final O2 and CO2 concentrations respectively.
Aril Transpiration Rate and Film Permeation Rate
The transpiration rate (TR) model developed for arils by Caleb et al. (2013b) was used for estimating the target water vapour transmission rate (WVTR) required to maintain optimal RH inside the package Eq. (3):
where TR is transpiration rate (mg kg−1 s−1); a w is the water activity of package headspace (RH/100); a wi is the water activity of the arils (0.984); K m is the mass transfer coefficient (89.96); and a is constant coefficient (0.09).The in-package RH is influenced by TR of produce as well as by the water vapour transmission of the packaging film as shown in Eq. (4). This is a mass balance equation that describes the rate of change of RH or moisture accumulation in the headspace.
where \( \dot{m_1} \) is the rate of water transpiration from produce to headspace (g kg−1 s−1); \( \dot{m_2} \) is the rate of water permeation from headspace to surrounding (g m−2 day−1); Wa is weight of dry air inside the package (g). At equilibrium when the rate of change in humidity is zero, Eq. (4) becomes \( \dot{m_1}=\dot{m_2}. \) Substituting the product TR and packaging material transmission rate, Eq. (3) becomes:
where W is weight of pomegranate arils (kg) and A is packaging film area (m2). Re-arranging Eq. (5) for determining packaging needs for pomegranate aril target WVTR was obtained as Eq. (6). This included an estimation of target WVTR required to achieve 65 to 95% RH inside a package containing 150 g of pomegranate arils (Caleb et al. 2013b):
Physicochemical and Microbial Quality Analysis
Mass Loss
Mass of pomegranate aril pack in different packaging designs taken on sampling day was measured using an electronic balance (CPA10035, Sartorius, Göttingen, Germany) with an accuracy of ± 0.01 g. Mass loss was expressed in percentage (Eq. (7)):
where WL is the mass loss (%), W o is the initial mass (g), and W f is the final mass (g) at a given time.
Aril Colour
The surface colour of pomegranate arils was measured and evaluated on the basis of Commission International del’ Eclairage (CIE) colour system using a digital Chroma-meter (CM-2600d, Konica Minolta Sensing Inc., Japan). Prior to each measurement, calibration of the colour meter was performed against a white and black tile background, respectively (Pathare et al. 2013). Colour measurements lightness (L*), redness/greenness (a*), and yellowness/blueness (b*) were taken using individual arils, mean of 20 measurements for each treatment, and the data was further analysed to describe Chroma (C*) and hue angle (h°) according to Caleb et al. (2016).
Aril Hardness Analysis
Softness of individual arils was measured using texture analyser (TA-XT Plus, Stable Micro Systems, Surrey, UK) with a 2-mm-diameter cylindrical probe SMSP/2. Magness-Taylor test (MTT), which is an empirical hardness indicator test, was performed according to Szychowski et al. (2015). This test included both compression and cutting effort (Chen and Opara 2013). Penetration rate was 0.3 mm s−1 for 5 s after contacting the surface of pomegranate arils and results were expressed in Newton (N). A total of 20 arils were measured per treatment.
Total Monomeric Anthocyanin
Total monomeric anthocyanin concentration (TAC) was quantified using the pH differential method as described by Fawole and Opara (2013). Pomegranate juice extracts (1 mL) were diluted with 9 mL potassium chloride buffer for pH 1.0 (0.025 M) and sodium acetate buffer for pH 4.5 (0.4 M), separately. Absorbance was measured at 510 and 700 nm in pH 1.0 and 4.5 buffers after 10-min incubation time and results were expressed as cyanidin-3-glucoside equivalents according to the following equation:
where A is (A520–A700); pH 1–pH 4.5 is (A520–A700); MW is molecular weight of cyanidin-3-glucoside (449.2 g mol−1; DF is dilution factor; ɛ is molar extinction coefficient (26,900); and L is path length in centimetres. All analyses were carried out in triplicate and results were expressed as mass cyanidin-3-glucoside equivalent.
Titratable Acidity
Fresh juice of pomegranate arils was used to measure titratable acidity (TA) according to the method described by Fawole and Opara (2013). The TA content of the juice sample was measured potentiometrically by titration with 0.1 mol L−1 NaOH, to an end-point of pH 8.2 using an automated titrator (T50 M) equipped with a 20-sample changer (Mettler Toledo, Switzerland). The TA value was expressed as milligrams per litre of malic acid, citric acid, and acetic acid based on fresh mass.
Individual Sugar Content and Total Soluble Solids
Individual sugar contents (glucose, fructose, and sucrose) were determined according to the HPLC method described (Magwaza and Opara 2015; Mphahlele et al. 2014). Individual sugar content was determined by method using a DIONEX Ultimate 3000 liquid chromatograph fitted with Analytical Auto-sampler WPS-3000TSL (Thermo Fisher Scientific GmbH, Dreieich, Germany). The system is equipped with a refractive index detector SHODEX RI-101 (Showa Denko Europe GmbH, Munich, Germany). Sugars were separated on a Eurokat H column (300 × 8 mm and 10 μm diameter) (KNAUER Wissenschaftliche Geräte GmbH, Berlin, Germany), with 0.01 M sulphuric acid as the mobile phase. The injection volume was 10 μL and the flow rate was maintained at 0.8 mL min−1 at 35 °C. Sample detection and identification were performed by comparing retention time obtained with the retention time of the calibration standards and results were quantitatively expressed in grams per litre of juice. Total soluble solid (TSS) of pomegranate juice was measured using a hand refractometer (DR301-95, Krüss Optronic, Hamburg, Germany) and expressed as percent.
Volatile Organic Compounds
Volatile compounds were extracted by static headspace sampling (SHS) according to the method described by Caleb et al. (2016). Pomegranate arils from each treatment were homogenized into puree and 7 g was placed in a 20-mL glass vial spiked with 100 μL of 3-octanol (diluted in absolute methanol to a concentration of 0.1 g L−1) as internal standard. The vials were tightly capped and allowed to equilibrate at 80 °C for 10 min in the headspace auto-sampler incubator (HS-20 automated-sampler, Shimadzu Europa GmbH, Duisburg, Germany). Analyses were carried out using helium as carrier gas with a constant flow rate of 0.03 mL s−1. The GC temperature was held at 50 °C, then ramped up to 110 °C at 5 °C min−1 and further ramped up to 180 at 20 °C min−1 held at this temperature for 2.5 min and finally ramped up to 220 at 10 °C min−1 held at this temperature for 3 min in total run time of 24 min. The mass selective detector in this study was operated in full-scan mode and mass spectra in the 35–500 m/z range were recorded. Individual volatile compounds were tentatively identified by their retention time and semi-quantification of the identified compounds was estimated using the following equation:
where RA is the relative abundances of the identified compound (g L−1), A ic is the peak area of the identified compound, A itc is the peak area of the internal standard, and C itc is the final concentration of internal standard in the sample (0.1 g L−1).
Microbial Analysis
Microbial quality of pomegranate arils was studied using total plate count method. Total aerobic mesophilic bacterial count was determined using plate count agar (PCA), while yeast and mould counts were determined using rosebengal chloramphenicol agar (RBCA). Pomegranate arils (10 g) were taken for each treatment and transferred into 90 mL peptone buffered water. Samples were homogenized for 2 min at a speed of 4 strokes s−1 in a lab blender (BagMixer1400CC1, Interscience, France) and thereafter threefold, serial dilution was made by adding 30 mL of each diluent into 270 mL of PS Rotilabo1-microtest plates (96er U-profile, Carl Roth GmbH & Co KG, Germany) and 100 mL from each dilution was pour-plated on respective growth media. PCA plates for aerobic mesophilic bacterial were incubated at 30 °C for 3 days, and RBCA plates for yeast and mould, respectively, were incubated at 25 °C for 5 days. After incubation, colonies between 30 and 300 colonies on each plate were counted (n = 6), and the results were expressed as log colony-forming unit per weight (log CFU mL−1).
Statistical Analysis
All the data obtained were subjected to analysis of variance (two-way ANOVA), and Duncan multiple range test was used to determine the difference between mean values at 95% confident interval. Results were presented as mean ± standard deviation.
Results and Discussions
In-package Atmosphere and Respiration Rate
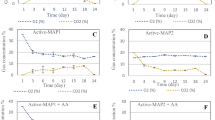
Packaging design and storage duration significantly (p ≤ 0.05) influenced the evolution of in-package headspace gas composition (Fig. 1). The highest reduction of O2 concentration (2.8%) at the end of the storage and highest CO2 accumulation from the initial concentration was observed for arils packed in NF film. Minimum changes in O2 and CO2 concentration were observed for arils packed under PF film. The decrease in O2 and CO2 concentration could be attributed to the respiration process in MAP during the storage period. However, due to efficient gas exchange maintained by using films fitted with fixed window (NF-PF and PF-NF), there was an equivalent balance between O2 consumption and CO2 production. The NF film has a higher oxygen transmission rate (OTR) and low permeability to CO2 properties (Somboonkaew and Terry 2010), which resulted in a higher reduction of O2 concentration and highest accumulation of CO2 inside the package throughout the storage period. Packaging materials have been shown in various studies on pomegranate arils to influence in-package headspace gas composition (Adiletta et al. 2017; Ayhan and Esturk 2009). Banda et al. (2015) reported for active MAP systems packed pomegranate arils the accumulation of 16 to 18% O2 inside the packages, which was above the recommended concentration (2 to 5%). This was attributed to the use of low-barrier BOP film for active MAP. Similarly, a high CO2 barrier film (polylid) resulted in continuous accumulation of CO2 (27 to 43%). However, the current study showed that packaging design via incorporation of fixed window films could maintain continuously optimal atmosphere for pomegranate arils.
Transpiration and Water Vapour Transmission Rates
Highest TR (0.15 ± 0.01 g kg−1 h−1) of pomegranate arils was observed in samples packed inside NF films, while lowest TR (0.03 ± 0.01 g kg−1 h−1) was found under PF films (Fig. 2). This implies that the RH level inside the NF film surrounding the arils was lower. Weight loss was also higher due to the higher vapour pressure difference between the fruit and the external atmosphere, which resulted in higher driving force for water evaporation. In contrast, under PF films, the lower produce TR could be attributed to the RH saturation inside the package (Fig. 3). Furthermore, TRs of 0.12 ± 0.00 and 0.07 ± 0.01 g kg−1 h−1 were recorded for arils packed in NF-PF and PF-NF films, respectively. These were relatively similar to the predicted TR of ≈ 0.06 g kg−1 h−1 at 10 °C under 96% RH.
Effect of packaging design on the water vapour transmission rate (WVTR) of packaging film (stripe bars) and transpiration rate of pomegranate arils (dotted bars) stored at 10 °C for 9 days. Mean value (n = 3) with standard deviation ≤ 0.01. Packaging design descriptions (NF, NF-PF, PF-NF, and PF) are listed in Table 2
Effect of packaging design on in-pack relative humidity (RH) during storage of pomegranate arils at 10 °C for 9 days. Mean RH (n = 2) with less than 0.01 standard deviation. Packaging design descriptions (NF, NF-PF, PF-NF, and PF) are listed in Table 2
Based on the application of Eq. (6), the packaging film with targeted WVTR of 0.009 g h−1 is required to match the TR of pomegranate arils. However, the calculated WVTR obtained was 0.14, 0.096, 0.05, and 0.01 g h−1 for NF, NF-PF, PF-NF, and PF films, respectively. The results obtained showed that NF film water vapour permeability was too high, which would lead to product shrivelling, colour change, and mass loss. On the other hand, the estimated lower WVTR at PF film have resulted in water vapour condensation inside the package. These results were in agreement with the study reported by Caleb et al. (2016). Therefore, findings from this study highlight the significance of selecting appropriate packaging films for minimally processed pomegranate arils.
Package Performance
Package designs had significant influence (p ≤ 0.05) on the in-package RH, moisture condensation on the film, and water absorption by the film. In-package RH widely varies across the MAP systems and ranged from 65 to 100% (Fig. 3). Samples packed in PF film RH reached saturation (100%) within 24 h of storage, while the lowest average RH (66%) was observed in NF films. Packages fitted with fixed window films (PF-NF and NF-PF) were effective in regulating the in-package RH. These findings were similar to observations reported by Caleb et al. (2016), for minimally processed broccoli under MAHP systems. However, the PF-NF packages better maintained in-package RH at 91% compared to the NF-PF packages. The capability of PF-NF film to maintain the recommended RH could be associated with the fact that the low WVTR property of PF film was optimized by incorporating NF window. This helped to increase the WVTR of the package. According to Kader and Rolle (2004), RH range of 90 to 95% was recommended for storage of fresh fruit and vegetables. Thus, this makes the PF-NF package a suitable design for pomegranate arils.
Previous reports have shown that product geometry and structure, storage temperature, and RH, as well as attributes of packaging material, can affect the intensity of the moisture condensation inside a package (Bovi et al. 2016). In this study, package designs had a considerable impact on the water vapour condensation inside the packages as shown in Fig. 4. Highest water vapour condensation and lowest water absorption were observed in PF packages. Under this condition, arils were excessively soft due to water vapour condensation both on the aril surface and in the packaging film. Kumar et al. (2012) also reported the incidence of moisture accumulation due to the presence of higher RH. On the other hand, highest water absorption was observed for NF film. This resulted in shrivelled arils due to excessive moisture loss at the end of the storage. Meanwhile, PF-NF film was characterised by average water vapour condensation and water absorption characteristics in comparison with NF and PF films. This assures that excess moisture is eliminated in the event that the condensations form within the package of PF film by fitting NF window film. The moisture uptake depends on water vapour permeability of the packaging material (Dak et al. 2014). Water vapour condensation has been identified as the main problem limiting the use of MAP as a postharvest preservation tool (Giuggioli et al. 2015). Therefore, this study has shown the ability of PF-based NF fitted window film ideally reduce moisture condensation in the package with a minimum weight loss.
Effect of packaging designs on water vapour condensed inside the package film and transmitted through the packaging film after 9 days of storage at 10 °C. Mean values (n = 3). Packaging design descriptions (NF, NF-PF, PF-NF, and PF) are listed in Table 2
Physicochemical Quality Analysis
Mass Loss
The gradual mass loss of pomegranate arils packed under different packaging films stored at 10 °C is shown in Fig. 5. Packaging design, storage duration, and their interaction significantly affected (p ≤ 0.05) mass loss of arils. Lower mass loss of 0.7 and 2.8% loss was observed for arils packaged under PF and PF-NF films, respectively, after 9 days of storage. On the contrary, mass loss was significantly higher for arils packed under NF and NF-PF films of 3.8 and 3.2%, respectively. The most probable explanation of highest moisture loss for NF package is due to high WVTR of the film (based on Innovia films, UK for NatureFlex) and the presence of significantly lower RH inside the package. Caleb et al. (2016) reported similar results of PF film on maintaining the weight loss but resulting water condensation on the film for broccoli branches at 10 °C. Mass loss of pomegranate arils associated with packaging types has been reported by various studies (Caleb et al. 2013a; Hussein et al. 2015). Hussein et al. (2015) reported higher mass losses (1.9 to 6.2%) for ‘Acco’ pomegranate arils under perforated package system. In general, a 3 to 10% loss in mass could have an adverse effect on appearance, saleable weight, and texture quality of fresh cut produce (Ben-Yehoshua et al. 1987). Therefore, the mass loss (> 3%) observed by NF and NF-PF fitted window could make these packaging conditions unfavourable for pomegranate aril packaging. On the other hand, Sepulveda et al. (2000) reported a significant mass loss for treated ‘Wonderful’ pomegranate arils packed in PE bags at 4 °C. Arils were treated with chlorinated water and both chlorinated water and antioxidant solution. In a recent study, Adiletta et al. (2017) reported a progressive increase in mass loss during storage for pomegranate arils placed into sterilized cellulosic trays and packed in two different conditions: semipermeable film (SP) and micro-perforated polymeric film (MPP) at 5 °C. However, the higher mass loss was observed for arils packed under MPP at the end of the storage period with a value up to 4.1 and 2.5% for ‘Kingdom’ and ‘MR-100’ varieties, respectively.
Normalize mass loss of pomegranate arils affected by packaging designs during storage at 10 °C for 9 days. Packaging design descriptions (NF, NF-PF, PF-NF, and PF) are listed in Table 2
Surface Colour
The results of ANOVA for colorimetry analysis indicated that storage duration has a significant effect on all colour parameters (L, C*, and h°) (Table 3). There was a significant (p ≤ 0.05) decrease of L value throughout the storage duration and a slight increase and highest lightness were observed for arils packed under PF and PF-NF films. Pomegranate aril lightness was lower under NF and NF-PF films; however, it was lowest for NF film, meaning that a darker colour was developed through time. Gil et al. (1996a) reported the increase in the lightness of ‘Mollar’ pomegranate arils stored at 1, 4, and 8 °C for 7 days under heat-sealed pouches. Packaging type, storage duration, and their interaction significantly affected the h°, C*, and h° values that significantly decreased throughout the storage with the fewer initial increment. Comparing all packages, highest h° was observed for arils packed in NF films. The highest h° and lower C* of arils under NF film could show the loss of colour intensity of the arils, and the lowest lightness of arils could be due to losses of high moisture and the presence of very low O2 (Palma et al. 2006). Furthermore, Li and Kader (1989) reported that 2 kPa O2 resulted in higher red colour on strawberries. On the other hand, Artés et al. (2000) found out the decrease in lightness and C* value of aril ‘Mollar de Elche’ at 5 °C and 95% RH.
Palma et al. (2006) reported a slight decrease of h° for ‘Primosole’ pomegranate arils. The study further explained that the increase in h° implies a little colour change from reddish-orange to red-magenta during storage. Ayhan and Eştürk (2009) demonstrated that MAP, duration, and their interaction had significant effect on the lightness of ‘Hicaznar’ pomegranate arils. The decrease of L and C* values and the increase of h° were correlated with browning and loss of red colour due to anthocyanin pigment degradation or polymerization with other phenolic compounds (Maghoumi et al. 2014). Furthermore, Caleb et al. (2016) found out that RH and modified atmospheres are important factors to maintain colour attributes of broccoli. The difference in fruit colour parameters found in this study may reflect the different physical attributes of the in-package condition (headspace gases changed with time, RH, and moisture condensation) or it could be due to the cultivar.
Aril Hardness
Aril hardness followed a declining trend corresponding with advancement in storage duration. Packaging design had significant effects (p ≤ 0.05) on the textural property of arils at end of the storage duration. Aril hardness was maintained significantly under PF-NF film compared to those packed in PF and NF-PF film (Table 3). For arils packed in PF film, hardness significantly decreased at the end of the storage by 24.8% from the initial value (1.45 N). On the contrary, dryness of the arils was observed under NF film where the hardness increased by 4.4% from the initial value. The difference in hardness between arils in different packaging conditions could be associated with the change in water condensation or water loss during storage (Ayhan and Eştürk 2009). Change in textural property due to water loss directly related to the decrease in turgor pressure or could be associated with the resistance of outer periderm or removal of the periderm to transpiration movement of water vapour (Ben-Yehoshua et al. 1987).
Similarly, Ayhan and Eştürk (2009) reported significant increase (21 to 24%) of ‘Hicaznar’ pomegranate aril hardness packed in polypropylene tray sealed with biaxial-oriented polypropylene (BOPP) film within 3 days of storage. Similar increase in pomegranate aril hardness from 76.10 to 77.50 N for ‘Acco’ and 85.55 to 102.36 N for ‘Herskawitz’ packed in polypropylene (PP) tray sealed with polylid films were reported by Caleb et al. (2013b). Furthermore, significant reduction of firmness was observed for Aloe vera gel-treated ‘Mollar de Elche’ pomegranate ails (Martínez-Romero et al. 2013), while Tayyari et al. (2017) showed that the texture profile of pomegranate arils was not significantly different between the low and enriched O2 MAP treatments until day 9. The authors observed that at the end of the storage, the highest hardness was obtained at low O2 MAP. However, in the current study, the effects of storage time and the interaction of storage time and packaging had no significant impact on the textural profile of the arils.
Total Monomeric Anthocyanin
The total monomeric anthocyanin concentration of pomegranate arils was affected by packaging design and storage condition (Table 3). The results obtained showed that anthocyanin concentration gradually decreased due to the type of packaging material used, which generated different levels of stress in the arils. Thus, arils packed in PF film had the highest (94.2 + 7.06 μg L−1) while arils packed in NF film had the lowest (79.3 + 11.57 μg L−1) anthocyanin concentration at the end of the storage duration. This finding can be linked to the higher O2 permeability of the film, as it has been reported that reducing the O2 availability and consequently increasing CO2 concentration affected the antioxidant capacity by decreasing anthocyanin biosynthesis (Sánchez et al. 2014). According to Horbowicz et al. (2008), anthocyanin undergoes structural transformations that are pH dependent and were found to be more stable in acidic media (pH < 2 to 3) than in neutral or alkaline media, where the colour goes towards bluish shade at hydroxyl group and reddish in methoxyl groups. In the current study, pH measurement has not been done; therefore, reaching a conclusion according to this theory could not be possible.
The presence of higher sugar may also affect the degradation of anthocyanin, in a mechanism associated with inhibition of enzyme activities of phenoloxidase and peroxidase (Delgado-Vargas and Paredes-López 2002). However, in the current study, the statistical analysis showed that the packaging types and storage duration or their interaction did not have a significant influence on the total monomeric anthocyanin except 9 days. This observation was consistent with the study reported by Gil et al. (1996a), where they observed no significant change of anthocyanin concentration in aril ‘Molar’ during active MAP storage using OPP film at 1 °C up to 7 days. On the other hand, Ayhan and Eştürk (2009) observed a significant difference due to MAP conditions (different low and super-atmospheric O2 and nitrogen atmospheres), duration, and their interaction on the anthocyanin concentration of pomegranate aril ‘Hicaznar’. Opara et al. (2017) reported the increase in total anthocyanin content of arils during storage for pomegranate aril ‘Wonderful’ stored under clamshell tray and PP trays heat sealed with perforated polymeric polylid polyethylene PP film at 5 °C. However, this variation could be due to fruit cultivar, fruit maturity, production area, and seasonal conditions, but cultivar has the most significant effect (Zaouay et al. 2012).
Titratable Acidity
Organic acids are important flavour components; malic acid was the most abundant organic acid for pomegranate arils in all packaging followed by acetic and citric acids. All organic acid concentration decreased initially and increased after 3 days. Malic acid (MA) concentration varied across different packaging conditions by the end of the storage (9 days). Highest malic acid concentration (5460 ± 0.4 mg L−1) was observed for arils packed in PF films and PF-NF fitted window films. Comparing to the initial concentration (5680 ± 0.4 mg L−1), highest losses of 7 and 9% were observed in NF-PF and NF films, respectively. However, the effect of packaging films was insignificant. Similar to malic acid, highest concentration of acetic acid (AA) and citric acid (CA) were observed at PF and PF-NF films, where the loss was 4% for both from the initial concentration (AA 1520 ± 0.1 mg L−1 and CA 1450 ± 0.1 mg L−1) (Table 4). Similarly, Tayyari et al. (2017) reported that OPP packaging film had no significant effect on the titratable acidity of ‘Malas-de-Saveh’ pomegranate arils at 4 and 8 °C.
On the other hand, the concentration of AA and CA had shown a similar decline from its initial concentration by 7 and 9% for NF and NF-PF films, respectively. It has been reported that respiration is a major factor that can convert sugars into energy in the presence of O2; therefore, the immediate increase after 3 days and minimum reduction from the initial values of acids could be due to the presence of low O2 and low RR. The progressive reduction in acidity with the advancement of storage period might be due to the increased catabolism of organic acids present in the fruit due to respiration process (Mahajan et al. 2015). Furthermore, the lowest loss of the acid under PF films could be associated with the effect of the packaging film for delaying the respiratory process. Organic acids inhibit the growth rate of microorganisms in fruit and their products and affect quality and shelf life (Dafny-Yalin et al. 2010). This could be shown by the lowest growth of bacteria and yeast and highest concentration of organic acids under PF films. However, in this study, statistical correlation of these parameters has not been done.
Individual Sugars and Total Soluble Solids
All sugar concentrations increased over 9 days, where fructose concentration was the most predominant followed by glucose and sucrose (Table 4). Arils packed under PF films showed the lowest concentrations of all sugars, compared to the other films at the end of storage. In contrast, arils in both PF-NF and NF-PF fitted windows had highest sugar concentrations. The concentration of sucrose increased from the initial 270 mg L−1 by 33, 37, 33, and 18% for NF, NF-PF, PF-NF, and PF films, respectively. Similarly, the initial concentrations of glucose (5190 ± 1.4 mg L−1) and fructose (6550 ± 1.7 mg L−1) were increased with the range of 57 to 63% and 64 to 69%, respectively.
Packaging types did not significantly affect the changes in individual sugar concentration, but storage duration had a significant impact on the observed changes. This result was in line with the study reported by Palma et al. (2006) for ‘Primosole’ pomegranate arils packed in polypropylene film stored at 5 °C for fructose, glucose, and sucrose. The authors showed that fructose concentration was the highest (7590 to 7750 mg L−1) followed by glucose (6420 to 6750 mg L−1). Similarly, Somboonkaew and Terry (2010) reported lowest glucose and sucrose concentration for polypropylene film packaged litchi fruit stored at 13 °C for 9 days. However, there is limited information available on literature about the effect of MAP on the sugar content of pomegranate arils.
Total soluble solids (TSS) of arils in NF and PF films increased from its initial concentration by 16.33%. The highest concentration was observed for arils packed in NF film (17.50%), while slight increase (16.66%) of TSS was observed at PF films. Packaging design, storage duration, and interaction of both had a significant effect (p ≤ 0.05). This result is in line with the study reported by Ghasemnezhad et al. (2013), where TSS significantly increased for ‘Tarom’ pomegranate arils stored at 4 °C for 12 days coated with chitosan. In contrast, a significant reduction of TSS was reported for ‘Wonderful’ pomegranate arils packed in low BOP film at the end of storage day 12 at 5 °C (Banda et al. 2015). On the other hand, Ayhan and Eştürk (2009) reported that different MAP did not affect TSS of arils over 9 days. In the current study, the highest increase in TSS under NF films might be attributed to changes in the solubility of large molecular weight insoluble pectin and non-pectin components such as cellulose, in the cell wall (Ghasemnezhad et al. 2013), due to loss of water and allowing sugar to become more concentrated in the arils (Selcuk and Erkan 2014).
Volatile Organic Compounds
A total of 20 volatile compounds (VOCs) were tentatively identified in the fresh and packaged pomegranate arils, which are under seven different functional groups (Table 5). The relative concentration of most of the identified VOCs was significantly influenced (p ≤ 0.05) by packaging design and storage duration (Table 6). Prior to packaging and storage, the predominant functional groups on processed arils were alkane derivatives, aldehydes, and alcohols. However, aldehyde and alcohol concentrations reduced in the elapse of time during storage. From alkane derivatives, the concentration of pentane was predominant throughout the storage across all the packages. Evolution of ethanol in all packaging was linearly increased except for arils packed in PF and NF films, where the concentration decreased after 6 days of storage. This could be associated with the conversion of alcohol to ester, where it was evident that the ester VOCs increased after 6 days. This result was in line with the study done by Rux et al. (2017), where the higher accumulation of ethanol was reported for rucola packed under MAP. The presence of high ethanol concentration can be as a result of low-O2 and high-CO2 packaging atmosphere (Belay et al. 2017; Caleb et al. 2016). On the other hand, several studies demonstrated that many C10 and C15 monoterpenes and sesquiterpenes compose the most abundant group of compounds present in the aroma profile and key compounds determining the characteristic aroma (El-Hadi et al. 2013; Edelenbos et al. 2009; Giuggioli et al. 2015).
Acetaldehyde concentration increased exponentially under NF-PF films and decreased under PF film, while there was no change observed for arils packaged in PF-NF films. On the other hand, β-pinene and D-limonene compounds were consistently emitted for arils under all packaging conditions throughout the storage time (Table 6). The retention of these compounds in the packages could be due to the low-O2 effect, which indicates that there was no oxidation. In addition, the condensation of moisture inside the PF film could be a reason to stimulate the leaching out of β-pinene (Edelenbos et al. 2009). Giuggioli et al. (2015) also reported the possible retention or increase of these VOCs under MAP using low-O2 atmosphere for raspberry fruit.
The higher emission of furan derivatives (3-furaldehyde and 2-furancarboxaldehyde, 5-(hydroxymethyl)-) which can be categorized under lactones was observed throughout the storage. According to Hu et al. (2013), the presence of these compounds can affect the flavour, colour, and nutritional value of fruit. El-Hadi et al. (2013) reported that lactones and esters are responsible for fruity aromas. In the current study, the concentration of ethyl acetate increases slightly from its initial concentration (0.001 g L−1) to the highest (0.013 g L−1) and the lowest (0.006 g L−1) concentrations observed for arils packed in PF and NF films, respectively (Table 6). This phenomenon indicated the development of off-flavour in-package arils. Esterification of alcohols to esters was observed in this study, wherein alcohol VOCs such as 2-methyl-1-phenyl-1H-benzimidazole and 3-hydroxy-2,3-dihydro-maltol were not detected after 3 days of storage, while the emission of ester VOCs such as ethyl acetate and acetic acid, and hydrazide increased until the end of storage. In line with these results, reduction of alcohol VOCs and increased emission of esters during storage for packaged produce were also reported in the literature (Belay et al. 2017; Caleb et al. 2013a; Rux et al. 2017). In addition, increase in the emission of esters for arils under the NF films could be associated with the increase in water loss or the presence of relatively lower O2 which leads to the production of ethyl ester (Giuggioli et al. 2015).
Microbial Analysis
Initial aerobic mesophilic bacteria, yeast, and mould count were 2.53 ± 0.15, 1.74 ± 0.11, and 2.87 ± 0.09 log CFU mL−1, respectively. Microbial load on arils increased through the storage period (Fig. 6). Packaging film, storage duration, and their interactions had a significant impact on the microbial growth. The total aerobic mesophilic bacteria and yeast had similar growth trend throughout the storage in all packaging conditions. Higher growth was observed for arils under NF film (bacteria 6.28 ± 0.013 log CFU mL−1 and yeast 6.19 ± 0.10 log CFU mL−1), while lower growth (5.9 ± 0.22 log CFU mL−1) was observed under PF films. NF and PF fitted window films showed similar growth (6.09 to 6.18 log CFU mL−1 and yeast 5.74 ± 0.29 log CFU mL−1) at the end of the storage. The use of polymeric film packaging in order to develop an atmosphere that reduces the respiratory intensity and maintains unfavourable conditions for the action of many contaminating microorganisms was reported by Sepulveda et al. (2000). Banda et al. (2015) reported similar observation for aerobic mesophilic bacteria for ‘Wonderful’ pomegranate arils. In the current study, all packaging films were able to maintain aerobic mesophilic bacteria below the maximum acceptable limit (7 log CFU mL−1) at the end of the storage according to the South African legislation, FCD Act 54 1979. However, yeast count exceeds (5 log CFU mL−1), which was established as a maximum acceptable limit for yeast growth at 9 days in all packaging. The reason for yeast exceeding the maximum acceptable limit could be associated with its capability to grow under lower pH environment comparing with aerobic mesophilic bacteria (Caleb et al. 2013a), which can be related to the presence of higher CO2 in the package. The effect of CO2 to change the intercellular pH and its bacteriostatic effect which limit the growth of aerobic mesophilic and yeast was reported by Ayhan and Eştürk (2009).
Effect of packaging designs on microbial growth for pomegranate arils’ a aerobic mesophilic bacteria, b yeast, and c mould growth stored at 10 °C for 9 days. Error bars represent standard deviation (SD) of mean values (n = 6). Bars with similar letters are not significantly different at 95% confident interval (p ≤ 0.05) according to Duncan’s multiple range test. Packaging design descriptions (NF, NF-PF, PF-NF, and PF) are listed in Table 2
Mould growth followed slightly different growth patterns from yeast and aerobic mesophilic bacteria. It showed an initial decrease until 6 days under all packaging films and the exponential increase was observed at day 9. Lowest mould growth was observed for arils packed under NF films (3.06 ± 0.16 log CFU mL−1) followed by NF-based PF fitted window (3.66 ± 0.92 log CFU mL−1). López-Rubira et al. (2005) reported similar observation, wherein the yeast count exceeded maximum acceptable limit after 6 days for ‘Mollar of Elche’ pomegranate arils harvested in December and treated with UV-C at 5 °C. Similarly, Krasnova et al. (2012) observed lowest growth of mould under NatureFlex-based films and highest growth under PropaFilm for mixed fruit salad. Furthermore, this study reported that PropaFilm-based film suppressed bacterial growth. The highest growth of mould in PF film could be a result of higher water vapour condensation inside the package.
Conclusions
The designed modified atmosphere and humidity packaging (MAHP) system showed significant potential to influence the quality of pomegranate arils stored at 10 °C for 9 days. The PF-based NF fitted window and NF-based PF fitted window films have prevented a build-up of CO2 and reduction of O2 concentration. However, the use of NF-based PF fitted window film was limited due to low RH inside the package, which affected the product appearance and quality. On the other hand, the use of BOPP-based NF window film (Fig. 7) successfully regulated the recommended in-package RH, thus assisted to control condensation as well as weight loss. Using absolute cellulose-based film has shown highest reduction of O2 concentration, lowest in-package RH, and increased moisture loss, which has resulted in aril hardness and colour change. 100% PF film helped to reduce the weight loss of the arils, but it was not efficient to control the in-package RH. This affected the appearance and aroma of pomegranate arils due to visible surface mould growth and higher concentration of ester compounds at day 9. These results indicated the importance of appropriate design of MAP to maintain the postharvest freshness of pomegranate arils as well as to reduce the economic loss. However, further study is necessary to identify the industrial applicability for compatibility with the packaging machines, saleability, and printing qualities of the improved packaging films.
References
Adiletta, G., Liguori, L., Albanese, D., Russo, P., Di Matteo, M., & Cerscitelli, A. (2017). Soft-seeded pomegranate (Punica granatum L.) varieties: preliminary characterization and quality changes of minimally processed arils during storage. Food and Bioprocess Technology, 10(9), 1631–1641.
Artés, F. (1993). Diseno y cálculo de polímeros sintéticos de interes para la conservación hortofrutícola en atmósfera modificada. In A. Madrid (Ed.), Nuevo curso de ingeniería del frío (pp. 427–454). Murcia: Colegio Oficial de Ingenieros Agronómos de Murcia.
Artés, F., Villaescusa, R., & Tudela, J. A. (2000). Modified atmosphere packaging of pomegranate. Journal of Food Science, 65(7), 1112–1116.
Ayhan, Z., & Eştürk, O. (2009). Overall quality and shelf life of minimally processed and modified atmosphere packaged ‘ready-to-eat’ pomegranate arils. Journal of Food Science, 74, 399–405.
Banda, K., Caleb, O. J., Jacobs, K., & Opara, U. L. (2015). Effect of active-modified atmosphere packaging on the respiration rate and quality of pomegranate arils (cv. Wonderful). Postharvest Biology and Technology, 109, 97–105.
Belay, Z. A., Caleb, O. J., & Opara, U. L. (2017). Impacts of low and super-atmospheric oxygen concentrations on quality attributes, phytonutrient content and volatile compounds of minimally processed pomegranate arils (cv. Wonderful). Postharvest Biology and Technology, 124, 119–127.
Ben-Yehoshua, S., Shapiro, B., & Moran, R. (1987). Individual seal-packaging enables the use of curing at high temperatures to reduce decay and heal injury of citrus fruits. Hort Science, 22, 777–783.
Bovi, G. G., Caleb, O. J., Linke, M., Rauh, C., & Mahajan, P. V. (2016). Transpiration and moisture evolution in packaged fresh horticultural produce and the role of integrated mathematical models: a review. Biosystems Engineering, 150, 24–39.
Caleb, O. J., Opara, U. L., & Witthuhn, C. R. (2012). Modified atmosphere packaging of pomegranate fruit and arils: a review. Food and Bioprocess Technology, 5(1), 15–30.
Caleb, O. J., Opara, U. L., Mahajan, P. V., Manley, M., Mokwena, L., & Tredoux, A. G. (2013a). Effect of modified atmosphere packaging and storage temperature on volatile composition and postharvest life of minimally processed pomegranate arils (cvs. Acco and Herskawitz). Postharvest Biology and Technology, 79, 54–61.
Caleb, O. J., Mahajan, P. V., Al-Said, F. A., & Opara, U. L. (2013b). Transpiration rate and quality of pomegranate arils as affected by storage conditions. CyTA-Journal of. Food, 11(3), 199–207.
Caleb, O. J., Ilte, K., Fröhling, A., Geyer, M., & Mahajan, P. V. (2016). Integrated modified atmosphere and humidity package design for minimally processed broccoli (Brassica oleracea L. var. italica). Postharvest Biology and Technology, 121, 87–100.
Charles, F., Sanchez, J., & Gontard, N. (2006). Absorption kinetics of oxygen and carbon dioxide scavengers as part of active modified atmosphere packaging. Journal of Food Engineering, 72(1), 1–7.
Chen, L., & Opara, U. L. (2013). Texture measurement approaches in fresh and processed foods—a review. Food Research International, 51(2), 823–835.
Dafny-Yalin, M., Glazer, I., Bar-Ilan, I., Kerem, Z., Holland, D., & Amir, R. (2010). Colour, sugars and organic acids composition in aril juices and peel homogenates prepared from different pomegranate accessions. Journal of Agricultural and Food Chemistry, 58(7), 4342–4352.
Dak, M., Sagar, V. R., & Jha, S. K. (2014). Shelf life and kinetics of quality change of dried pomegranate arils in flexible packaging. Food Packaging and Shelf Life, 2(1), 1–6.
Delgado-Vargas, F., & Paredes-López, O. (2002). Natural colorants for food and nutraceutical uses. USA: CRC Press. Taylor & Francis group.
Del-Valle, V., Almenar, E., Hernández-Muñoz, P., Lagarón, J. M., Catala, R., & Gavara, R. (2004). Volatile organic compound permeation through porous polymeric films for modified atmosphere packaging of foods. Journal of the Science of Food and Agriculture, 84(9), 937–942.
Edelenbos, M., Balasubramaniam, M., & Pedersen, H. T. (2009). Effects of minimal processing and packaging on volatile compounds and other sensory aspects in carrots. Acta Horticulturae, 876, 269–277.
El-Hadi, M. A. M., Zhang, F. J., Wu, F. F., Zhou, C. H., & Tao, J. (2013). Advances in fruit aroma volatile research. Molecules, 18(7), 8200–8229.
Fawole, O. A., & Opara, U. L. (2013). Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Scientia Horticulturae, 150, 37–46.
Ghasemnezhad, M., Zareh, S., Rassa, M., & Sajedi, R. H. (2013). Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature. Journal of the Science of Food and Agriculture, 93(2), 368–374.
Gil, M. I., Artés, F., & Tomas-Barberan, F. A. (1996a). Minimal processing and modified atmosphere packaging effects on pigmentation of pomegranate seeds. Journal of Food Science, 61(1), 161–164.
Gil, M. I., Martínez, J. A., & Artés, F. (1996b). Minimally processed pomegranate seeds. Lebensm Wiss U Technol, 29(8), 708–713.
Giuggioli, N. R., Briano, R., Baudino, C., & Peano, C. (2015). Effects of packaging and storage conditions on quality and volatile compounds of raspberry fruit. CyTA-Journal of Food, 13, 512–521.
Gomes, M. H., Beaudry, R. M., Almeida, D. P., & Malcata, F. X. (2010). Modelling respiration of packaged fresh-cut ‘Rocha’ pear as affected by oxygen concentration and temperature. Journal of Food Engineering, 96(1), 74–79.
Horbowicz, M., Kosson, R., Grzesiuk, A., & Dębski, H. (2008). Anthocyanins of fruit and vegetables—their occurrence, analysis and role in human nutrition. Vegetable crops research bulletin, 68, 5–22.
Hu, G., Hernandez, M., Zhu, H., & Shao, S. (2013). An efficient method for the determination of furan derivatives in apple cider and wine by solid phase extraction and high performance liquid chromatography-diode array detector. Journal of Chromatography A, 1284, 100–106.
Hussein, Z., Caleb, O. J., & Opara, U. L. (2015). Perforation-mediated modified atmosphere packaging of fresh and minimally processed produce—a review. Food Packaging and Shelf Life, 6, 7–20.
Kader, A. A., & Rolle, R. S. (2004). The role of post-harvest management in assuring the quality and safety of horticultural produce (Vol. 152). Rome: Food and Agriculture Organization (FAO).
Krasnova, I., Dukalska, L., Seglina, D., Juhnevica, K., Sne, E., & Karklina, D. (2012). Effect of passive modified atmosphere in different packaging materials on fresh-cut mixed fruit salad quality during storage. International Journal of Biological, Bio molecular, Agricultural, Food and Biotechnological Engineering, 6, 1095–1104.
Kumar, A. K., Babu, J. D., Bhagwan, A., & Raj Kumar, M. (2012). Effect of modified atmosphere packaging on shelf life and quality of ‘Bhagwa’ pomegranate in cold storage. Acta Horticulturae, 1012, 963–969.
Li, C., & Kader, A. A. (1989). Residual effects of controlled atmospheres on postharvest physiology and quality of strawberries. Journal of the American Society for Horticultural Science, 114, 629–634.
López-Rubira, V., Conesa, A., Allende, A., & Artés, F. (2005). Shelf life and overall quality of minimally processed pomegranate arils modified atmosphere packaged and treated with UV-C. Postharvest Biology and Technology, 37(2), 174–185.
Maghoumi, M., Mostofi, Y., Zamani, Z., Talaie, A., Boojar, M., & Gómez, P. A. (2014). Influence of hot-air treatment, super-atmospheric O2 and elevated CO2 on bioactive compounds and storage properties of fresh-cut pomegranate arils. International Journal of Food Science and Technology, 49(1), 153–159.
Magwaza, L. S., & Opara, U. L. (2015). Analytical methods for determination of sugars and sweetness of horticultural products—a review. Scientia Horticulturae, 184, 179–192.
Mahajan, B. V. C., Dhillon, W. S., Kumar, M., & Singh, B. (2015). Effect of different packaging films on shelf life and quality of peach under super and ordinary market conditions. Journal of Food Science and Technology, 52(6), 3756–3762.
Mangaraj, S., Goswami, T. K., & Mahajan, P. V. (2009). Applications of plastic films for modified atmosphere packaging of fruit and vegetables: a review. Food Engineering Reviews, 1(2), 133–158.
Martínez-Romero, D., Castillo, S., Guillén, F., Díaz-Mula, H. M., Zapata, P. J., Valero, D., & Serrano, M. (2013). Aloe vera gel coating maintains quality and safety of ready-to-eat pomegranate arils. Postharvest Biology and Technology, 86, 107–112.
Mphahlele, R. R., Stander, M. A., Fawole, O. A., & Opara, U. L. (2014). Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolic acids, vitamin C and antioxidant activity of pomegranate juice (cv. Wonderful). Scientia Horticulturae, 179, 36–45.
Mphahlele, R. R., Caleb, O. J., Fawole, O. A., & Opara, U. L. (2016). Effects of different maturity stages and growing locations on changes in chemical, biochemical and aroma volatile composition of ‘Wonderful’ pomegranate juice. Journal of the Science of Food and Agriculture, 96(3), 1002–1009.
Opara, U. L., Al-Ani, M. R., & Al-Shuaibi, Y. S. (2009). Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food and Bioprocess Technology, 2(3), 315–321.
Opara, U. L., Hussein, Z., & Caleb, O. J. (2017). Phytochemical properties and antioxidant activities of minimally processed “Acco” pomegranate arils as affected by perforation-mediated modified atmosphere packaging. Journal of Food Processing and Preservation, 41(3), e12948.
Palma, A., Schirra, M., D'Aquino, S., La Malfa, S., & Continella, G. (2006). Chemical properties changes in pomegranate seeds packaged in polypropylene trays. Acta Horticulturae, 818, 323–330.
Pathare, P. B., Opara, U. L., & Al-Said, F. A. J. (2013). Colour measurement and analysis in fresh and processed foods: a review. Food and Bioprocess Technology, 6, 1–25.
Rux, G., Caleb, O. J., Geyer, M., & Mahajan, P. V. (2017). Impact of water rinsing and perforation-mediated MAP on the quality and off-odour development for rucola. Food Packaging and Shelf Life, 11, 21–30.
Sánchez, L. C. A., Real, C. P. V., & Perez, Y. B. (2014). Effect of an edible cross-linked coating and two types of packaging on antioxidant capacity of ‘castilla’ blackberries. Food Science and Technology, 34(2), 281–286.
Selcuk, N., & Erkan, M. (2014). Changes in antioxidant activity and postharvest quality of sweet pomegranates (cv. Hicrannar) under modified atmosphere packaging. Postharvest Biology and Technology, 92, 29–36.
Sepulveda, E., Galletti, L., Saenz, C., & Tapia, M. (2000). Minimal processing of pomegranate var. Wonderful. In: Melgarejo P, Martinez J J, Martinez T J, editors. Symposium on production, processing and marketing of pomegranate in the Mediterranean region: advances in research and technology. Zaragosa, Spain: CIHEAM-IAMZ. pp. 237–42.
Somboonkaew, N., & Terry, L. A. (2010). Physiological and biochemical profiles of imported litchi fruit under modified atmosphere packaging. Postharvest Biology and Technology, 56(3), 246–253.
Song, Y., Vorsa, N., & Yam, K. L. (2002). Modelling respiration transpiration in a modified atmosphere packaging system containing blueberry. Journal of Food Engineering, 53(2), 103–109.
South African Legislation. (1979). Foodstuff, Cosmetics and Disinfectant (FCD) Act 54. Department of Health.
Szychowski, P. J., Frutos, M. J., Burló, F., Pérez-López, A. J., Carbonell-Barrachina, Á. A., & Hernández, F. (2015). Instrumental and sensory texture attributes of pomegranate arils and seeds asaffected by cultivar. LWT-Food Science and Technology, 60(2), 656–663.
Tayyari, F., Khazaei, J., Rajaei, P., & Jouki, M. (2017). Effects of modified atmosphere packaging systems, low temperature and storage time on the quality of fresh minimally processed pomegranate arils. Carpathian Journal of Food Science and Technology, 9(1), 16–26.
Zaouay, F., Mena, P., Garcia-Viguera, C., & Mars, M. (2012). Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Industrial Crops and Products, 40, 81–89.
Funding
This work is based on research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation. Ms. Zinash Belay recognizes the award of PhD Fellowship by the Organisation for Women in Science for the Developing World (OWSD) and Swedish International Development Cooperation Agency (SIDA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belay, Z.A., Caleb, O.J., Mahajan, P.V. et al. Design of Active Modified Atmosphere and Humidity Packaging (MAHP) for ‘Wonderful’ Pomegranate Arils. Food Bioprocess Technol 11, 1478–1494 (2018). https://doi.org/10.1007/s11947-018-2119-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2119-0