Abstract
The purpose of this study was to investigate the inhibitory effect of essential oils (thyme, clove and cinnamon) in vapour phase against the major fungal diseases of mango in vitro and in vivo. Thyme oil vapour (5 μL/Petri plate) completely inhibited the mycelial growth of Colletotrichum gloeosporioides and Lasiodiplodia theobromae under in vitro condition. Thyme oil vapour at 66.7 μL L−1 significantly reduced artificially inoculated C. gloeosporioides and L. theobromae in mangoes for 4 days. GC/MS analysis revealed thymol, eugenol and benzofuran, 3-methyl as the dominant compounds in thyme, clove and cinnamon oils, respectively. The activities of defence and antioxidant enzymes including peroxidase, chitinase, phenylalanine ammonia-lyase, β-1,3-glucanase, catalase and superoxide dismutase were enhanced by thyme oil (66.7 μL L−1) treatment and also help to maintain the phenolic content. Hence, postharvest thyme oil vapour treatment may prove to be an alternative means of controlling disease in mangoes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mango (Mangifera indica L.) is considered as a functional tropical fruit due to its delicacy flavour, nutritional value and health benefits and high market demand (Sivakumar et al. 2011). However, mango is highly susceptible to several diseases, resulting in significant economic losses and quality deterioration (Tian et al. 2010). The Colletotrichum gloeosporioides [Penz] causing anthracnose and Lasiodiplodia theobromae [Pat] causing stem-end rot are the most serious postharvest mango pathogens. Disease control is attained primarily by the postharvest application of fungicides such as prochloraz, carbendazim, thiabendazole and benomyl (Khan et al. 2001), though continuous use of these fungicides has resulted in the emergence of resistant strains of pathogens and potential risks on the environment and human health (Droby et al. 2009). Due to increasing consumer demand for organically produced fruits, the quest for natural environmental friendly alternative products and processes has become important for the fruit industry (Sivakumar et al. 2011).
Plant extracts, including essential oils, have been examined as alternative measures against pathological breakdown (Ahmed et al. 2007). Essential oils are natural volatile aromatic compounds, characterized by a strong odour, and are formed by aromatic plants as secondary metabolites. The report suggests that essential oils have fungicidal properties against postharvest diseases of tropical fruits and vegetables, and they are also safer to the environment than synthetic fungicides (Imelouane et al. 2009). The application of essential oil in the vapour phase is preferred than in the liquid phase owing to its volatility, which leads to better activity, requires less amount and does not change sensory properties of foodstuffs (Laird and Phillips 2011).
Regnier et al. (2008) reported that using essential oil of Lippia scaberrima, rich in (d)-limonene, R-(−)-carvone and 1,8-cineole, was an effective in vivo control measure against two mango postharvest spoilage pathogens. Previously, it has been also reported that some essential oils have the potential function of enhancing antioxidant capacities in various kinds of fruits like raspberries (Jin, Wang et al. 2012a) and strawberries (Shao et al. 2013) and also defence-related enzyme activities in avocado fruits (Periyar Selvam, Sivakumar, Soundy and Lise Korsten 2013b). However, to the best of our knowledge, no data are available on the inhibitory effects of thyme oil vapour against mango pathogens and no reports on enhancing antioxidant enzyme activities by essential oils in mango fruits.
Only little information is available on the effect of essential oils on induced defence-related enzymes such as chitinase, phenylalanine ammonia-lyase (PAL), β-1,3-glucanase, peroxidase (POD) activity and total phenols and their impact on reactive oxygen species (ROS) metabolism (superoxide anion, hydrogen peroxide [H2O2] and hydroxyl radical) [Wang et al. 2008] and ROS scavenging activity catalysed by enzymes (Periyar Selvam, Sivakumar, Soundy and Lise Korsten 2013b). These enzymes are the major antioxidant enzymatic systems for protecting cells against oxidative damage caused by free radicals. Hence, the development of appropriate application of essential oil treatment in the vapour phase, the effects of essential oil vapours on disease control, defence and antioxidant enzyme activity need to be investigated.
The objectives of the study were (1) to evaluate the effects of essential oil treatment (vapour phase) against fungal mango postharvest pathogens such as C. gloeosporioides and L. theobromae by in vitro; (2) to test the effects of essential oil against the postharvest pathogens in inoculated mangoes (in vivo); and (3) to determine the responses of defence-related enzymes (chitinase, PAL, β-1,3-glucanase, POD), phenolic compounds and ROS metabolism (superoxide dismutase and catalase) in treated mangoes (cv. Banganapalli and Totapuri).
Materials and Methods
Materials
Thyme (Thymus vulgaris), clove (Syzygium aromaticum) and cinnamon (Cinnamomum zeylanicum) essential oils obtained by hydrodistillation were acquired from Cyrus Enterprises, Chennai, Tamil Nadu, India, and stored at 4 °C until further use. Media were obtained from HiMedia, Mumbai, India. All chemicals and reagents were purchased from Sigma-Aldrich, USA, and Sisco Research Laboratories, Mumbai, India.
Isolation of Pathogens
The postharvest pathogens C. gloeosporioides and L. theobromae were isolated from equatorial and stem-end rot regions of the infected mango fruits, respectively. The pure cultures were maintained in sterile potato dextrose agar (PDA) medium at 25 °C with occasional subculturing.
In Vitro Antifungal Assay
The essential oils were evaluated for in vitro antifungal activity using the disc volatilization method (Dafarera et al. 2000; Tzortzakis and Economakis 2007). The Petri plates (90 × 15-mm diameter) containing the sterile PDA medium were inoculated with 6-mm diameter disc of 7-day-old culture of each tested pathogen. To these Petri plate lids, a 6-mm diameter sterile Whatman filter paper disc was placed using a double-sided tape. Different volume concentrations of oils were added onto this filter paper disc (1 to 8 μL), and plates with disc containing sterile distilled water served as control (Arrebola et al. 2010). The plates were sealed with parafilm immediately to avoid vapour fuges and incubated at 25 °C for 7 days. The radial mycelia growth of C. gloeosporioides and L. theobromae was measured using a Vernier caliper (mm) and denoted as percentage inhibition of radial mycelial growth (IRMG %) using the following formula:
where dc and dt are the radial mycelial growth measurements in control and treatments, respectively. Assays were performed thrice with three replicates per treatment.
Effects of Essential Oils on Artificially Pathogen-Inoculated Mango Fruit
Freshly harvested, unblemished mango fruit cvs. of Banganapalli and Totapuri were acquired from a local farm (Kattankulathur village, Chennai, India) based on uniformity in size and colour and absence of damage and decay. The fruits were disinfected by dipping them in 70% ethanol for 1 min, rinsed with sterilized distilled water and air-dried before wounding. Spore suspensions of the tested pathogen were prepared by flooding 14-day-old culture with sterile distilled water and gently rubbing with a glass rod. The suspension was filtered through a muslin cloth for the removal of mycelial fragment, and it was adjusted to 1 × 108 spores L−1 using a haemocytometer.
Following which, the fruits were wounded uniformly (2 mm deep and 6 mm wide) with a sterile cork borer at three adjacent places on stem-end region and three adjacent wounds on equatorial region. The spore suspension (20 μL) of C. gloeosporioides (108 spores L−1) was inoculated at the equatorial region and L. theobromae (108 spores L−1) at the stem-end region and was left to air-dry. After inoculation, the fruits were placed in 27 × 19.5 × 9-cm boxes. Petri plate containing a Whatman no. 1 filter paper (35-mm diameter) was placed inside the box (90% RH) containing the inoculated fruits, and the corresponding essential oil was spotted on the sterile filter paper. The specific volume concentrations of essential oils: thyme (16.7, 33.3 and 66.7 μL/L), clove (26.7, 53.33 and 106 μL/L) and cinnamon (26.7, 53.33 and 106 μL/L) were calculated according to the volume of the box. The boxes were tightly closed with lid immediately after loading the essential oil to avoid vapour fuges. The untreated fruits were used as control, and commercial fungicide carbendazim-treated (0.05% for 5-min dip) fruits were used as a positive control. One set of fruits was opened on day 4 and another set on day 6. The experiment was done twice with 12 replicate fruits per specific essential oil for each volume concentration. Fungal growth was recorded on days 4 and 6. The severity of disease in the inoculated fruits was measured, and the results were expressed as lesion diameter in millimetre. The average was calculated from all the wounds [24 fruits × 3 (3 wounds per fruit per pathogen)]. The disease incidence was calculated according to Xing et al. (2010) using the following equation:
Determination of Defence Response-Related Enzymes and Total Phenolic Content in Treated Mango Fruits
Fruit exposed to 66.7 μL/L thyme oil and 106 μL/L clove and cinnamon oil vapour treatments were subjected to the analysis of enzyme activity (PAL, β-1, 3-glucanase, chitinase, POD, superoxide dismutase (SOD), catalase (CAT)) and total phenolic content. Sample preparation for the determination of enzyme activity: 1 g of fruit tissue samples from 15 fruits were collected (2 mm away from the wound) and homogenized with specific chilled buffers using a mortar and pestle. The resulting suspension was centrifuged at 15,000×g for 30 min at 4 °C, and a supernatant was used to determine the enzyme activity. For the measurement of POD and CAT activities, sodium phosphate buffer (100 mM, pH 7.0) was used whereas sodium phosphate buffer (100 mM, pH 7.8) was used for SOD activity determination. For chitinase and β-1,3-glucanase, the samples were extracted with 50 mM sodium acetate buffer (pH 5.0). The PAL activity was assayed using borate buffer (100 mM, pH 8.8) having 5 mM β-mercaptoethanol and 2 mM EDTA.
PAL (EC 4.3.1.5) activity was estimated according to Assis et al. (2001), with minor modification following Periyar Selvam, Sivakumar, Soundy and Lise Korsten (2013b). The 75 μL of enzyme extract was incubated with 150 μL of 50 mM borate buffer (pH 8.8) consisting of 20 mM l-phenylalanine for 60 min at 37 °C. The enzyme reaction was stopped by adding 75 μL of 1 M HCl after incubation time. The production of cinnamate was measured at 290 nm (Multiskan GO Microplate Reader, Thermo Scientific, Finland). Specific enzyme activity (U) was defined as the nanomolar amount of cinnamic acid produced per milligram of protein in 1 min, U mg−1 protein.
The activity of β-1,3-glucanase (EC 3.2.1.39) was assayed, following the method of Abeles et al. (1971) with a slight modification reported by Periyar Selvam, Sivakumar, Soundy and Lise Korsten (2013b). The enzyme extract (100 μL) was added to equal volume of 2% (w/v) laminarin (Sigma-Aldrich, USA) and incubated at 40 °C for 24 h. Then, 25 μL 3,5-dinitrosalicylic reagent was added and mixtures were heated in boiling water for 5 min to stop the reaction. The amount of reducing sugar released from the substrate laminarin was recorded at 540 nm (Multiskan GO Microplate Reader, Thermo Scientific, Finland). Enzyme activity was expressed in units: one unit was defined as the amount of enzyme required to catalyse the formation of micromolar amount of glucose equivalent per milligram of protein in 1 min, U mg−1 protein.
The activity of chitinase (EC 3.2.1.14) was analysed according to Abeles et al. (1971) with slight modification. The reaction mixture contained 125 μL of 2% (w/v) dye-labelled chitin azure (Sigma-Aldrich, USA) in 50 mM sodium acetate buffer (pH 5.0) and 600 μL of enzyme extract and incubated at 40 °C for 120 min. The reaction was terminated by adding 25 μL of 1 M HCl after the incubation period. The supernatant of the sample was measured at 550 nm (Multiskan GO Microplate Reader, Thermo Scientific, Finland). One unit was denoted as the amount of enzyme needed to mediate the formation of a nanomolar amount of product per milligram of protein in 1 min, U mg−1 protein.
POD (EC 1.11.1.7) activity was determined by following the Jiang et al. (2002) method with some modifications reported by Periyar Selvam, Sivakumar, Soundy and Lise Korsten (2013b). Thirty-six microlitres of enzyme extract was added to 144 μL buffered substrate (100 mM sodium phosphate, pH 7.0, and 20 mM guaiacol) and incubated for 5 min at 30 °C. Thereafter, 72 μL of H2O2 (100 mM) was added and the increase in absorbance was recorded at 460 nm for 2 min (Multiskan GO Microplate Reader, Thermo Scientific, Finland). One unit of POD enzyme activity was expressed as the amount of enzyme that causes a change in absorbance per milligram of protein per minute, U mg−1 protein.
Total Phenolic Content Analysis
The fruit samples were extracted with acetone/water (1:1), and the total phenolic content was determined by the Singleton et al. (1999) method with slight modification. The sample extract (9 μL) and Folin-Ciocalteu reagent (109 μL) were added to the microtiter plate well and incubated for 3 min. Thereafter, 180 μL of sodium carbonate (7.5%) solution was added to each well and incubated at 50 °C for 5 min. The absorbance was measured at 760 nm (Multiskan GO Microplate Reader, Thermo Scientific, Finland). Gallic acid was used as standard, and the results were expressed as milligram of gallic acid equivalent per gram of fruit (mg/g).
Determination of Antioxidant Enzyme Activities
CAT (EC 1.11.1.6) activity was estimated by the Beers and Sizer (1952) method with a slight modification shown by Periyar Selvam, Sivakumar, Soundy and Lise Korsten (2013b). The reaction mixture comprised 150 μL sodium phosphate buffer (100 mM, pH 7.0), 50 μL of H2O2 (100 mM) and 50 μL of enzyme extract. The decomposition of hydrogen peroxide was recorded at 240 nm (Multiskan GO Microplate Reader, Thermo Scientific, Finland). The activity of enzyme was expressed as units per milligram of protein per minute, U mg−1 protein. One unit was defined as the amount of catalase required to convert 1 μmol of H2O2 min−1.
SOD (EC 1.15.1.1) activity was determined according to the Constantine and Stanley (1977) method with minor modification reported by Periyar Selvam, Sivakumar, Soundy and Lise Korsten (2013b). The reaction mixture (200 μL) consists of 100 mM sodium phosphate buffer (pH 7.8), methionine (13 mM), 75 μM of nitroblue tetrazolium (NBT), EDTA (10 μM), riboflavin (2 μM) and 100 μL of enzyme extract. The mixture was illuminated using a fluorescent lamp (60 μmol m−2 s−1) for 10 min, and the absorbance was noted at 560 nm (Multiskan GO Microplate Reader, Thermo Scientific, Finland). Identical solutions were kept under the dark which served as a blank. The activity of enzyme was expressed as units per milligram of protein, U mg−1 protein where one unit of SOD activity was stated as the amount of enzyme that caused 50% inhibition of NBT. All the enzyme assays were carried out for each treatment in triplicates per sample.
The total protein content of the enzyme extracts was estimated according to the dye-binding method of Bradford (1976), and bovine serum albumin (BSA) was used as a standard.
Statistical Analysis
An entirely randomized design was adopted, and the data were analysed by ANOVA using SPSS v.19.0 (SPSS Inc., Chicago, IL, USA). The mean values were compared by Duncan’s HSD test at p < 0.05.
Result and Discussion
In Vitro Antifungal Assay
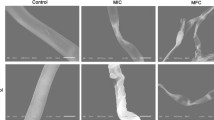
The antifungal activities of different amounts of tested essential oils after 7 days of incubation are depicted in Fig. 1a, b. The obtained results revealed that the type of essential oil and its different volume concentrations significantly affect the mycelial growth (p < 0.05) of C. gloeosporioides and L. theobromae. Thyme oil completely (100%) inhibited the mycelial growth at a volume concentration of 5 μL/Petri plate (Fig. 2a, b), while 8 μL/Petri plate volume concentration of clove and cinnamon oils were needed. Thyme essential oil was most effective in inhibiting the evaluated pathogens. Similar results were reported previously for thyme oil exerting potent antifungal activity against avocado and peach postharvest pathogens (Periyar Selvam, Sivakumar and Soundy 2013a; Elshafie et al. 2015). Bosquez-Monila et al. (2010) reported that 0.06% thyme oil inhibited C. gloeosporioides with a slight rate of growth in food poison technique. However, in our investigation, 5 μL/Petri plate thyme oil showed complete inhibition of C. gloeosporioides in the vapour phase method (Fig. 1a). Hence, our study supports the previous evidence that the vapour phase shows greater efficiency than when applied by direct addition as large amount is needed in biological effect than in gaseous form (Laird and Phillips 2011). Similarly, in our previous work, thyme oil vapour (5 μL/Petri plate) showed complete inhibition against C. gloeosporioides and L. theobromae isolated from mango (Anand Babu et al. 2016). The antimicrobial mechanism or mode of action of essential oils may be due to their direct inhibitory effect on pathogen growth, spore germination (Arrebola et al. 2010), disruption of cellular membrane and cellular metabolism, which leads to cell death (Ultee et al. 1999).
Essential Oil Composition
GC-MS analysis revealed a prevalence of aromatic monoterpenes: thymol (23.88%) and o-cymol (23.88%), aliphatic monoterpene: terpinolene (23.88%) and cyclic monoterpene: β-pinene (7.83%) as major components in thyme oil. These compounds are common in plant essential oil and are attributed to provide them various biological properties such as antimicrobial activity (Bill et al. 2014). The composition of essential oil is similar with the earlier report suggested by Asllani and Toska (2011) where thyme oil of Elbasan region showed the presence of thymol (32.02%). Variation in essential oil components may be influenced by geographical variables, harvesting time, extraction process and quantification conditions (Burt 2004; Gende et al. 2010). Predominant components of clove oil were eugenol (37.42%), phenol, 2-methoxy-3-[2-propenyl] (29.33%) and caryophyllene (16.15%). Similarly, the main constituents of cinnamon oil were benzofuran, 3-methyl, caryophyllene, curcumene, cinnamyl acetate and linalool in the proportion of 17.97, 13.34, 12.85, 12.29 and 11.04%, respectively, which mainly attribute the antimicrobial activity of these essential oils.
Effect of Essential Oil Vapours on Artificially Infected Mangoes
All treated mango fruits showed significant (p < 0.05) inhibition of decay when compared to the control fruits (Tables 1 and 2). The incidence and severity of disease caused by C. gloeosporioides and L. theobromae in both cultivars of mango fruits decreased by increasing the volume concentration of essential oils. Even though, clove and cinnamon oils (106 μL/L) showed significant (p < 0.05) reduction of infection, thyme oil at 66.7 μL/L showed highest reduction in disease incidence and severity of anthracnose and stem-end rot (Fig. 3a, b), which increased with incubation time (Tables 1 and 2). It is notable that thyme oil (66.7 μL/L) showed prominent control on anthracnose and stem-end rot disease than the commercial treatment (carbendazim 0.05% for 5-min dip) in both the mango cultivars after day 4 (Tables 1 and 2).
Inoculated fruits exposed to all postharvest treatments showed an increase in disease incidence on day 6. Thyme oil (66.7 μL/L) treatment showed significantly (p < 0.05) more effects on postharvest diseases in both Banganapalli and Totapuri fruits than other treatments including carbendazim treatment on day 6 (Tables 1 and 2). This result is consistent with the earlier studies which emphasized on the action of thyme oil against postharvest decay in apricot and plum (Liu et al. 2002). The inhibitory activity of thyme oil against fungal pathogens observed under vapour treatments could be due to hydroxyl groups in antimicrobial components forming hydrogen bonds with active enzymes resulting in deactivation and affecting the biosynthesis of mycotoxins (Juglal et al. 2002). The advantage of vapour phase treatment over solution treatment is that the microbial growth and sporulation could be inhibited by low volume concentration of essential oil. The amount of essential oil vapour and its duration time are the important factors in the inhibition of the growth of pathogens by essential oils. The present study confirmed the effects of thymol which showed significant (p < 0.05) inhibition against the postharvest pathogen in mango fruits and supports previous evidence that monoterpenoid phenols have a broad-spectrum antimicrobial activity (Davidson and Naidu 2000).
Effect of Essential Oils on PAL, β-1,3-Glucanase, Chitinase and POD Activity
Among all the essential oil treatments and carbendazim treatment, thyme oil vapour (66.7 μL/L) significantly (p < 0.05) induced the PAL enzyme activity on day 4 and after which it decreased gradually (Table 3). However, higher PAL enzyme activity was observed in Totapuri when compared to Banganapalli, but the thyme oil (66.7 μL/L) induced PAL activity in Banganapalli was almost similar to that of carbendazim. β-1,3-Glucanase activity increased significantly in inoculated Totapuri and Banganapalli, exposed to thyme (66.7 μL/L) and carbendazim (0.05%) on day 4, which then gradually decreased (Table 3), though higher activity was observed in Totapuri fruits. Clove (106 μL/L) and cinnamon oils (106 μL/L) also exhibited a significant induction of enzyme activity but lower than thyme oil vapour treatment. Chitinase activity was significantly higher in Totapuri fruits when exposed to thyme oil vapour (66.7 μL/L) than carbendazim (0.05%) or other treated fruits (Table 3). Banganapalli showed lower chitinase activity when compared to Totapuri fruits in the presence of thyme oil vapour (66.7 μL/L). Conversely, higher chitinase activity was observed in Banganapalli exposed to thyme oil (66.7 μL/L) when compared to other treatments. These results are in agreement with the findings of Periyar Selvam, Sivakumar, Soundy and Lise Korsten (2013b) who reported that thyme oil vapour induced chitinase and β-1,3-glucanase activity in avocado fruits. β-1,3-Glucanase belongs to the PR-8 family; pathogenesis-related (PR) proteins have potential defence mechanisms against fungal infection, which degrade the pathogen cell wall directly or by releasing oligosaccharide elicitors of defence reactions indirectly (Zhao et al. 2008). Chitinase belonging to the PR-2 family can also degrade chitin, a component of the pathogen cell wall.
POD exhibited significantly higher activity in thyme oil (66.7 μL/L) and carbendazim (0.05%) treated mango fruits when compared to other oil-treated and oil-untreated control fruits (Table 3) on day 4, which increased gradually till day 6 except untreated control. However, POD activity was higher in Banganapalli fruits exposed to all treatments including untreated control when compared to Totapuri. It is evident from this research that the thyme oil treatment (66.7 μL/L) enhanced the defence response-related enzyme activities. POD is involved in various processes associated with cell wall reinforcement, including oxidation of phenols and lignification (Brisson et al. 1994). Previous researchers have demonstrated the relationship between the higher activity of chitinase and β-1,3-glucanase and stimulated disease resistance against postharvest decay (Mauch et al. 1988). Our results clearly state that thyme oil (66.7 μL/L) treatment can induce the activities of POD, PAL, β-1,3-glucanase and chitinase, which plays a significant role in disease resistance in mango fruits. The constitutive increases in antioxidant titre (enzymatic and non-enzymatic) in tissues may be due to the activity of essential oils as “signalling molecules”. It is possible that different methods of application indicate varied results and different fruit crops respond differently to the treatments.
Effect of Essential Oils on Total Phenolic Content
Total phenolic content was higher in infected Totapuri fruits when compared to infected Banganapalli fruits, when subjected to different treatments on day 4 of storage, which then declined gradually (Table 3). Furthermore, the application of thyme oil (66.7 μL/L) treatment helped to maintain the total phenolic content in infected mango fruits than carbendazim-treated fruits during the incubation period. Clove (106 μL/L) and cinnamon oils (106 μL/L) also maintained the total phenolic content when compared to untreated control fruits. Phenolic compounds play a significant role in scavenging free radicals. Thus, these compounds may help to protect cells against the oxidative damage caused by free radicals (Zhang et al. 2008). PAL is the primary enzyme involved in the biosynthesis of active metabolites such as phenols, tannin and lignin (Singh et al. 2010). The current study indicates that the increasing activity of both phenolic content and PAL occurred concurrently, and similar findings were reported in strawberry (Shao et al. 2013).
Effect of Essential Oils on Antioxidant Enzymes
The influence of thyme oil treatment (66.7 μL/L) on SOD activity was significantly (p < 0.05) higher among all the treatments (Fig. 4). The fruits exposed to different postharvest treatments exhibited a slight increase in SOD activity on day 4, and a slight decrease in activity was observed on day 6. Thyme oil (66.7 μL/L) and carbendazim (0.05%)-treated Totapuri fruits showed increased SOD activity than the Banganapalli cultivar, when compared to clove (106 μL/L), cinnamon (106 μL/L) and untreated control fruits. Banganapalli fruits subjected to thyme oil (66.7 μL/L) treatment showed increased SOD activity, which was almost similar to carbendazim (0.05%) treatment. SOD contains metal-binding motifs that mediate the dismutation reaction of O2·− into H2O2, and the CAT converts H2O2 to O2 and H2O (Gill and Tuteja 2010). Our results indicate that mango fruits exposed to essential oils had increased antioxidant enzyme activities (SOD and CAT) than the control without treatment, which is concordant with the findings suggested by Bill et al. (2014). Thymol or eugenol essential oil components could induce constitutive increases in the antioxidants of plant tissues, including enzymatic and non-enzymatic systems (Jin, Wu et al. 2012b). Hence, it may be concluded that the fruits subjected to thyme oil (66.7 μL/L) treatment enhanced the antioxidant mechanism or secondary metabolites, which triggers the antioxidant capacity and free radical scavenging activity. CAT enzyme activity also showed similar trend as SOD, where thyme oil (66.7 μL/L) and carbendazim (0.05%) treatment enhanced the enzyme activity in both Totapuri and Banganapalli fruits than those of the control and other treated fruits on day 4 and declining on day 6 (Fig. 5). However, thyme oil (66.7 μL/L)-treated Totapuri fruits showed higher CAT activity than Banganapalli. Hence, all treatments significantly (p < 0.05) enhanced the activities of antioxidant enzymes in mango fruits compared to untreated control fruits. In our investigation, we observed that thymol showed higher impact on antioxidant enzyme activities, which concur to the previous findings of Jin et al. (2012a, b) and Periyar Selvam, Sivakumar, Soundy and Lise Korsten (2013b), where the essential oils induced the antioxidant enzyme activities in raspberry, bayberry and avocado fruits, respectively.
Effect of essential oil treatment on antioxidant enzyme activities (catalase) in artificially pathogen-inoculated mango fruit cvs. Banganapalli and Totapuri. a Day 4. b Day 6. Different letters indicate significant difference (p < 0.05) analysed by Duncan’s multiple range test. Vertical bars represent the standard error of the mean
Conclusion
Our findings suggest that the application of thyme oil (66.7 μL/L) significantly reduced the disease incidence and severity of anthracnose and stem-end rot caused by C. gloeosporioides and L. theobromae than cinnamon (106 μL/L) and clove (106 μL/L) in both the mango cultivars. However, the effectiveness of thyme oil (66.7 μL/L) on the anthracnose and stem-end rot decay control was greater in Totapuri fruits than in Banganapalli fruits. This correlated with the enhanced activity of antioxidant and defence enzymes, higher levels of phenolic content and resistance of fruit tissue against infection during storage in Totapuri fruits. It is evident from our experiment that the effect of thyme oil vapour treatment differs among cultivars. Further investigation needs to be carried out in the future in naturally infected fruits in order to offer an effective decay control measure to the organic mango fruit industry. Due to its lesser price, thyme oil could be reported as cost-effective for commercial application. Thyme oil fumigation treatment can be considered as a better alternative treatment due to the low volume concentration used in anthracnose and stem end rot decay control. Thus, these essential oils have the great potential to preserve the quality and safety of fresh produce.
References
Abeles, F. B., Bosshart, R. P., Forrence, L. E., & Habig, W. H. (1971). Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiology, 47(1), 129–134.
Ahmed, D. M., El-Shami, S. M., & El-Mallah, M. H. (2007). Jojoba oil as a novel coating for exported Valencia orange fruit. Part 1. The use of trans (isomerized) jojoba oil. American-Eurasian Journal of Agricultural & Environmental Science, 2, 173–181.
Anand Babu, P., Periyar Selvam, S., Reshma N. B., & Rotimi Sadiku, E. (2016). Antifungal activity of five different essential oils in vapour phase for the control of Colletotrichum gloeosporioides and Lasiodiplodia theobromae in vitro and on mango. International Journal of Food Science and Technology, 51(2), 411–418.
Arrebola, E., Sivakumar, D., Bacigalupo, R., & Korsten, L. (2010). Combined application of antagonist Bacillus amyloliquefaciens and essential oils for the control of peach postharvest diseases. Crop Protection, 29(4), 369–377.
Asllani, U., & Toska, V. (2011). Chemical composition of Albanian thyme oil (Thymus vulgaris L.) Journal of Essential Oil Research, 15(3), 165–167.
Assis, J. S., Maldonado, R., Munoz, T., Escribano, M. I., & Merodio, C. (2001). Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biology and Technology, 23(1), 33–39.
Beers, R. F., & Sizer, I. W. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biological Chemistry, 195(1), 133–140.
Bill, M., Sivakumar, D., Korsten, L., & Thompson, A. K. (2014). The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Protection, 64, 159–167.
Bosquez-Monila, E., Ronquillo-de Jesus, E., Bautista-Banos, S., Verde-Calvo, J. R., & Morales-Lopez, J. (2010). Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biology and Technology, 57(2), 132–137.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Brisson, L. F., Tenhaken, R., & Lamb, C. J. (1994). Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell, 6(12), 1703–1712.
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods: a review. International Journal of Food Microbiology, 94(3), 223–253.
Constantine, N. G., & Stanley, K. R. (1977). Superoxide dismutases. Plant Physiology, 59(2), 309–314.
Dafarera, D., Ziogas, B. N., & Polissiou, M. G. (2000). GC/MS analysis of essential oils from Greek aromatic plants and their fungi toxicity on Penicillium digitatum. Journal of Agricultural and Food Chemistry, 48(6), 2576–2581.
Davidson, P. M., & Naidu, A. S. (2000). Phyto-phenol. In A. S. Naidu (Ed.), Natural food antimicrobial systems (pp. 265–294). Boca Raton, Florida: CRC Press.
Droby, S., Wisniewski, M., Macarisin, D., & Wilson, C. (2009). Twenty years of postharvest biocontrol research: it is time for a new paradigm? Postharvest Biology and Technology, 52(2), 137–145.
Elshafie, H. S., Mancini, E., Sakr, S., De Martino, L., Mattia, C. A., De Feo, V., et al. (2015). Antifungal activity of some constituents of Origanum vulgare L. essential oil against postharvest disease of peach fruit. Journal of Medicinal Food, 18(8), 929–934.
Gende, L. B., Maggi, M., Van Baren, C., Di Leo Lira, A., Bandoni, A., Fritz, R., et al. (2010). Antimicrobial and miticide activities of Eucalyptus globulus essential oils obtained from different Argentine regions. Spanish Journal of Agricultural Research, 8(3), 642–650.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930.
Imelouane, B., Elbachiri, A., Ankit, M., Benzeid, H., & Khedid, K. (2009). Physico-chemical compositions and antimicrobial activity of essential oil of Eastern Moroccan Lavandula dentata. International Journal of Agriculture and Biology, 11(2), 113–118.
Jiang, A. L., Tian, S. P., & Xu, Y. (2002). Effects of controlled atmospheres with high O2 or high CO2 concentrations on postharvest physiology and storability of “Napoleon” sweet cherry. Acta Botanica Sinica, 44(8), 925–930.
Jin, P., Wang, S. Y., Gao, H., Chen, H., Zheng, Y., & Wang, C. Y. (2012a). Effect of cultural system and essential oil treatment on antioxidant capacity in raspberries. Food Chemistry, 132(1), 399–405.
Jin, P., Wu, X., Xu, F., Wang, X., Wang, J., & Zheng, Y. (2012b). Enhancing antioxidant capacity and reducing decay of Chinese bayberries by essential oils. Journal of the Science of Food and Agriculture, 60(14), 3769–3775.
Juglal, S., Govinden, R., & Odhav, B. (2002). Spice oils for the control of co-occurring mycotoxin-producing fungi. Journal of Food Protection, 65(4), 683–687.
Khan, S. H., Aked, J., & Magan, N. (2001). Control of the anthracnose pathogen of banana (Colletotrichum musae) using antioxidants alone and in combination with thiabendazole or imazalil. Plant Pathology, 50(5), 601–608.
Laird, K., & Phillips, C. (2011). Vapour phase: a potential future use for essential oils as antimicrobials? Letters in Applied Microbiology, 54(3), 169–174.
Liu, W. T., Chu, C. L., & Zhou, T. (2002). Thymol and acetic acid vapors reduce postharvest brown rot of apricot and plums. Hortscience, 37(1), 151–156.
Mauch, F., Mauch-Mani, B., & Boller, T. (1988). Antifungal hydrolases in pea tissue. Plant Physiology, 88(3), 936–942.
Periyar Selvam, S., Sivakumar, D., & Soundy, P. (2013a). Antifungal activity and chemical composition of thyme, peppermint and citronella oils in vapor phase against avocado and peach postharvest pathogens. Journal of Food Safety, 33(1), 86–93.
Periyar Selvam, S., Sivakumar, D., Soundy, P., & Lise Korsten, L. (2013b). Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biology and Technology, 81, 66–72.
Regnier, T., du Plooy, W., Combrinck, S., & Botha, B. (2008). Fungi toxicity of Lippia scaberrima essential oil and selected terpenoid components on two mango postharvest spoilage pathogens. Postharvest Biology and Technology, 48(2), 254–258.
Shao, X., Wang, H., Xu, F., & Cheng, S. (2013). Effects and possible mechanisms of tea tree oil vapor treatment on the main disease in postharvest strawberry fruit. Postharvest Biology and Technology, 77, 94–101.
Singh, R., Rastogi, S., & Dwivedi, U. N. (2010). Phenylpropanoid metabolism in ripening fruits. Comprehensive Reviews in Food Science and Food Safety, 9(4), 398–416.
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Sivakumar, D., Jiang, Y., & Yahia, E. (2011). Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Research International, 44(5), 1254–1263.
Tian, S. P., Liu, J., Zhang, C. F., & Meng, X. H. (2010). Quality properties of harvested mango fruits and regulating technologies. In D. Sivakumar, (Ed.), New Trends in postharvest management of fresh produce II (pp. 49–54). Fresh Produce 4 (Special Issue 1). Kagawa ken, Japan: Global Science Books, Ltd.
Tzortzakis, N. G., & Economakis, C. D. (2007). Antifungal activity of lemongrass (Cymbopogon citratus L.) essential oil against key postharvest pathogens. Innovative Food Science and Emerging Technologies, 8(2), 253–258.
Ultee, A., Kets, E. P. W., & Smid, E. J. (1999). Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Applied Environmental and Microbiology, 65(10), 4606–4610.
Wang, C. Y., Wang, S. Y., & Chen, C. T. (2008). Increasing antioxidant activity and reducing decay of blueberries by essential oils. Journal of Agricultural and Food Chemistry, 56, 3587–3592.
Xing, Y., Li, X., Xu, Q., Yun, J., & Lu, Y. (2010). Antifungal activities of cinnamon oil against Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit test. International Journal of Food Science and Technology, 45(9), 1837–1842.
Zhang, W. S., Li, X., Zheng, J. T., Wang, G. Y., Sun, C. D., Ferguson, I. B., et al. (2008). Bioactive components and antioxidant capacity of Chinese bayberry (Myrica rubra Sieb. and Zucc.) in relation to fruit maturity and postharvest storage. European Food Research and Technology, 227(4), 1091–1097.
Zhao, Y., Tu, K., Shao, X., Jing, W., & Su, Z. (2008). Effects of the yeast Pichia guilliermondii against Rhizopus nigricans on tomato fruit. Postharvest Biology and Technology, 49(1), 113–120.
Acknowledgements
This research was financially supported by the Department of Science and Technology (DST), India, and National Research Foundation (NRF), South Africa, under the India and South African Bilateral project collaboration program (DST/INT/South Africa/P-07/2014), which are greatly acknowledged for the funding. The authors express their thanks to Prof. C. Muthamizhchelvan, director (Engineering and Technology), and Dr. M. Vairamani, dean, School of Bioengineering, SRM University, for providing facilities and their cordial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perumal, A.B., Sellamuthu, P.S., Nambiar, R.B. et al. Effects of Essential Oil Vapour Treatment on the Postharvest Disease Control and Different Defence Responses in Two Mango (Mangifera indica L.) Cultivars. Food Bioprocess Technol 10, 1131–1141 (2017). https://doi.org/10.1007/s11947-017-1891-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1891-6