Abstract
The effect of the combination of maltodextrin DE 10 (MD) and native agave fructans (FR) in concentrations of 0, 2, and 4% (w/v) on the rheological properties, microstructure, and water sorption of spray-dried chayote and pineapple powders was evaluated. A 10% (w/v) maltodextrin treatment was used as a control to compare treatments added with fructans. The scanning electron micrographs revealed spherical particles in a range from 16 to 105 μm with shrinkage, whereby greater caking and agglomeration occurred among particles in treatments with native agave fructans. The flow behavior of all juices can be described by the Bingham model with low plastic viscosities (0.0026 to 0.0030 Pa s−1); the isotherms of the powders show a sigmoid shape pointing to the easy union of the fructans with water molecules. These types of isotherms are common for non-porous foods, and are indicative of physical adsorption in multi-layers where the adsorbate conserves its identity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pineapple and chayote are highly regarded due to their taste and nutritional value. Both pineapple and chayote are recognized because of its high content in antioxidants such as polyphenols, carotenoids, and vitamin C, and chayote is additionally an important source of folic acid. There is an increasing interest in highly nutritious and instant foods, which is why the concentrated juice powder of these fruits could be an excellent and profitable option for consumption. In this way, chayote is mainly produced in Mexico and unknown in most countries. Due to the physical characteristics of the different types of fruit and the environmental conditions in the growing regions, large amounts of the harvested fruit fall short of the minimum quality standards for direct use and result in significant losses.
In recent years, new processes have been under development in order to achieve commercialization for fruit that fails to meet quality standards—the idea is to create products higher in quality and with greater added value. In this sense, the production of fruit powders is a better alternative for the use of raw material that fails to meet quality standards for exportation. Spray-drying is an alternative process for products which are sensitive to heat and has been used successfully in fruit juices (Bhandari et al. 1993; Caliskan and Dirim 2016); the result is a product that has acceptable sensory characteristics and that is also stable for storage. The use of additives is necessary to increase Tg in order to ensure proper control of the spray-drying process in fruit juices and the quality of the obtained products (Mujumdar 1995). The average added amount has been reported between 30 and 75% (w/v) (Fernandes et al. 2013a; Fernandes et al. 2013b; Shrestha et al. 2007), which stands in function of the total solid content of the particular juice to be processed. One of the challenges in the production of fruit powders is the reduction of viscosity to improve their drying, handling, and storage. The stickiness of fruit powders is mainly due to the presence of low molecular weight sugars such as fructose, glucose, sucrose, and some organic acids in the fruit. These sugars and organic acids are very hygroscopic in their amorphous state and have low glass transition temperatures (Sablani et al. 2008). To overcome this undesirable situation, the use of different substances high in molecular weight, such as gum Arabic and maltodextrin with different dextrose equivalents (DE), has been noted—the aim being to increase glass transition temperature of the sample, reduce stickiness, and produce free-flowing powders with improved handling and enhanced quality (Mosquera et al. 2010). Maltodextrin is currently the most widely used additive employed to obtain fruit powders; it satisfies production demands and is low in cost (Dibtaxi et al. 2000; Tan et al. 2015). Maltodextrins consist of d-glucose units with mainly 1,4-glycosidic bonds and are classified according to their dextrose equivalent value.
The DE of a maltodextrin determines its stabilizing capacity and is inversely related to its mean molecular weight (Caliskan and Dirim 2013). Recent studies have attributed agave fructans with thermal protective and encapsulating properties, as well as technological functions as stabilizers (Espinosa-Andrews and Urias-Silvas 2012). However, little information is available on the influence of native agave fructan concentration as a spray-drying stabilizer, nor on their effect on water sorption and on the reconstituted powder’s microstructure and rheology. These effects can be studied through moisture absorption characterization of the products with water sorption isotherms. This contributes to the correct assessment of storage conditions, packaging, prediction of shelf life, and comprehension of physico-chemical changes that intervene in the production process (Tunc and Duman 2007). In this sense, numerous mathematical models have been proposed to describe the behavior of moisture absorption of foods. In general, these models can be divided into various categories: kinetic models based on a monolayer (BET model), and kinetic models based on a multilayer and condensed film (Guggenheim-Anderson-de Boer, GAB model).
The reconstituted powders are fluids that can be classified according to their response to deformation in the linear (LVR) and non-linear viscoelastic (n-LVR) region. In particular, large amplitude oscillatory shear (LAOS) deformation permits varying experiments depending on whether suitable conditions for examining the material response within LVR are present, and to obtain additional information in n-LVR. LVR is related to the structural arrangement, while n-LVR is associated with the mechanical response when the food structure has been mostly deformed (Harte et al. 2007). The n-LVR is more related to consumer perception than the LVR (Guggisberg et al. 2009; Núñez-Santiago et al. 2001).
Currently, few reports on the use of agave fructans as additives in the spray-drying process of fruit, or their use in the reduction of conventionally used materials, such as MD exist. In this sense, the objective of this study was to evaluate the effect of adding agave fructans and maltodextrin DE 10 on rheology, microstructure, and water sorption in spray-dried chayote (Sechium edule) and pineapple (Ananas comosus) juices.
Materials and Methods
Raw Materials
Chayote and pineapple fruit in consumption degree of ripeness were obtained in Tepic, Nayarit, and subsequently washed and disinfected in a sodium hypochlorite solution (NaClO) at 200 ppm (0.02%). Then, they were peeled and the juice of these fruits was obtained using a conventional extractor; maltodextrin D10 or native agave fructans were added for homogenization using an electric laboratory homogenizer (PRO Scientific. Inc. 300 PC) at 350 rpm during 15 min and filtered in a No. 50 filter sieve. One liter of homogenized juice was used for each test.
Spray-Drying
A pilot dryer (model LPG-5, CIMA Industries Inc.) equipped with a rotatory nozzle was used for the spray-drying process with a feed rate of 15 mL min−1, 2.5 bar and juice feed temperature 25 °C, air inlet 9.4 m/s and temperature 120 °C, maltodextrin concentration 10% (m/v), and three fructan concentrations 0, 2, and 4%. The inlet temperature and maltodextrin concentrations were determined in preliminary studies.
Physico-Chemical Properties of Powders
Moisture Content
The content of moisture was determined using the thermobalance method (Nollet and Toldrá 2015). A thermobalance Sartorius MA 35 was used and operated at a temperature of 75 °C at constant weight.
Water Activity
Water activity was determined by an Aqualab 4TEV (Decagon devices). Five grams of sample was placed inside the chamber where the water activity is determined by the dew point principle.
Hygroscopicity
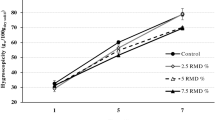
This analysis was carried out according to the methodology described by Al Kahatani and Hassan (Al-Kahtani and Hassan 1990; Tonon et al. 2011).
Solubility
The Eastman and Moore methodology, modified by Cano et al., was used (Cano-Chauca et al. 2005).
Bulk Density
Twenty grams of sample was placed in a test tube of 100 mL, then stirred in a vortex for 5 min. Bulk density was calculated by the powder mass flow rate and the final volume; the result was reported in grams per cubic centimeter (Goula and Adamopoulos 2008).
Wettability
The static method described by Freuding was used. The wettability was expressed as the necessary time for 1 g of powder to disappear from the water surface (Fuchs et al. 2006).
Drying Yield
Spray-drying yield was evaluated by the determination of the product recovery given by the percentual ratio (dry basis) between the total mass of product recovered by the mass of juice fed to the system (Fazaeli et al. 2012).
Scanning Electron Microscope
A scanning electron microscope (model SEC 3200 M, SEMICOM Korea) with an accelerating voltage of 20 kV was used. Two to 5 mg of sample powder were fixed on a double-sided adhesive tape and placed onto a metallic slide. The samples were then metalized with gold during 2 min.
Rheological Properties of Juices
The rheometric measurements were carried out with a controlled stress rheometer (Model AR-G2 TA Instruments with Software Trios v4.0.1) using concentric cylinder geometry (exterior cylinder diameter 21.96 mm, interior cylinder diameter 20.38 mm, height 59.50 mm, gap 500 μm), under a constant temperature of 25 °C, maintained in a circulating water bath and AG2 heater. The flow behavior was analyzed in a simple shear flow and low cutting speed in a range of 5 to 200 s−1. Juices were reconstituted based on the total soluble solids of the fresh juice. The experimental data of fresh or reconstituted juices were fitted to different flow models such as Newtonian (Eq. 1), Bingham (Eq. 2), and Ostwald-de-Waele (power law model) (Eq. 3).
where τ is the shear stress (Pa), \( \dot{\gamma} \) is the shear rate (s−1), η is the viscosity (Pa s−1), η′ is the plastic viscosity (Pa s−1), τ 0 is the yield stress (Pa), K is the consistency index (Pa1/2 sn), and n is the flow behavior index.
The best rheological model for describing the flow behavior of juices was selected by comparing the value of correlation coefficient, R 2.
Analysis of Moisture Adsorption
The isotherms were determined using a vapor sorption analyzer (VSA) (AquaLab, Decagon Devices, Inc. Pullman, W.A.) SOFTWARE VSA Dowlander. The isotherms were obtained at a temperature of 45 °C using approximately 300 mg of the sample—models were adjusted with BET and GAB models (Eq. 4 and Eq. 5).
whereby:
- M :
-
moisture content, kg water/kg dry mass
- m 0 :
-
monolayer moisture content
- C :
-
material constant, related to heat sorption.
- a w :
-
water activity
whereby
- M :
-
moisture content, kg water/kg dry mass
- m 0 :
-
monolayer moisture content
- C :
-
Guggenheim constant, product characteristics and related to monolayer heat sorption
- K :
-
Correction factor related to multi-layer heat sorption
- a w :
-
water activity
The performance of non-linear models has been approved in different branches as irrigation (Valipour 2016a), climate change (Valipour et al. 2017), meteorology (Valipour 2016b), and food engineering (Shamsudin et al. 2013).
Differential Scanning Calorimetry
The glass transition temperatures (Tg) of the treatments at different concentrations of native agave fructans were estimated using a calorimeter (DSC, Q2000 TA-Instruments, New Castle, DE, USA). In general, 4 mg of sample equilibrated at 25 °C was heated in hermetic aluminum crucibles. A heating ramp of 20 to 200 °C at 10 °C/min was used. An empty hermetically sealed aluminum crucible was used as reference. The DSC was calibrated with metallic Indian standard for temperature. The calorimeter was purged with nitrogen at a flow rate of 50 mL/min. All measurements were made in triplicate. The data were analyzed using the Universal Analysis 2000 software, version 4.7a (TA Instruments, New Castle, USA); Tg was calculated by evaluating the midpoint of the inflection region in the heat flow signal.
Results and Discussion
The physicochemical properties of juices with different concentrations of maltodextrin showed that there is no difference between juices at 10 and 30%, which is the percentage of maltodextrin used in the fruit powder industry. This data allows selection of 10% maltodextrin concentration with which the combinations with the agave fructans were made. The increase in the concentration of maltodextrin has a significant effect causing a decrease in the moisture content, and being the variable which other physicochemical properties depend on, these variables also have a decrease in relation to the concentration of maltodextrin (Table 1).
Powder Microstructure
The microcapsule micrographs obtained with maltodextrin presented rounded outer surfaces with cavities and dents; this is attributed to mechanical atomization, air-drop interaction, and capsule cooling, and are stages that occur during spray-drying (Rosenberg et al. 1985; Phisut 2012; Crispín-Isidro et al. 2014). Irregular surfaces were observed for the chayote particles; this corresponds to findings in previous studies that found cracks and cavities in the spray-dried mango powder particles, while pineapple particles presented a smooth surface without cracks (Caparino et al. 2012).
The electron scanning microscope showed particles with shrinkage, lumps, and caking between them. It also showed a size distribution range between 17 and 105 μm. According to the obtained microscopy, the samples with fructans showed the highest agglomeration and caking. Temperature and the water absorption capacity of the stabilizer are factors that control structure collapse, adhesiveness, and caking; these factors are time-dependent in the drying process (Le Meste et al. 2002; Roos 2010). Thus, the irregularity found in the particles is caused by the hygroscopicity of the native agave fructans and the temperature of the spray-drying process. At temperatures below 130 °C, the fructans have a mass loss that is related to water evaporation and thermal decomposition or stickiness causing shrinkage in the particles (Espinosa-Andrews and Urias-Silvas 2012).
The ramified structure of the agave fructans allows for a greater water absorption capacity and leads to higher particle plasticization in powders containing native agave fructans (Espinosa-Andrews and Urias-Silvas 2012). The effect that the ramified structure of native agave fructans has as a reducer of the glass transition temperature (Tg) is a known result of internal plasticization caused by spray-drying (Bizot et al. 1997; Kurozawa et al. 2009). The presence of β (2–6) bonds in native agave fructans leads to higher flexibility in the biopolymer chains that decrease the Tg value (Espinosa-Andrews and Urias-Silvas 2012). The agglomeration and caking of the particles treated with fructans could be attributed to the temperature (120 °C) used in the spray-drying process. The glass transition temperatures for pineapple were 42.45, 52.68, and 55.24 °C and 48.04, 49.68, and 52.95 for chayote for 0, 2, and 4% fructan concentrations, respectively. The Tg values obtained in this study indicate a positive effect of the addition of fructans since these have a higher molar mass than maltodextrin and Tg is positively correlated to the molar mass (Truong et al. 2005; De Barros Fernandes et al. 2014); consequently, the treatments with fructans increased the value of Tg. It has also been established that the water absorption of the carbohydrates and their plasticization product implies not only the formation of hydrogen bridge bonds and the structural disorganization of the particles but also changes in matrix volume, resulting in amorphous particles (Kilburn et al. 2004; Roussenova et al. 2010). This explains the deformations and caking observed in the spherical particles of the chayote and pineapple powders as observed in the micrographs (Fig. 1).
Rheological Analysis
Table 2 shows the fitted parameters of the Newtonian, Bingham, and Power Law rheological models for chayote and pineapple fresh or reconstituted juices with different contents of fructans. For all the considered juices, the best adjustment of flow data was obtained for the Bingham model with high values of R 2 ranging between 0.9992 and 0.9999. These results are in agreement are in agreement with those of Shamsudin et al. (2013) who found that the flow curves of Yankee pineapple juices were described by the Bingham model.
Bingham model describes plastic fluid that behaves as a rigid body at low stresses but flows as a viscous fluid at high stress. Then this type of fluid is characterized by a yield stress and a plastic viscosity. These parameters were statistically compared in Table 3 for each formulation of juices. Whether for pineapple or chayote juices, the addition of maltodextrin (10% w/v) and fructans (0–4% w/v) and the step of spray-drying had no significant influence on the plastic viscosity of juices as compared to fresh juices. Moreover, all the considered juices (Table 3) presented a low plastic viscosity that ranged from 0.0026 to 0.0030 Pa s−1 at 25 °C. In contrast, yield stresses were impacted by the addition of maltodextrin or fructans or by the step of spray-drying. Yield stress values were correlated to the presence and the degree of entanglement of colloids in fluids. For chayote juices, the addition of maltodextrin decreases significantly the value of the yield stress from 0.0145 Pa (fresh juice) to 0.0029 Pa. The addition of maltodextrin to chayote juice induces a significant decrease of the yield stress of juice. This effect may be attributed to the interactions between maltodextrin and the other polysaccharides, which do not allow these polysaccharides to fully extend in solution (Goula and Adamopoulos 2008). According to Grabowski et al. 2008 who studied the rheological properties of spray-dried sweet potato-maltodextrin powders, a maltodextrin polysaccharide interaction facilitates a decreased solution viscosity, since longer molecules have a larger hydrodynamic volume, which increases solution viscosity.
The additional supplementation of fructans up to 4% induced a slight but significant increase of the yield stress of fresh chayote juice up to 0.0078 Pa. Furthermore, spray-drying did not seem to deeply impact the rheological behavior of chayote juices (Table 3). Pineapple juices presented higher yield stresses than chayote, while they had the same plastic viscosity. These findings could be due to the fact that fruit juices contain colloidal systems. These colloids are part of the fruit itself or may be formed by microorganisms during fruit ripening. Most of the colloids come from the plant itself. The amount of colloids present in fruit juice is in the range of 100–1000 mg/L. An examination of colloids in the juice after pressing show that they are basically polysaccharides such as pectins and starch (Barrosi et al. 2004; Echavarría et al. 2012). In this sense, pineapple contains more pectins than chayote and pectins are responsible for the turbidity and high viscosity of fruit juices. The addition of maltodextrin and fructans up to 4% (w/v) also induced a slight but significant increase of the yield stress of pineapple juices. However, a decrease of this rheological parameter was observed for reconstituted juices after spray-drying especially for samples with 4% of fructans, which could be due to the solubility of fructans above 95% (Espinosa-Andrews and Urias-Silvas 2012).
The viscosity of agave fructan solutions (Agave tequilana Weber blue var.) at different concentrations and temperatures has been studied by Ponce et al. (2008). They observed that solutions with concentrations inferior to 30% (w/v) at temperatures ranging between 30 and 60 °C presented a low viscosity—similar to water. Meanwhile, the viscosity of solutions with concentrations superior or equal to 30% (w/v) increased when increasing the concentration up to 70%, forming a highly viscous fluid. The results obtained in this work are in agreement with previous findings; lower viscosity was observed in fructan powders with lower concentrations. This shear thinning behavior at low shear rate (20 s−1) followed by thickening behavior at high shear rate (300 s−1) in low fructan concentrations (Cfructans ≤ 30% in weight) could be due to flow instabilities such as vortices, since the viscosity of the samples is close to that of water.
The effect of adding maltodextrin on tomato pulp during spray-drying has been studied by Goula and Adamopoulos (2008). They observed that reconstituted tomato pulp from the resulting powder showed a non-Newtonian behavior with low stress, and a decrease in viscosity when the maltodextrin concentration and dextrose equivalent increase. This same effect is observed in the reconstituted chayote and pineapple powders and is attributed to the fact that the 10% maltodextrin concentration is higher in comparison to that of fructans.
Chayote and pineapple juices (fresh and reconstituted) did not present the same rheological behavior (specially for the yield stress). In this sense, studies have found that a decrease in sweet potato solids, as well as an interaction between maltodextrin and sweet potato polysaccharides, contributed to a decrease in viscosity of the reconstituted purée (Grabowski et al. 2008). In general, the flow behavior of fresh and reconstituted sweet potato solutions was different suggesting that the solid concentration was modified by the molecular changes during spray-drying. This could also explain the distinct rheological behavior for each reconstituted fruit powder in the present study.
Sorption Isotherms
Each analyzed sample of pineapple and chayote presented a different behavior. One to four days were required within the AquaLab VSA equipment in order to obtain a complete sorption isotherm. The required time for each sample depends on the grade or sorption; the obtained isotherms correspond to the sigmoid form and to the type II and III isotherms according to the classification by Brunauer (Brunauer et al. 1940; Yang 1987), where they proposed that this type of isotherms commonly represents non-porous foods. This is indicative of a physical adsorption in multilayers. The same behavior was reported for cornmeal without nixtamalization (Vega et al. 2006). A similar behavior was also observed in camu-camu pulp studies and with spray-dried pineapple pulp using maltodextrin (Silva et al. 2006; Gabas et al. 2007). Two regions stand out in this sigmoid-shaped adsorption isotherm; the first region is found around the a w values between 0.2 and 0.4, and the second at values 0.6 and 0.7, and is a result of the changes in magnitude that go from the physical to the chemical effects; this would be the formation of multilayers and the filling of small pores in the region with low a w values followed by the filling of large pores and dilution of solutes in the region of elevated a w values (Chen and Lai 2008). The studied variable in each spray-dried fruit juice was fructan concentration. The effect of the native agave fructans at a higher concentration reflects an increase in the degree of water adsorption, this with an approximate 14% increase and thereby projecting the curve above 50% water retention for the higher fructan concentration in chayote (Fig. 2) and 60% in the case of pineapple with 2% fructan concentration. In this way, these results indicate the effect of fructans on powder stability in chayote at 4% and in pineapple since 2%. The isotherms of chayote with an increase in the concentration of fructans indicated a deficient desorption, which was evidenced by the greater percentage of humidity at the end of desorption, indicating a greater saturation of the pores and greater size in the hysteresis loop. For pineapple, water absorption capacity followed an increasing fructan concentration; however, the final region of the desorption isotherm was below the isotherm of the treatment using only maltodextrin, since they reached lower percentages of humidity. For all fruits and in all treatments, isotherms indicate acceptable stability. This behavior can be credited to the different sizes and forms of the obtained particles, as well as the amount of fructans of a low, medium, and high grade of polymerization present in the native agave fructans. The chayote particles with only maltodextrin presented smooth surfaces with greater dispersibility while treatments added with fructans presented amorphous particles with contractions, as well as agglomeration; this prevents complete desorption and causes the powder to rehydrate as shown by the isotherms. In the case of pineapple, the treatments with fructans showed smooth spherical particles, caking, and agglomeration; this agglomeration causes a greater hysteresis loop due to the capacity of the fructans to bond with water molecules. With the addition of fructans, the powders presented larger particles, meaning a lower contact surface and therefore a lower efficiency in the mass transfer process. It has also been found that tomato powder with larger particles presented lower mass transfer. Due to the hydroxyl groups of the native fructans, however, a higher water retention capacity was observed (Goula and Adamapoulos 2005; Cal and Sollohub 2010).
The characteristic stages were observed in the two fruit powder isotherms. First, the hydration of highly polar hydrophilic groups related to the hydration of the monolayer in stage I. Stage II is characterized by the conformation of small water molecule conglomerates. This stage includes multi-layer hydration in which water is relatively mobile. In the third stage, empty capillaries were filled and swelling of the amorphous region was observed. This phenomenon gives way to a future hydration. This water located in the capillaries is relatively free to react (Labuza et al. 1985; Vega-Gálvez et al. 2008).
The adsorption and desorption curves did not coincide, thus enabling the formation of a hysteresis that indicated that more water was retained during desorption compared to adsorption, according to Fig. 2; chayote has a minor degree of adsorption with respect to pineapple and due to the final values of moisture in desorption in fructan-added treatments, where each is always on the maltodextrin value treatments. Labuza et al. (1985) established three reasons that give rise to the differences in water content between the two isotherms when dealing with foods: super-saturation of a w during drying, and capillaries which can empty differently towards desorption due to the differences in shape of the capillary and the effects of surface tension (Labuza et al. 1985; Vega-Gálvez et al. 2010). Due to the hysteresis representing a moment in which capillary condensation occurs, it has been used to evaluate pore-form in porous materials (Do 1998).
Hysteresis
Foods are complex matrices; the different components that constitute them behave differently and make it difficult to generalize water sorption behavior for a determined group of foods. The study of water sorption presents two phenomena: adsorption and desorption. The desorption isotherm is always larger than the adsorption isotherm, forming a loop called hysteresis that generally decreases when the temperature of the isotherm increases (Martínez et al. 1998; Aguirre-Cruz et al. 2010). This is due to the moisture content decreasing at a higher temperature to a constant aw. An exothermic phenomenon in moisture adsorption exists; increasing the isotherm temperature translates to a loss in moisture in equilibrium with a given relative humidity (Dalgıç et al. 2012). The hysteresis loop is observed beginning at the monolayer in the a w range from 0.18 to 0.90 (Fig. 2). In the same manner, it was observed that the hysteresis zone is larger in those samples that had the highest agave fructan concentration—showing a higher moisture content for the same a w value; this shows that the samples rehydrate. This is because foods that have capillaries in their structure close or contract upon water extraction. This makes it more difficult for water to re-enter and in many occasions not entering at all. This leaves a larger amount of water available and increases the value of water activity (Barbosa-Canovas et al. 2005; Caparino et al. 2012).
In order to interpret this difference, it is necessary to consider that the hysteresis is frequently explained in terms of filling and emptying empty spaces in foods’ structure. Thus, the dynamics of water molecules moving into capillaries’ interiors during the adsorption process can be illustrated. However, it is clearly observed that these capillaries are not efficiently emptied during the desorption process, thus giving rise to the hysteresis phenomenon. In this sense, the larger hysteresis observed in the chayote and pineapple samples with higher fructan concentration suggests that the capillaries of this porous material were not emptied the same way as the samples without fructans. This significant difficulty in eliminating water from the capillaries could have been caused by diverse factors such as pore-form or marked differences in the mobility of the powders’ structure.
The hysteresis curves can vary for different foods (Bello 2000); even identical foods can present different isotherm patterns when a variable in the process is altered. This diversity can be interpreted as a consequence of the variability that comes with different concentrations of chemical components, as well as some changes produced in the factors influencing capillary porosity that characterize the food.
This hysteresis loop presented in foods’ sorption isotherms as in pineapple and chayote powders is generally influenced by factors such as isotherm generation temperature. Considering that for two sorption isotherms obtained at different temperatures, different hysteresis loops have been observed for the same product, the existence of an exothermic phenomenon in moisture adsorption provides an explanation. Increasing the isotherm’s temperature translates to a loss of moisture at equilibrium with a given relative humidity, with an approximation of the isotherm towards the axis of water activity with the same moisture content.
GAB and BET Modeling
The degree of adjustment of each model was evaluated with the help of the correlation coefficient (R 2), which is recommended to be above 0.85 to achieve an adequate model of the experimental data. The isotherm predicted by the GAB model presents a better adjustment with the adsorption isotherm’s experimental data; a maximum R 2 value of 0.998 correlating the data was obtained in a majority of the samples. Meanwhile, the BET model presented a maximum R 2 of 0.965 (Tables 4 and 5).
For the GAB model, the value of C (Guggenheim constant) increased alongside an increment to fructan concentration with the exception of pineapple, with 2% fructans that presented a C value greater than the 4% fructans. The K-value (correction factor related to heat sorption of the multi-layer) showed a tendency to increase with the addition of fructans.
The parameter that represents the water content of the monolayer (m 0) rose with the increase of fructan concentration—indicating that a higher concentration increases the bonding energy of the first adsorbed layer; that is to say, the adsorbent-adsorbate interactions are weak and endothermic. This can be linked to the endothermic dissolution of fruit sugar in the absorbed water (Quirijns et al. 2005; Moreira et al. 2008). This agrees with recent studies that reported endothermic reactions for spray-dried tamarind pulp powder (Muzaffar and Kumar 2016). In some fructan varieties, a higher concentration of monosaccharides can exist, increasing the m 0 values in the spray-dried powder (Caparino et al. 2012). The amount of moisture in the monolayer was greater during the desorption process and linked to the increase in fructan concentration, indicating that water removal was not that efficient. In this sense, the samples with 4 and 2% fructans retained a larger amount of water during desorption as compared to the samples that only contained maltodextrin.
In all cases, as was expected, the value of the C parameter of the GAB model was much greater than 1, and the K constant had values smaller than or equal to 1. K considers the modified properties of the sorbate in the multi-layer region and the properties of the liquid. The results obtained in this study were found to be within the range reported for foods in different studies compiled by Rahman (2009). They also coincide with results in which the values of m 0 for pure pineapple pulp were reduced from 14.6–16.6 to 6.0–6.3% in juice containing 18% maltodextrin (Gabas et al. 2007).
The BET model is applicable in predicting the sorption isotherms in a range of water activity from 0.1 to 0.495. For water activity ranging from 0.1 to 0.9, the GAB model was applied since it is attributed with obtaining a better correlation. The GAB model makes better predictions, because it includes an additional constant (K) with a small average deviation of 6.5% from experimental results (Lomauro et al. 1985; Perdomo et al. 2009).
The glass transition of a product has been described as a phase change of a solid structure to a semi-solid or pseudo-plastic (Slade and Levine 1991; Roos 2010). Upon analysis of the isotherms of each one of the samples with different fructan concentrations, one can observe linearity in the water activity range from 0.2 to 0.82 in the sample without agave fructans, from 0.2 to 0.78 with 2% fructans and from 0.2 to 0.72 with 4% fructans. This shows that when fructan concentration is increased, an inflection in a lower water activity level is observed. Based on this inflection on the sample’s isotherm, it is believed that there is a transition phase equivalent to the glass transition phase in the sample. At the end of the test, the sample presented a plasticized or semi-solid texture. This inflection zone provides the critical a w information for a phase change in the fruit powder.
Conclusion
The addition of native agave fructans contributes to a significant decrease in the mass fraction of maltodextrin added in this type of industrial products. All fresh or reconstituted juices present a flow behavior typical of plastic fluids (Bingham model). The addition of maltodextrin (10%) and fructans (up to 4%) as well as the step of spray-drying did not change significantly the plastic viscosity of juices. Only the yield stresses, which represent the behavior of fluids at rest, were impacted by these parameters. The combination of native agave fructans with maltodextrin as a stabilizer produced spherical particles with shrinkage, lumps, and caking between them. The isotherms showed evidence of a non-porous food or a food with micropores. The sorption properties were modified in the multi-layer region as a result of physical adsorption. Consequently, the adsorbent-adsorbate interactions are weak (hydrogen bonds, dipole-dipole bonds, or Van der Waals forces), and therefore endothermic. These findings represent a breakthrough in the use of agave native fructans in the spray-drying process. However, agave native fructans are a mixture of fructans with high, medium, and low polymerization degrees. In this way, the behavior observed in this study gives the guideline to evaluate fractions with different degrees of polymerization, in order to determine the fraction with better technofunctionality.
References
Aguirre-Cruz, A., Alvarez-Castillo, A., Castrejón-Rosales, T., Carmona-García, R., & Bello-Pérez, L. A. (2010). Moisture adsorption behavior of banana flours (Musa paradisiaca) unmodified and modified by acid-treatment. Starch-Stärke, 62(12), 658–666.
Al-kahtani, H. A., & Hassan, B. H. (1990). Spray drying of roselle (Hibiscus sabdariffa L.) extract. Journal of Food Science, 55(4), 1073–1076.
Barbosa-Canovas, G., Ortega-Rivas, E., Juliano, P., & Yan, H. (2005). Food powders: physical properties, processing and functionality. Nueva York: Kluwer Academic/Plenum Publisher New York.
Barrosi, S. T., Mendes, E. S., & Peres, L. (2004). Influence of depectinization in the ultrafiltration of West Indian cherry (Malpighia glabra L.) and pineapple (Ananas Comosus (L.) Meer) juices. Food Science and Technology (Campinas), 24(2), 194–201.
Bello, G. J. (2000). Ciencia bromatológica—principios generales (1st ed., pp. 439). Madrid: Díaz de Santos S. A.
Bhandari, B. R., Senoussi, A., Dumoulin, E. D., & Lebert, A. (1993). Spray drying of concentrated fruit juices. Drying Technology, 11(5), 1081–1092.
Bizot, H., Lebail, P., Leroux, B., Davy, J., Roger, P., & Buleon, A. (1997). Calorimetric evaluation of glass transition in hydrated, linear and branched polyanhydroglucose compounds. Carbohydrates Polymers, 32, 33–50.
Brunauer, S., Deming, L. S., Deming, W. E., & Teller, E. (1940). On the theory of Vander Waals adsorption of gases. Journal of the American Chemical Society, 41, 1755–1760.
Cal, K., & Sollohub, K. (2010). Spray drying technique. I: hardware and process parameters. Journal of Pharmaceutical Sciences, 99(2), 575–586.
Caliskan, G., & Dirim, S. N. (2016). The effect of different drying processes and the amounts of maltodextrin addition on the powder properties of sumac extract powders. Powder Technology, 287, 308–314.
Caliskan, G., & Dirim, S. N. (2013). The effects of the different drying conditions and the amounts of maltodextrin addition during spray drying of sumac extract. Food and Bioproducts Processing, 91(4), 539–548.
Cano-Chauca, M., Stringheta, P. C., Ramos, A. M., & Cal-Vidal, J. (2005). Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innovative Food Science & Emerging Technologies, 6(4), 420–428.
Caparino, O. A., Tang, J., Nindo, C. I., Sablani, S. S., Powers, J. R., & Fellman, J. K. (2012). Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’var.) powder. Journal of Food Engineering, 111(1), 135–148.
Chen, C., & Lai, L. (2008). Mechanical and water vapor barrier properties of tapioca starch/ decolorized hsian-tsao leaf gum films in the presence of plasticizer. Food Hydrocolloids, 22, 1584–1595.
Crispín-Isidro, G., Lobato-Calleros, C., Espinosa-Andrews, H., Alvarez-Ramirez, J., & Vernon-Carter, E. J. (2014). Effect of inulin and agave fructans addition on the rheological, microstructural and sensory properties of reduced-fat stirred yogurt. LWT-Food Science and Technology, 62(1), 438–444.
Dalgıç, A. C., Pekmez, H., & Belibağlı, K. B. (2012). Effect of drying methods on the moisture sorption isotherms and thermodynamic properties of mint leaves. Journal of Food Science and Technology, 49(4), 439–449.
De Barros Fernandes, R. V., Borges, S. V., & Botrel, D. A. (2014). Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydrate Polymers, 101, 524–532.
DibTaxi, M. C. A., Santos, A. B., Menezes, H. C., & Grosso, C. R. F. (2000). Efeito da temperatura de secado e da percentagem de encapsulante no rendimento del jugo de camu-camu (Mirciaria dubia). In Anais do XVII Congresso Brasileiro de Ciencia e Tecnologia de Alimentos, SBCTA, Fortaleza, pp. 6–113.
Do, D. D. (1998). Adsoprtion analysis: equilibria and kinetics. London: Imperial College Press.
Echavarría, A. P., Falguera, V., Torras, C., Berdún, C., Pagán, J., & Ibarz, A. (2012). Ultrafiltration and reverse osmosis for clarification and concentration of fruit juices at pilot plant scale. LWT-Food Science and Technology, 46(1), 189–195.
Espinosa-Andrews, H., & Urias-Silvas, J. E. (2012). Thermal properties of agave fructans (Agave Tequilana Weber var. Azul). Carbohydrate Polymers, 87, 2671–2676.
Fernandes, R. V. B., Borges, S. V., Botrel, D. A., Silva, E. K., Costa, J. M. G., & Queiroz, F. (2013a). Microencapsulation of rosemary essential oil: characterization of particles. Drying Technology, 31, 1245–1254.
Fernandes, R. V. B., Borges, S. V., & Botrel, D. A. (2013b). Influence of spray drying operating conditions on microencapsulated rosemary essential oil properties. Ciência e Tecnologia de Alimentos, 33, 171–178.
Fazaeli, M., Emam-Djomeh, Z., Ashtari, A. K., & Omid, M. (2012). Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food and Bioproducts Processing, 90(4), 667–675.
Fuchs, M., Turchiuli, C., Bohin, M., Cuvelier, M. E., Ordonnaud, C., Peyrat-Maillard, M. N., & Dumoulin, E. (2006). Encapsulation of oil in powder using spray drying and fluidised bed agglomeration. Journal of Food Engineering, 75(1), 27–35.
Gabas, A. L., Telis, V. R. N., Sobral, P. J. A., & Telis-Romero, J. (2007). Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. Journal of Food Engineering, 82(2), 246–252.
Goula, A. M., & Adamapoulos, K. G. (2005). Spray drying of tomato pulp in dehumidified air: II—the effect on powder properties. Journal of Food Engineering, 66, 35–42.
Goula, A. M., & Adamopoulos, K. G. (2008). Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified air: II. Powder properties. Drying Technology, 26(6), 726–737.
Grabowski, J. A., Truong, V. D., & Daubert, C. R. (2008). Nutritional and rheological characterization of spray drying sweet potato powder. Food Science and Technology, 41, 206–216.
Guggisberg, D., Cuthbert-Steven, J., Piccinali, P., Bütikofer, U., & Eberhard, P. (2009). Rheological, microstructural and sensory characterization of low-fat and whole milk set yoghurt as influenced by inulin addition. International Dairy Journal, 19, 107–115.
Harte, F., Clark, S., & Barbosa-Canovas, G. V. (2007). Yield stress for initial firmness determination on yogurt. Journal of Food Engineering, 80, 990–995.
Kilburn, D., Claude, J., Mezzenga, R., Dlubek, G., Alam, A., & Ubbink, J. (2004). Water in glassy carbohydrates: opening it up at the nanolevel. Journal of Physical and Chemistry B, 108, 12436–12441.
Kurozawa, L. E., Park, K. J., & Hubinger, M. D. (2009). Effect of maltodextrin and gum arabic on water sorption and glass transition temperature of spray dried chicken meat hydrolysate protein. Journal of Food Engineering, 91(2), 287–296.
Labuza, T. P., Kaanane, A., & Chen, J. Y. (1985). Effect of temperatura on the moisture sorption isotherms and water activity shift of two dehydrated foods. Journal of Food Science, 50, 385–391.
Le Meste, M., Champion, D., Roudaut, G., Blond, G., & Simatos, D. (2002). Glass transition and food technology: a critical appraisal. Journal of Food Science, 67(7), 2444–2458.
Lomauro, C. J., Bakshi, A. S., & Labuza, T. P. (1985). Effect of temperatura on the moisture sorption isotherm equations. Part I: fruit, vegetable and meat products. Lebensmittel Wissenschaft und Technologie, 18, 111–117.
Martínez, N. N. Grau, M. A., Chirlat, B., & Fito, P. (1998). Termodinámica y cinética de sistemas alimento entorno (1st ed. pp. 144–213). Universidad Politécnica de Valencia, España.
Moreira, R., Chenlo, F., Torres, M. D., & Vallejo, N. (2008). Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. Journal of Food Engineering, 88(4), 514–521.
Mosquera, L. H., Moraga, G., & Martinez Navarrete, N. (2010). Effect of maltodextrin on the stability of freeze-dried borojó (Borojoa patinoi Cuatrec.) powder. Journal of Food Engineering, 97, 72–78.
Mujumdar, A. S. (1995). Handbook of industrial drying (pp. 263–309). New York: Ed. Marcel Dekker, Inc..
Muzaffar, K., & Kumar, P. (2016). Moisture sorption isotherms and storage study of spray dried tamarind pulp powder. Powder Technology, 291, 322–327.
Nollet, L. M., & Toldrá, F. (Eds.). (2015). Handbook of food analysis, -two volume set. Boca Raton: CRC Press.
Núñez-Santiago M. C., Méndez-Montealvo M. G. C., & Solorza-Feria J. (2001). Introducción a la Reología. México: Instituto Politécnico Nacional.
Perdomo, J., Cova, A., Sandoval, A. J., García, L., Laredo, E., & Müller, A. J. (2009). Glass transition temperatures and water sorption isotherms of cassava starch. Carbohydrate Polymers, 76(2), 305–313.
Phisut, N. (2012). Spray drying technique of fruit juice powder: some factors influencing the properties of product. International Food Research Journal, 19(4), 1297–1306.
Ponce S. J. A., Macías B. E. R., Soltero M., J. F. A., Fernández E. V. V, Zúñiga P. V. , & Escalona B. H. B. (2008). Physical-chemical and non-linear rheological properties of aqueous solutions of agave fructans. e-Gnosis, 6, 1–23.
Quirijns, E. J., Van Boxtel, A. J. B., Van Loon, W. K. P., & Van Straten, G. (2005). Sorption isotherms, GAB parameters and isosteric heat of sorption. Journal of the Science of Food and Agriculture, 85, 1805–1814.
Rahman, M. S. (Ed.). (2009). Food properties handbook. Florida: CRC press. Inc.
Roos, Y. H. (2010). Glass transition temperature and its relevance in food processing. Annual Review of Food Science and Technology, 1, 469–496.
Rosenberg, M., Kopelman, I. J., & Talmon, Y. (1985). A scanning electron microscopy study of microencapsulation. Journal of Food Science, 50(1), 139–144.
Roussenova, M., Murith, M., Alam, A., & Ubbink, J. (2010). Plasticization, antiplasticization, and molecular packing in amorphus carbohydraye-glycerol matrices. Biomacromolecules, 11(12), 3237–3247.
Sablani, S. S., Shrestha, A. K., & Bhandari, B. R. (2008). A new method of producing date poder granules: physicochemical characteristics of powder. Journal of Food Engineering, 87(3), 416–421.
Shamsudin, R., Ling, C. S., Adzahan, N. M., & Daud, W. R. W. (2013). Rheological properties of ultraviolet-irradiated and thermally pasteurized Yankee pineapple juice. Journal of Food Engineering, 116(2), 548–553.
Shrestha, A. K., Ua-Arak, T., Adhikari, B. P., Howes, T., & Bhandari, B. R. (2007). Glass transition behavior of spray dried orange juice powder measured by differential scanning calorimetry (DSC) and thermal mechanical compression test (TMCT). International Journal of Food Properties, 10(3), 661–673.
Silva, M. A., Sobral, P. J. A., & Kieckbusch, T. G. (2006). State diagrams of freeze-dried camu-camu (Myrcaria dubia (HBK) Mc Vaugh) pulp with and without maltodextrin addition. Journal of Food Engineering, 77(3), 426–432.
Slade, L., & Levine, H. (1991). Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Critical Reviews in Food Science and Nutrition, 30, 115–360.
Tan, S. P., Tuyen, C. K., Parks, S. E., Stathopoulos, C. E., & Roach, P. D. (2015). Effects of the spray-drying temperatures on the physicochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technology, 281, 65–75.
Tonon, R. V., Freitas, S. S., & Hubinger, M. D. (2011). Spray drying of açai (Euterpe oleraceae Mart.) juice: effect of inlet air temperature and type of carrier agent. Journal of Food Processing and Preservation, 35(5), 691–700.
Truong, V., Bhandari, B. R., & Howes, T. (2005). Optimization of co-current spray drying process of sugar-rich foods. Part I—moisture and glass transition temperature profile during drying. Journal of Food Engineering, 71(1), 55–65.
Tunc, S., & Duman, O. (2007). Thermodynamic properties andmoisture adsorption isotherms of cottonseed protein isolateand different forms of cottonseed samples. Journal. Food Engineering, 81, 133–143.
Valipour, M. (2016a). How much meteorological information is necessary to achieve reliable accuracy for rainfall estimations? Agriculture, 6(4), 53.
Valipour, M. (2016b). Variations of land use and irrigation for next decades under different scenarios. Irriga, 1(01), 262–288.
Valipour, M., Sefidkouhi, M. A. G., & Raeini, M. (2017). Selecting the best model to estimate potential evapotranspiration with respect to climate change and magnitudes of extreme events. Agricultural Water Management, 180, 50–60.
Vega, G. A., Lara, A. E., & Lemus, M. R. (2006). Isotermas de adsorción de harina de maíz (Zea mays L). Ciencia y Tecnología de Alimentos, Campinas, 26, 821–827.
Vega-Gálvez, A. L. M. S., Lemus-Mondaca, R., Bilbao-Sáinz, C., Fito, P., & Andrés, A. (2008). Effect of air drying temperature on the quality of rehydrated dried red bell pepper (var. Lamuyo). Journal of Food Engineering, 85(1), 42–50.
Vega-Gálvez, A., Miranda, M., Díaz, L. P., Lopez, L., Rodriguez, K., & Di Scala, K. (2010). Effective moisture diffusivity determination and mathematical modelling of the drying curves of the olive-waste cake. Bioresource Technology, 101(19), 7265–7270.
Yang, R.T. (1987). Gas Separation by Adsorption Processes. Stoneham: Butterworths Publishers.
Acknowledgements
The authors thank CONACyT (Mexico) for their support in conducting the work throughout project number 210874 and for the scholarship granted to JIMENEZ-SANCHEZ D. E.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jimenez-Sánchez, D.E., Calderón-Santoyo, M., Picart-Palmade, L. et al. Effect of Addition of Native Agave Fructans on Spray-Dried Chayote (Sechium edule) and Pineapple (Ananas comosus) Juices: Rheology, Microstructure, and Water Sorption. Food Bioprocess Technol 10, 2069–2080 (2017). https://doi.org/10.1007/s11947-017-1974-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1974-4






 ) 2% FT, and (
) 2% FT, and ( ) 4% FT
) 4% FT