Abstract
There are currently limitations to storing and feeding distillers wet grains (DWG) due to their potential for spoilage and mycotoxin contamination in storage. In this study, in-package treatments of DWG using high-voltage atmospheric cold plasma (HVACP) treatment and storage in modified atmosphere with carbon dioxide were investigated with the primary purpose of increasing product shelf-life. The conditions investigated were (1) HVACP treatment and modified atmosphere packaging (MAP) storage, (2) carbon dioxide-modified atmosphere storage, and (3) HVACP treatment and carbon dioxide-modified atmosphere storage, compared with a control sample with no treatment under MAP storage. Treated samples and controls were stored for 0, 7, 14, 21, and 28 days at 10 and 25 °C, after which treated samples were evaluated for their efficacy to control mold growth indicated by pH, microbial count (CFU/g)/log reduction, and peroxide (H2O2) level. There was a significant difference among the treatments indicated by CFU/g (P < 0.003) and pH (P < 0.0001). The HVACP treatment alone and its combination with carbon dioxide-modified atmosphere storage provided a better control of microbial growth and preservation in the wet substrate (>60% moisture), DWG for up to 28 days of storage at 10 and 25 °C. Both HVACP and the combination treatment showed high peroxide levels (100 mg/L) after treatment, which degraded in storage over time. It is thought that a better strategy for the combination treatment would be to treat substrate using HVACP with MAP having a high O2 concentration (65%) and store samples post-treatment in-package in a high CO2 environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Direct feeding of corn distillers wet grains with solubles (DWGS), a co-product of fuel ethanol manufacture from corn, is common due to their rich nutrient content, which after starch extraction is concentrated threefold (Klopfenstein et al. 2007). In the USA, it is common for cattle feedlots within an 80-km radius of an ethanol plant to source distillers grains in its wet form, DWGS. Due to the high moisture (65–70%, wet basis) of DWGS, spoilage can occur after 3–5 days in warm summer temperatures and 5–7 days in cold winter temperatures in the Midwest USA, and these storage periods have been set as the shelf-life values for DWGS (Lehman and Rosentrater 2012). This is one of the primary reasons why DWGS is preferably utilized in its dried form, distillers dried grains with solubles (DDGS). Spiehs et al. (2002) reported the chemical composition of DDGS from 118 samples collected from 10 ethanol plants in 1997, 1998, and 1999: 30.2% for protein, 10.9% for oil, 5.8% for ash, 8.8% for crude fiber, 16.2% for ADF, and 42.1% for NDF (% dry matter basis).
Over the years, there have been several modifications of the dry-grind corn-to-ethanol process, which have changed the final composition of the co-products, DWGS and DDGS. The changes mainly involved removing one or more of the unfermentable fraction of corn (germ and fiber) at the front-end prior to fermentation or at the back-end after fermentation. Stripping the oil from the condensed distillers solubles (CDS) by centrifuging is the most common industrial process used to extract more of the oil from DDGS, which is sold for feed or biodiesel production. In general, modified DDGS by these new processes have higher protein levels, and lower fat and fiber levels than the conventional DDGS (Liu and Rosentrater 2012).
Shelf-life is defined as the amount of time during which a food product will be certain to retain desired physical and microbiological characteristics (IFST 1993). Although shelf-life can be preserved when DWGS is dried to DDGS, drying DWGS increases feed cost because of the enormous amounts of fossil energy, primarily natural gas that is used in drying. Also, drying DWGS using high-temperature rotary drum dryers has been reported to affect the quality of DDGS (Kingsly et al. 2010). There are currently no standards for determining the shelf-life of DWG or defining what it is. Lehman and Rosentrater (2012) monitored carbon dioxide production and mold colonization to determine the storage period for DWG. They showed that the aerobic stability of DWG decreased with an increase in temperature, from >10 days at 12 °C to 2 days at 32 °C. Harding (2012) noted that spoilage of grains is classified as a process that causes food to be undesirable or unacceptable for human or animal consumption. Factors such as visible bacteria, molds, or yeasts growing on the food and metabolizing it for their own energy mean the shelf-life of the product has been reached or expired. McClurkin and Ileleji (2015) showed that colony-forming units (CFU/g) and pH can be used as indicators of deterioration of DWG in storage.
Carbon dioxide has been used in modified atmospheric storage to suppress the growth of aerobic microorganisms including mold in food products (Pardo and Zufia 2012). Carbon dioxide concentration above 14% can be detrimental to mold growth (Christensen and Kalscheur 1974). Likewise, ozone (O3) gas has also been shown to significantly reduce microbial growth in corn (McClurkin 2009). Ozone along with many other reactive gas species (RGS) such as nitrogen oxides, singlet oxygen, and peroxides can be formed in atmospheric plasma. Atmospheric cold plasma can be formed by discharging electrical energy through a dielectric medium into a gas causing ionization, dissociation, and energizing of the gas molecules (Grabowski et al. 2007). Plasma consists of ions, electrons, neutral species, and UV-visible light. Gordillo-Vazquez (2008) used a chemical model to determine that air plasmas can produce more than 75 ionizing species and 500 chemical reactions. Research into inactivation of Escherichia coli, Salmonella sp., and Listeria monocytogenes (Ziuzina et al. 2013) has shown successful results in reducing the loads of these pathogens. Because of environmental compliance, cost, and ease of processing, the use of atmospheric cold plasma (ACP), that is, plasma generated at room temperature and pressure, is being explored in the areas of environmental, biological, food processing, and clinical and health care (Lu et al. 2013; Muller and Zahn 2007; Eto et al. 2008; Sensenig et al. 2008; Dobrynin et al. 2011).
Unlike the previous systems of ozone application reported, Klockow and Keener (2009) developed an atmospheric cold plasma device, which utilized much higher voltages (>15 kV) and could generate ozone and other reactive gas species inside sealed packages. It was referred to as high-voltage atmospheric cold plasma (HVACP). HVACP was demonstrated to reduce E. coli O157:H7 populations on packaged spinach. HVACP was unique in its ability to generate ozone and other RGS inside the container. More recent HVACP studies have been performed with voltages greater than 100 kV. The product is introduced to the HVACP system in a bag (in-package), which contains air or modified gas consisting of nitrogen, oxygen, and carbon dioxide at various ratios. HVACP treatment generates a variety of RGS including but not limited to ozone (O3), singlet oxygen (O or O−), superoxide (O2 −), peroxide (O2 −2 or H2O2), and hydroxyl radicals (OH) (Keener and Klockow 2010). Of these species listed, ozone has the longest half-life; however, as the gas composition changes other RGS may have a long half-life. Advantages of the HVACP system are as follows: (1) very high RGS concentrations (>10,000 ppm) are achieved at the target sight without transport to the target area and (2) the containment of the RGS for an extended period of time inside the treatment container. Therefore, HVACP treatment of dry and wet grains, processed grains, and packaged foods could be a novel approach to sterilizing products in order to increase shelf-life stability and prevent storage molds and contamination from mycotoxins. Previous research has shown HVACP to be effective in microbial inactivation (Fernandez et al. 2012; Ziuzina et al. 2013; Han et al. 2014). Currently, there are no published studies on the efficacy of using HVACP treatment for increasing the shelf-life of distillers wet grains (DWG).
The primary objectives of this research were to investigate the efficacy of (1) HVACP treatment, (2) carbon dioxide-modified atmosphere storage, and (3) HVACP treatment and carbon dioxide-modified atmosphere storage, on microbial deactivation and suppression in order to extend the shelf-life of wet distillers grains (DWG). The efficacy of these treatments conducted in-package was evaluated based on their effect on pH changes, microbial count (CFU/g)/log reduction, and peroxide levels on the treated substrate, DWG. A control sample that was not treated but had substrate stored at 10 and 25 °C under the same in-package modified gas blend used in the HVACP treatment was compared alongside the treatments in a post-treatment storage evaluation for various periods up to 28 days. We hypothesized that the shelf-life of DWG can be increased by eradicating any microorganisms on the product using HVACP treatment and suppressing the growth of any remaining aerobic microorganisms in storage with carbon dioxide-modified atmosphere.
Materials and Methods
Distillers wet grains were obtained from a local ethanol plant (New Energy Corp, South Bend, IN) and stored in a freezer at −20 °C until the time of the experiment. Three different treatment conditions and a control sample (no treatment) were applied to a 25 g sample of DWG. In order to evaluate the effect of the treatments on shelf-life in storage, the treated samples were stored for various periods (7, 14, 21, and 28 days) at two different storage temperatures (10 and 25 °C). These treatment combinations were implemented using a completely randomized design. The conditions applied were: (1) HVACP treatment in a modified gas blend (65% O2/30% CO2/5% N2) referred to as MAP, (2) carbon dioxide-modified atmosphere storage, and (3) HVACP treatment in a MAP (32.5% O2/65% CO2/2.5%N2). A sample which was not treated but stored under the same MAP gas environment (65% O2/30% CO2/5% N2) as the HVACP treatment was used as the control to compare with the three treatments. The O, CO2, and N ratios in the MAP blends were determined based on preliminary studies, which indicted that MAP with 65% O2/30% CO2/5% N2 produced a high level of ozone, one of the reactive gas species (RGS) found to be effective in deactivating fungi (not published). Thus, the 3 treatments and the control (4 treatments) yielded a total of 120 test samples for the storage tests: 4 treatments × 2 storage temperatures × 5 storage periods = 40 experimental runs × 3 replications = 120.
In-Package Treatments of DWG
High-Voltage Atmospheric Cold Plasma
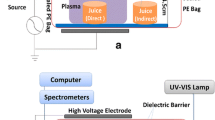
The HVACP treatments of samples were conducted as shown in Fig. 1 (Lu et al. 2013). The system consists of a high-voltage generator, which applies the alternating current potential to a pair of metallic plates. To conduct treatments of samples, 25 g of DWG was placed in a weigh boat and the weigh boat was placed in a 30.5 cm × 28.0 cm Cryovac® bag (Sealed Air, Duncan, SC). The Cryovac® bag (packages) that were to be ionized were filled with a modified gas blend (MAP) of 65% O2/30% CO2/5% N2 and purged in order to ensure purity of the gas in the bag. The Cryovac® bag was then filled with MAP for 90 s to obtain a gas volume of 3.89 L. Flow was measured with a flow meter (Gilmont Instruments, Inc., Barrington, IL). This volume provided a gap space of 4.6 cm between the electrodes of the HVACP system, which preliminary tests showed was capable of reducing microbial load. Gap space is the distance from the bottom to the top of the package when measured between the electrodes. The dielectric barriers used in the HVACP system are described in Fig. 1. The sealed packages were placed between the electrodes of the HVACP system for treatment at 70 kV for 360 s. After treatment, the bags were removed and one end was rolled up and taped to reduce gas loss during storage, after which it was placed in a Ziploc® bag in preparation for shelf-life test. For shelf-life studies, the bags were placed in a temperature chamber and stored at either 10 or 25 °C for a set period of time (7, 14, 21, or 28 days).
Schematic diagram of a high-voltage atmospheric cold plasma system. Note that image is not to scale (adapted from Lu et al. 2013).
Carbon Dioxide-Modified Atmosphere Storage
Cryovac® bags of DWG used for storage under modified atmosphere with carbon dioxide gas only were filled with 7 g of dry ice. They were then sealed and placed inside Ziploc® bags in preparation for shelf-life test. As the dry ice came to room temperature, the bag was filled with the carbon dioxide gas. Bag volume reached 3.89 L. After carbon dioxide was incorporated, one end of the Cryovac® bag was rolled up, taped, and placed in a Ziploc® bag, in preparation for shelf-life test. The bags were then placed in a temperature chamber and stored at either 10 or 25 °C for a set period of time (7, 14, 21, or 28 days).
HVACP Treatment and Carbon Dioxide-Modified Atmosphere Storage (Combination Treatment)
DWG samples in Cryovac® bags for combination treatments were filled with 3.5 g of dry ice and sealed, then filled with 1.95 L of MAP using a syringe, so that the total volume in the package was still ∼3.89 L, resulting in a final gas composition concentration of 32.5% O2/65% CO2/2.5% N2. The procedures used for the HVACP treatment were repeated for the combination treatment. The bag was then placed on the HVACP system as in Fig. 1 and treated at 70 kV for a treatment time of 360 s. After treatment, the same procedure used for preparing treated samples for the shelf-life studies was implemented.
The Control Sample
Control samples of DWG in bags were filled with MAP (65% O2/30% CO2/5% N2) to obtain the 3.89 L gas volume found in all treatments and sealed. Neither dry ice or HVACP treatments were applied. It should be noted that the MAP gas was made up of the same concentration of gases as in the HVACP treatment. One end of the Cryovac® bag was rolled up, taped, and placed in a Ziploc® bag in preparation for shelf-life test. The bag was then placed in a temperature chamber and stored at either 10 or 25 °C for a set period of time (7, 14, 21, or 28 days).
Storage Tests for Shelf-Life Evaluation
Three replications were performed per treatment and storage condition under two temperatures (10 and 25 °C). The two temperatures were selected from a previous research by the authors McClurkin and Ileleji (2015) to evaluate the shelf-life of DWGS stored in cool (10 °C) and warm (20 and 25 °C) temperatures. Storage at 20 °C was not conducted because the previous study indicated that deterioration results of storage at 20 and 25 °C were not significantly different. The sealed treated Cryovac® bags inside the Ziploc® bags were placed in a single layer, in a Percival Scientific biological incubator (Percival Scientific Inc., 1-36VL, Boone, IA) and stored under 10 and 25 °C for periods of 0, 7, 14, 21, and 28 days.
For each sample treatment and storage condition, three replicate samples were used to determine moisture content, pH, and microbial load (CFU/g), which were the parameters used to evaluate the effectiveness of microbial control by the treatments. Initial moisture content of the DWG samples treated was 59 to 64% (w.b.). HVACP treatment of products with high moistures can result in the formation of peroxides, H2O2 (Chen 2014). Because peroxides have bactericidal effects, the level of peroxides formed due to HVACP treatments was measured. Therefore, during the storage tests, H2O2 levels were measured in the HVACP-treated bags only (HVACP and combination treatments) using Quantofix® Peroxide test strips (Macherey-Nagel GmbH & Co., KG). Values of H2O2 levels were averaged from two test strips, which measured peroxide levels at 0, 1, 3, 10, 30, or 100 mg/L. To determine H2O2 levels, the H2O2 strip was swabbed on the inside of the bag where condensation had formed. After the storage period, the sample was removed from the incubator and from the exterior Ziploc® bag. The Cryovac® bag was then cut open, and the DWG sample in the weigh boat was removed and set aside for conducting tests used in the assessment of microbial control as a measure of shelf-life improvement.
Assessment of Shelf-Life for Treatments
To assess the shelf-life of DWG for the three treatments and the control investigated, analysis for pH and microbial load (CFU/g) before storage (day 0) and after 7, 14, 2,1 and 28 days of storage was determined. Moisture content (MC) was determined using the NFTA 2.2.2.5 method (Shreve et al. 2006) where 2 g of the sample was placed in an air oven for 3 h at 105 °C. All moistures were determined on a wet basis. Since DWG come from the processing plant acidic and microorganisms grow best at more neutral pH levels, pH was monitored throughout the tests. The pH was measured with a probe pH meter (pH Electrode LE427, Mettler Toledo, Columbus, OH) used for solids. The microbial load was attained through dilution plating followed by counting colony-forming units per gram of DWG (CFU/g). To measure the change in microbial populations, 5-g samples were taken and placed in a sterile stomacher bag filled with 45 mL of 0.1% peptone water. Samples were stomached for 2 min, and aliquots were removed from the bag and transferred to a sterile 2-mL tube. The wash was then serially diluted, and 10 μL of each dilution was plated onto plate count agar (Sigma-Aldrich, St. Louis, MO) to obtain a count of microbial growth. After 3 days of incubation at 28 °C, the total number of colonies present was counted, giving CFU per gram values.
Statistical Analysis
Statistical analysis was conducted with two-way ANOVA with heterogeneous variances using SAS v9.1® (SAS Institute, Cary, NC, 2004). Analyzed classes were for HVACP treatment, carbon dioxide-modified atmosphere storage, HVACP treatment, carbon dioxide-modified atmosphere storage, and MAP storage (control). The effects analyzed as a measure of shelf-life improvement due to the treatments were CFU per gram, pH, and peroxide levels.
Results and Discussion
The objectives of this study were to examine the effect of HVACP treatment and its combination with carbon dioxide-modified atmosphere in storage on DWG shelf-life as measured by mold growth on stored samples post-treatment from 0 to 28 days in 7-day intervals. Changes in properties of the fungal species can be influenced by environmental factors as well as sample conditions, similar to that noted by Beuchat et al. (1999). Mold growth was expressed as CFU per gram, and pH change from acidic to basic was also measured as an indicator of mold growth on the stored samples. The log reduction in microbial load from day 0 to other storage periods (7, 14, 21, and 28) was determined and used to evaluate the treatment effectiveness. The H2O2 level, which is formed in the package when RGS reacts with moisture, was also used to evaluate the treatment effects of HVACP treatments. H2O2 is known to have bactericidal effects (Linley et al. 2012).
The Effect of Treatments on pH Levels During Storage
Within both the storage temperature and treatment and the storage period, the mean pH (P < 0.0001) was significantly different (Table 1). In general, the HVACP treatment had the lowest increase in pH after 28 days for treated DWG samples stored at both 10 and 25 °C. While pH increased up to 7.21 (by 3.46 points) for DWG stored under CO2-modified atmosphere storage at 25 °C after 28 days, this was not the case for storage at 10 °C, which increased to a pH of 4.73 (by 0.42 points). For the combination treatments of HVACP and CO2-modified atmosphere, pH increased by about 1.56 and 1.63 for treated DWG samples stored at 10 and 25 °C, respectively. It appears that the HVACP contributed to the low change in pH for these treatments.
Previous literature indicates that an increase in pH over time correlates to microbial proliferation. As the microbial species continue to reproduce, they continually create an environment more suitable for their growth, thereby adjusting the pH of the substrate on which they are growing (Kung 2010). The pH of the control samples of DWG increased in storage over time from 3.76 at the beginning of storage (0 day) to 7.29 (up by 3.5 points) and 6.40 (up by 2.64 points) for samples stored at 10 and 25 °C, respectively, after 28 days of storage. This corroborates with the work by Christensen et al. (2010) and Yelden et al. (2011) indicating that spoilage is likely to have occurred (Rosentrater and Lehman 2010; Kung 2010). Vylkova et al. (2011) found that pH of Candida albicans can increase from 4 to >7 within 12 h. Prusky and Yakoby (2003) noted that fungi could increase or decrease its surrounding pH to create an environment more suitable for its growth. Over time as the spoilage species proliferate, they adjust the pH in the substrate creating conditions conducive to their survival and continued growth (Jay et al. 2005).
The Effect of Treatments on CFU/g Counts During Storage
Within both the storage temperature and treatment and the storage period, the mean CFU per gram counts (P < 0.0003) were significantly different (Table 2). Interpreting CFU per gram counts on a substrate like distillers grains can be a challenge because of the potential population of residual yeast cells from fermentation that could compete with microbial populations in storage. Unfortunately, the microbial populations on our substrate, DWG, was not identified. Table 2 shows the changes in CFU per gram counts for the three treatments and the control stored at 10 and 25 °C over five storage periods from 0 to 28 days. The results should be interpreted based on the reduction in CFU per gram at the beginning of storage (0 days) immediately after the treatment and the suppression of microbial growth over time. As was expected, immediately after the treatments, the HVACP-treated samples had the lowest microbial counts, followed by the combination-treated samples (HVACP- and CO2-modified atmosphere), while the control samples had the highest initial microbial counts followed by the CO2-modified atmosphere storage. However, suppression of microbes by the treatments over time cannot be clearly differentiated by observing the CFU per gram alone. In order to understand the effects of the treatments on the CFU per gram, the log reduction was determined for the treatments.
Table 3 shows the log reduction of CFU per gram counts for days 0, 7, 14, 21, and 28 for samples with different treatment conditions. The log reduction represents the difference between the treated sample and the control sample for each of those days as expressed using Eq. 1.
where A c represents the microbial load value (CFU/g) for the control sample and B T represents the microbial load value (CFU/g) for the treated samples. The highest log reduction for HVACP and the combination treatment was observed for day 0 and 7, respectively. While the initial log reduction for the HVACP treatment was higher than that for the combination treatment, the combination-treated samples were able to suppress microbial growth better over time than the HVACP-treated samples. For some reason that could not be explained, there was a rebound in microbial growth for HVACP-treated samples measured for CFU per gram at 14 days. CO2 alone appeared to be effective in suppressing microbial growth over the storage time of 28 days. Given that the combination-treated samples gave the best efficacy with respect to suppressing microbial growth over the storage time (2.36 and 1.20 logs stored at 10 and 25 °C, respectively), this could be explained by the synergistic effect of using HVACP to reduce the initial microbial loads on the stored substrate (DWG) prior to storage and suppressing growth (or rebound) on the substrate by using a CO2-rich environment.
Previous research has shown HVACP to be effective in microbial inactivation (Deng et al. 2007; Joshi et al. 2011; Ziuzina et al. 2013; Han et al. 2014). The use of carbon dioxide as a modified atmosphere to reduce microbiological growth in packaged grains has great potential. Carbon dioxide is related to the availability of oxygen within the microenvironment where the microbiological species are growing. An oxygen-depleted environment, with high levels of carbon dioxide, can result in reduced growth of these common stored grain molds. This can occur when the product is placed in a sealed package. Carbon dioxide concentration above 14% is detrimental to mold growth (Nofsinger et al. 1983); however, for some mold species, growth can occur at an oxygen concentration of about 0.2%. To date, there is no literature on the treatment of DWG with HVACP or a combination of HVACP- and CO2-modified atmosphere storage. Also, there are no known studies, which have investigated the efficacy of using HVACP or ionization for increasing the shelf-life of grains, oilseeds, and processed grains, especially for products highly susceptible to spoilage such as wet granular feed. The generation of plasma inside a sealed package using HVACP produces other reactive gas species besides the singular production of ozone gas, thus providing a better microbial load reduction. The synergistic effect of using both HVACP treatment and CO2-modified atmosphere storage has some potential, especially in fuel ethanol plants that readily have a cheap access of CO2 produced during fermentation. The potential for utilization of HVACP treatment and its combination with CO2-modified atmosphere extends beyond packaged food applications to other industrial applications, medical equipment, biofilms, and beyond.
The Effect of HVACP Treatments on Peroxide (H2O2) Production
Although HVACP treatments with the MAP maximize ozone species produced, there are many other species that are created when HVACP is used (Keener and Klockow 2010). These species can contribute to the bactericidal changes on the product. Other species ionized in the MAP that could have contributed to reducing the microbial loads on the DWG samples include peroxides and nitrides that are produced with HVACP. The science is currently not developed to identify the concentration of many other species produced by HVACP treatments in various gases due to its complexity (Keener and Klockow 2010).
Due to the high initial moisture content of DWG, it was important to monitor the peroxide (H2O2) levels, which also have bactericidal effects (Linley et al. 2012). Peroxides are created during the HVACP process when there is water present in the package, which was the case for the treated substrate, DWG, used in this study. The moisture content of DWG used in all treatments was above 50% (wet basis) (Fig. 2a, b). There was a minimal change in moisture content of the substrate post-treatment and throughout the storage period. Water produces OH radicals, which also have strong bactericidal effects (Tanino et al. 2007). It is therefore important to monitor the peroxide levels in high moisture products. Table 4 gives the peroxide levels from residual water droplets inside the package at the time of testing from the beginning of the storage post-treatment to 28 days of storage. Over the course of the 28-day storage period, the peroxide levels reduced from 100 mg/L (the maximum scale on the test strip) to 1 mg/L (the minimum scale on the strip is 0). Higher levels of peroxides present in the package indicate the retaining of bactericidal effect from the HVACP treatment. This shows that as long as there is still moisture in the sample and RGS, some level of peroxides will still be present in the package for up to 28 days.
The levels of peroxides retained reduced over time for both HVACP and the combination of HVACP and CO2 treatment. HVACP treatment alone appears to retain more peroxides over time than the combination treatment at both storage temperatures. However, the warmer (25 °C) storage condition retained more peroxides than the cooler (10 °C) storage condition. Since peroxides are formed from the reaction between OH radicals produced from water generated in HVACP, more free water released from the DWG under the warm storage condition created more peroxides than was created due to low amounts of water released under the cool storage condition. Because the scale on the strip used for peroxide measurement was not continuous, accurate quantification of the actual levels of peroxides created post-treatment (0 day) and retained over time for the 28 days of storage was not possible.
Theoretically, the levels of peroxides created in the package would depend on the amount of available free water in the package that the plasma converts to OH radicals to produce peroxides. Also, the amount of RGS created depends on the gas composition concentration in the package, that is, MAP. McClurkin-Moore (2015) showed that a MAP of 60% O2/35% CO2/5% N2 created more RGS and was more potent at inactivating microbes as expressed by methylene blue discoloration and CFU per gram count compared to “air” used as the in-package gas. A higher oxygen concentration is necessary for the production of RGS, especially ozone (O3), which is one of the RGS produced in HVACP treatment that has been identified in deactivating microbes and for its bactericidal effect. The MAP (32.5% O2/65% CO2/2.5%N2) used for the combination treatment had about half of the concentration of oxygen compared to the MAP (65% O2/30% CO2/5% N2) used for HVACP treatment alone. Therefore, the peroxide level in the HVACP treatment was most likely higher than the level in the combination treatment, which is reflected by the higher residual levels for the HVACP after 28 days. A better approach for the combination treatment is to treat the substrate (DWG) using the MAP with the higher oxygen concentration, that is, 65% O2/30% CO2/5% N2, and then add CO2 in the package (CO2-modified atmosphere) post-treatment to suppress microbial growth in storage. This might give a better synergistic treatment than the combination treatment conducted in this study, which applied HVACP with a CO2-enriched MAP.
Conclusions
This investigation shows that HVACP treatment alone or in combination with carbon dioxide-modified atmosphere storage could be effective in extending the storage shelf-life of DWG or, in general, wet food and feed. The three measures used to evaluate the efficacy of the treatments on shelf-life—pH, mold count (CFU/g)/log reduction, and peroxide level—showed that HVACP alone or in combination with carbon dioxide-modified atmosphere storage controlled microbial growth for up to 28 days of storage at both 10 and 25 °C. Because of the complex nature of the substrate (DWG), a co-product residue after fermentation of corn starch to ethanol using the dry-grind process, the microbial counts (CFU/g) showed no clear differentiation in efficacy between the three treatments. However, in comparison to the control sample (no treatment), HVACP alone and its combination with CO2-modified atmosphere storage provided better control of microbial growth through the storage period. In particular, the log reduction showed that the combination treatment gave a better control of microbial growth in comparison to HVACP alone or CO2-modified atmosphere storage. The pH, which was used as an indicator of microbial growth, showed that a better control was achieved for both HVACP alone and its combination with CO2-modified atmosphere storage. Using the peroxide level created in the package from the interaction of RGS with moisture, an initially high bactericidal environment was created for both HVACP alone and its combination with CO2-modified atmosphere. However, it appears that the peroxide levels for the combination treatment degraded faster or might have not been as high as that created in the HVACP treatment. This can be explained by the lower concentration of O2 (32.5%) in the MAP used for the combination treatment compared with 65% O2 used in the MAP for HVACP alone. A better strategy for the combination treatment would be to treat the substrate using a MAP with a high O2 concentration (65%) and store samples in-package post-treatment in a high CO2 environment. This would better reflect the strategy of the combined treatment of HVACP with CO2-modified atmosphere storage: use HVACP to reduce microbial load and carbon dioxide-modified atmosphere in storage to suppress any rebound of microbial growth.
References
Beuchat, L., Chmielewski, R., Keswani, J., Law, S. E., & Frank, J. F. (1999). Inactivation of aflatoxigenic Aspergilli by treatment with ozone. Letters in Applied Microbiology, 29, 202–205.
Chen, Y. (2014). High voltage atmospheric cold plasma treatment of refrigerated chicken eggs for control of salmonella enteritidis on external surface. MS Thesis, Purdue University, 2014, Dissertations & Theses @ CIC Institutions, ProQuest.
Christensen, C. M., & Kalscheur, K. F. (1974). Storage of cereal grains and their products. American Association of Cereal Chemists Microflora, 158–192.
Christensen, D. L., Rolfe, K. M., Klopfenstein, T. J., & Erickson, G. E. (2010). Evaluation of storage of covers when wet distillers byproducts are mixed and stored with forages. Nebraska Beef Rep, MP93, 21.
Deng, S., Ruan, R., Mok, C. K., Huang, G., Lin, X., & Chen, P. (2007). Inactivation of Escherichia coli on almonds using nonthermal plasma. Journal of Food Science, 72(2), 62–66.
Dobrynin, D., Wasko, K., Friedman, G., Fridman, A., & Fridman, G. (2011). Cold plasma steriliza of open wounds: live rat model. J Plasma Med, 1(2), 109–114.
Eto, H., Ono, Y., Ogino, A., & Nagatsu, M. (2008). Low temperature internal sterilization of medical plastic tubes using a linear dielectric barrier discharge. Plasma Processes and Polymers, 5(3), 269–274.
Fernandez, A., Shearer, N., Wilson, D. R., & Thompson, A. (2012). Effect of microbial loading on the efficiency of cold atmospheric gas plasma inactivation of Salmonella enterica serovar Typhimurium. International Journal of Food Microbiology, 152(3), 175–180.
Gordillo-Vazquez, F. J. (2008). Air plasma kinetics under the influence of sprites. Journal of Physics D: Applied Physics, 41, 234016.
Grabowski, L. R., Van Veldhuizen, E. M., Pemen, A. J. M., & Rutgers, W. R. (2007). Breakdown of methylene blue and methyl orange by pulsed corona discharge. Plasma Sources Science Technology, 16, 226–232.
Han, L., Patil, S., Keener, K. M., Cullen, P. J., & Bourke, P. (2014). Bacterial inactivation by high voltage atmospheric cold plasma: influence of process parameters and effects on cell leakage and DNA. Journal of Applied Microbiology, 116(4), 784–794. doi:10.1111/jam.12426.
Harding, J.L. (2012). Spoilage of wet distillers grains plus solubles when stored in a bunker. MS. Thesis, University of Nebraska, Theses and Dissertation. http://ag.udel.edu/anfs/faculty/kung/documents/05AerobicStability.pdf.
IFST (1993). Shelf-life of foods—guidelines for its determination and prediction. Institute of Food Science and Technology, London. ISBN 0 905367 11 1.
Jay, J. M., Loessner, M. J., & Golden, D. A. (2005). Modern food microbiology (7th ed.). New York, NY: Springer.
Joshi, S. G., Cooper, M., Yost, A., Paff, M., Ercan, U. K., Fridman, G., Friedman, G., Fridman, A., & Brooks, A. D. (2011). Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrobial Agents Chemotherapy., 55(3), 1053–1062.
Keener, K. M., & Klockow, P. A. (2010). Non-equilibrium plasma generation in a sealed package. U.S. patent no. U.S. patent application 64796. Washington, D.C: U.S. Patent and Trademark Office.
Kingsly, A. R. P., Ileleji, K. E., Clementson, C. L., Garcia, A., Maier, D. E., Stroshine, R. L., & Radcliff, S. (2010). The effect of process variables during drying on the physical and chemical characteristics of corn dried distillers grains with solubles (DDGS)—plant scale experiments. Bioresource Technology, 101, 193–199.
Klockow, P. A., & Keener, K. M. (2009). Safety and quality assessment of packaged spinach treated with a novel ozone-generation system. LWT - Food Science and Technology, 42, 1047–1053.
Klopfenstein, T. J., Erickson, G. E., & Bremer, V. R. (2007). Feeding corn milling byproducts to feedlot cattle. Veterinary Clinics of North America: Food Animal Practice, 23, 223–245.
Kung, L. (2010) Aerobic stability of silages. Proceedings of the Conference on Silage for Dairy Farms. Retrieved from http://alfalfa.ucdavis.edu/+symposium/proceedings/2010/10-89.pdf
Lehman, R. M., & Rosentrater, K. A. (2012). Aerobic stability of distillers wet grains as influenced by temperature. Journal of Science Food Agriculture, 93, 498–503.
Linley, E., Denyer, S. P., McDonnell, G., Simons, C., & Maillard, J.-Y. (2012). Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. Journal of Antimicrobial Chemotherapy, 67(7), 1589–1596.
Liu, S. and K. A. Rosentrater. 2012. Chemical composition of DDGS. In: Distillers grains—production, properties and utilization. CRC Press, Copyright by Taylor and Francis Group LLC.
Lu, H., Patil, S., Keener, K. M., Cullen, P. J., & Bourke, P. (2013). Bacterial inactivation by high-voltage atmospheric cold plasma: influence of process parameters and effects on cell leakage and DNA. Journal of Applied Microbiology, 116, 784–794.
McClurkin, J. D. (2009). Control of stored grain fungi and off odors with ozone in a grain treatment system. MS Thesis Purdue University, 2009. Dissertations & Theses @ CIC Institutions, ProQuest.
McClurkin-Moore, J.D. (2015). Shelf-life improvement of distillers wet grains with solubles. PhD Diss. Purdue University, 2015. Dissertations & Thes]ProQuest.
McClurkin, J.D. and K.E. Ileleji. (2015). The effect of storage temperature and percentage of condensed distillers solubles on the shelf-life of distillers wet grains stored aerobically. Journal of Stored Product Research, 62(2015), 58–64.
Müller, S., & Zahn, R. J. (2007). Air pollution control by non-thermal plasma. Contributions to Plasma Physics, 47(7), 520–529.
Nofsinger, G. W., VanCauwenberge, J. E., Bothast, R. J., & Kwolek, W. F. (1983). An evaluation of chemical methods to extend the allowable storage time of wet distillers’ grains. J. Agric. Food Chemisty, 31, 276–279.
Pardo, G., & Zufia, J. (2012). Life cycle assessment of food-preservation technologies. Journal of Cleaner Production., 28, 198–207.
Prusky, D., & Yakoby, N. (2003). Pathogenic fungi: leading or led by ambient pH? Molecular Plant Pathology, 4(6), 509–516.
Rosentrater, K., & Lehman, R. (2010). Predicting stability of distillers wet grains (DWG) with color analysis. Food and Bioprocess Technology, 3(2), 204–212.
Sensenig, R., Kalghatgi, S., Fridman, G., Shereshevsky, A. Brooks, A., Vailets, V., Gutsol, A., Fridman, A., & Friedman, G. (2008). Induction of apoptosis in melanoma cells by non-thermal atmospheric plasma discharge. SSO’s 61st Annual Cancer Symposium, Chicago, USA. March 13–16.
Shreve, B., Thiex, N., & Wolf, M. (2006). National forage testing association reference method, dry matter by oven drying for 3 hr at 105 C. NFTA reference methods. Omaha, NB: National Forage Testing Association URL:www.foragetesting.org.
Spiehs, M. J., Whitney, M. H., & Shurson, G. C. (2002). Nutrient database for distiller’s dried grains with solubles produced from new ethanol plants in Minnesota and South Dakota. Journal of Animal Science, 2002(80), 2639–2645.
Tanino, M., Xilu, W., Taskashima, K., Katsura, S., & Mizuno, A. (2007). Sterilization using dielectric barrier discharge at atmospheric pressure. International Journal of Plasma Environmental Science and Technology, 1, 102–107.
Vylkova, S., Carman, A. J., Danhof, H. A., Collette, J. R., Zhou, H., & Lorenz, M. C. (2011). The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by reusing extracellular pH. MBio, 2(3), 00055–00011. doi:10.1128/mBio.00055-11.
Yelden, J. R., Buckner, C. D., Rolfe, K. M., Christensen, D. L., Klopfenstein, T. J., & Erickson, G. E. (2011). Nutrient composition of spoiled and non-spoiled wet by-products mixed and stored with straw. Nebraska Beef Ref, MP94, 18.
Ziuzina, D., Patil, S., Cullen, P. J., Keener, K. M., & Bourke, P. (2013). Atmospheric cold plasma inactivation of Escherichia coli in liquid media inside a sealed package. Journal of Applied Microbiology, 114(3), 778–787.
Acknowledgments
We would like to thank Mr. Russ Abarr of the New Energy Corp in South Bend, Indiana (New Energy Corp is no longer in operation), for supplying the distillers wet grains that were used in this study. We would also like to thank Jeanette Jensen, Research Associate/Lab Manager for the Food Technology and Development Laboratory at Purdue University, for assisting and training in using the Purdue high-voltage and atmospheric cold plasma (HVACP) system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McClurkin-Moore, J.D., Ileleji, K.E. & Keener, K.M. The Effect of High-Voltage Atmospheric Cold Plasma Treatment on the Shelf-Life of Distillers Wet Grains. Food Bioprocess Technol 10, 1431–1440 (2017). https://doi.org/10.1007/s11947-017-1903-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1903-6