Abstract
In this research, our chief aim was to survey possible improvements in thermophysical properties of nanofluids when they are used as heating mediums for time reduction and energy saving in food industries for the first time. Accordingly, three different variables of temperature (70, 80, and 90 °C), alumina nanoparticle concentration (0, 2, and 4 %), and time (30, 60, and 90 s) were selected for thermal processing of tomato juice by a shell and tube heat exchanger. Our results revealed that incorporation of nanoparticles could raise density, viscosity, and thermal conductivity and decrease heat capacity, but this increasing/decreasing trend was linear or non-linear depending on the diameter of the nanoparticles. Four percent Al2O3–water, compared with 2 % nanofluid and pure water (0 % nanofluid), had the highest overall heat transfer coefficients for all Re numbers. Incorporating nanoparticles into the base heating fluid of water could augment the effectiveness of the heat exchanger by 49 %. Thermal processing time of tomato juice was shorter for 2 and 4 % nanofluids, compared with water, by 22.23 and 46.29 %, respectively; this time reduction caused energy saving rates for 2 and 4 % nanofluids to be improved by 22.3 and 48.76 %, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat exchangers could be divided into two direct and indirect contact types. As their names suggest, in indirect contact heat exchangers, the product and hot or cold fluid are separated by a thin layer physically. As an example, in a direct contact heat exchanger so-called steam injection systems, steam is injected into the product directly. However, in plate heat exchangers, a thin layer of metal plate separates hot from cold fluid, and heat transfer is carried out without mixing. After plate heat exchangers, shell and tube is the most frequent and common heat exchanger in the industry; the reason of this inclination is their tolerance with high temperatures and pressures (Worth and Mascone 1991; Pritiviraj and Andrews 1996). Constructing components of a shell and tube heat exchanger include tube, tube sheet, shell, front-end head, rear-end head, and baffles.
Most conventional fluids in heating and cooling systems, e.g., water and water vapor in the food industry, or ethylene glycol and engine oil in other industries, have internal limits in their heat capacity and properties, creating multitude barriers to their application in heating equipment, especially heat exchangers. So, there is an urgent and obvious need to develop a new strategy to amend thermal properties of these fluids. Meanwhile, compounds having millimeter or micrometer particles cause intense pressure drop, rapid particle sedimentation, and passage clogging or erosion (Nasiri et al. 2011). Fortunately, emergent technologies have recently made it possible to produce particles in nanoscales, facilitating the synthesis of nanofluids. Expression of nanofluid refers to a two-phase compound, usually comprising a saturate fluid and some very small solid particles with dimensions below 40 nm. Tiny and low volume fractions of particles used in these fluids remove problems of sedimentation and reduce necessary costs of maintenance and transfer of these fluids, and there is minimal friction or damages to equipment because of the trivial size of particles. Besides, as thermal conductivity is one of the critical and determining parameters for heat transfer enhancement, a series of studies were carried out on thermal conductivity of nanofluids, demonstrating the enhancement of thermal conductivity by addition of nanoparticles (Farajollahi et al. 2010).
Hence, a lot of researches have surveyed experimental usage of nanoparticles, especially alumina, to enhance heat transfer rates by now. Palm et al. (2006) studied heat transfer enhancement with the use of nanofluids in radial flow cooling systems considering temperature-dependent properties. The results clearly indicated that considerable heat transfer increase was possible with the use of these fluid/solid particle mixtures. Water/Al2O3 nanofluid with the volume fraction of nanoparticles as low as 4 % could produce a 25 % increase in the average wall heat transfer coefficient in comparison with the base fluid of water. Ho et al. (2010) investigated forced convective cooling performance of a copper microchannel heat sink with Al2O3/water nanofluid as the coolant. Their results showed that the nanofluid-cooled heat sink outperformed the water-cooled one with significantly higher average heat transfer coefficient and thereby considerably lowered thermal resistance and wall temperature, especially at high pumping powers. Farajollahi et al. (2010) compared heat transfer characteristics of γ-Al2O3–water and TiO2–water nanofluids in a shell and tube heat exchanger under turbulent flow condition and investigated the effects of Peclet number, volume concentration of suspended nanoparticles, and particle type on their heat characteristics. Based on their results, adding naoparticles to the base fluid enhanced heat transfer characteristics remarkably. In details, at a certain Peclet number, heat transfer characteristics of TiO2–water nanofluid were greater than those of γ-Al2O3–water nanofluid at its optimum nanoparticle concentration while the latter possessed better heat transfer features at higher nanoparticle concentrations than the former. Zeinali Heris et al. (2011) investigated laminar flow-forced convective heat transfer of Al2O3–water nanofluid in a triangular duct under constant wall temperature condition. The results obtained by the numerical solutions showed that decreasing the nanoparticle size increased Nusselt number at a specific concentration, and raising the nanoparticle concentration increased Nusselt number at a constant particle size. Peyghambarzadeh et al. (2011) carried out an experimental study of heat transfer enhancement using water–ethylene glycol-based nanofluids as a new coolant for car radiators. The results demonstrated that 0–1 (%vol) nanofluids (γ-Al2O3) clearly enhanced heat transfer compared to their own base fluid, and, in the best conditions, this heat transfer enhancement could reach nearly 40 %. These findings convinced us that alumina could be a suitable nanoparticle to enhance heat transfer in thermal processing of the food products.
In Iran, approximately 35 % of agricultural products are annually converted into waste since there is shortage in suitable storage conditions and lack of appropriate processing industries to prepare fruit juices from raw fruits (Jafari et al. 2016). Besides, in some cities of our country, internal production is much higher than the demand, and this surplus rate certainly wastes due to the shortage of suitable storage conditions. However, proper processing industries can not only prevent spoiling of fruits but also prosper diverse food markets. Nowadays, in the food industry, fruit juices are pasteurized by the HTST method to inactivate microorganisms and enzymes and prolong storage time. These thermal processes, carried out at high temperatures and short times, decrease bacteria population [FDA 2004]. However, it should not be left unsaid that although conventional thermal pasteurization guarantees safety and long-storage conditions of fruit juices, it leads to palpable drop in quality and nutritional properties of food products [Cortes et al. 2008]. Thus, there is an obvious need to deploy new emerging thermal technologies, e.g., nanotechnology as nanofluids, in the global food industry to reduce thermal processing time significantly and keep natural properties of products more effectively. As a result, the current article was defined to inspect the application of a shell and tube heat exchanger with circulating alumina–water nanofluids in the food processing for the first time and to survey heat transfer aspects of this project to evaluate possible shortening of processing duration and optimization of energy consumption in the processing. Besides, we selected tomato juice since, firstly, long duration of conventional processing might result in massive destruction of lycopene in this product, and secondly, as it is nearly always under the threat of being spoiled by dangerous microorganisms such as Bacillus coagulans, Clostridium, Salmonella, yeasts, and fungi, e.g., Geotrichum, effective treatment of this product is of paramount importance.
Materials and Methods
Preparing the Product
Fresh tomatoes were purchased from a local fruit market (Gorgan, Iran) and stored at 3 ± 1 °C. At the appropriate time, they were crushed using a domestic juice extractor (MJ-W176P, Panasonic, Japan). The juice was filtered on a sterile double-layer cheese cloth to remove seeds from the juice and processed subsequently (Adekunte et al. 2010).
Nanofluid Preparation
Alumina nanoparticles with 99 % purity (US Research Nanomaterials, Inc.) were purchased and dispersed with different volume concentrations of 0, 2, and 4 % w/v in deionized distilled water. Then, they were stirred completely for an hour with a heater-stirrer at 1500 rpm in order to ensure nanofluid stability. No sedimentation was observed in the prepared nanofluids after 24 h.
Applied Intelligent Thermal Processing System
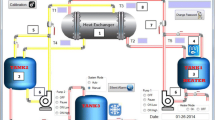
This system is composed of a shell and tube heat exchanger, two separate reservoirs, one for food product, and the other one equipped with a 1-kW heater for heating the fluid (water or nanofluid), and flow loop tubes equipped with two sanitary centrifugal pumps for transferring the fluids from the reservoir to the heat exchanger. All the components were made from 316 L stainless steel and were insulated by aluminum foam to reduce heat loss. Required power to overcome the pressure drop was supplied by two 0.55-kW steel centrifugal pumps (three-phase induction motor, Western Electric, Australia). Control, return, and drain valves were installed in proper places. Heating performance and temperatures of nanofluid and food product were checked by PT100 sensors (Fig. 1) (Jabbari et al. 2016).
a Mechanical components of intelligent thermal/heating system for nanofluids and b PLC section: 1 insulated stainless steel shell and tube heat exchanger, 2 food liquid reservoir, 3 nanofluid reservoir, 4 heater, 5 pipes of fluid flow, 6 stainless steel centrifugal pump, 7 stainless steel valves for fluid flow controlling, 8 thermocouple, and 9 PT100 sensors
Nanofluid and food liquid exchanged their thermal energy by passing through 13 tubes with external diameter, thickness, and length of 8, 2, and 800 mm (respectively) and passing a shell with internal diameter of 100 mm in a counter-current way. Controlling flow speed of two fluids and converting three-phase electricity of electropumps to a single-phase state were carried out through a computer and two N700E vector inverters (Hyundai Heavy Industries Co., Ltd., Korea). Temperature changes in fluids were monitored by PT100 sensors. Heating relays, inverters, and PT100 thermal sensors were attached to the entrance of a microcontroller and were connected to the monitoring system through USB ports. Sending commands to the system and depicting temperature–time diagrams were carried out by Visual Studio 2012 software and Microsoft Excel 2010, respectively. The latter was implemented online and automatically every 10 s (Fig. 1b).
Predicting Thermophysical Properties
Density (ρnf) and specific heat capacity \( {\left({\mathrm{C}}_{\mathrm{p}}\right)}_{nf} \) properties of alumina–water nanofluid were calculated according to the following empirical equations (Pak and Cho 1998; Xuan and Roetzel 2000):
Einstein equation for determining viscosity (μ nf ) of nanofluids with concentrations below 5 % was represented as follows (Luciu et al. 2009):
There are numerous equations to estimate viscosity of nanofluids in different resources, each of which has its own special applications and constraints. Some of complex relations were surveyed by Nguyen et al. (2005), and polynomial approximation was selected finally for alumina–water nanofluid according to empirical data:
Changes in thermal conductivity of nanofluids with temperatures have been estimated in many researches while numerous parameters including concentration, morphology, particle size, and distribution style could affect that factor. Changes in thermal conductivity of alumina–water nanofluids with temperature were calculated according to following empirical equations (Nguyen et al. 2005):
Overall heat transfer coefficient of each fluid is proportional to Nusselt number, estimated according to the following equation, directly (Farajollahi et al. 2010):
Following equations were developed to find the Nusselt numbers of laminar and turbulent flows (Xuan and Li 2003):
The Peclet number of particles, the Reynolds and Prandtl numbers of nanofluids, and thermal diffusivity were obtained as follows (Farajollahi et al. 2010):
Heat quantity was calculated by this equation (Farajollahi et al. 2010):
Heat transfer coefficient on external surface was calculated by Bell’s method and heat transfer coefficient on internal surface by the following equation (Coulson and Richardson 1999):
in which Di, Do, Ui, and kw are the inner diameter of the tube, the outer diameter of the tube, overall heat transfer coefficient, and thermal conductivity of the inner wall material, respectively. Ui is obtained by following equation (Choi et al. 2001):
∆Tlm is the logarithmic mean temperature differential (LMTD). In fact, in heat exchanger analysis, if the fluid inlet and outlet temperatures are specified or can be determined by a simple energy balance, the LMTD method can be used; but when these temperatures are not available, the NTU or the effectiveness method is used. The Number of Transfer Units (NTU) method is used to calculate the heat transfer rate in heat exchangers, especially counter-current exchangers, when there is insufficient information to calculate the log-mean temperature difference (LMTD). Effectiveness is defined as the ratio between the actual heat transfer rate and the maximum possible heat transfer rate (Q max). The general equations to calculate the effectiveness are shown in Eqs. 24 and 25 (Putra et al. 2013):
where C min is the lowest specific heat capacity between the hot and cold fluid and ΔTmax is the difference between the temperatures at the inlet of the hot side (TH,in) and at the inlet of the cold side (TC,in ).
NTU (number of transfer units) is a non-dimensional number that shows thermal size or heat transfer size of a heat exchanger. Equation 26 shows the relationship between NTU with UA and C min (Putra et al. 2013):
Coefficient of friction was calculated based on the following equations:
Average velocities of nanofluid and pressure drop were calculated by Eq. 29 and 30, respectively:
Finally, pump power was calculated by the following equation:
Results and Discussion
Thermophysical Properties of the Nanofluid
Initial properties of the applied nanoparticle have been represented in Table 1. As Table 2 indicates, incorporation of nanoparticles could raise density, viscosity, and thermal conductivity and decrease heat capacity. The same ranges and trends were observed by Pandey and Nema (2012) for density, heat capacity, and thermal conductivity of 0–4 % alumina–water nanofluids. The important point is that the increasing/decreasing trends in this research were linear for each one of four properties; however, non-linear trends for changes in density, heat capacity, and viscosity of alumina nanofluids were reported by Jafari et al. (2016a, b). who added alumina nanoparticles with 50 nm diameter to the base fluid of water and used these to enhance heat transfer into watermelon juices. In other words, we expect that this difference could be due to the diameter of nanoparticles. Ho et al. (2010) found a non-linear increase in viscosity of water after addition of alumina nanoparticles with diameter of 33 nm, as well.
Heat Transfer Coefficient
Figure 2 illustrates that the heat transfer coefficient escalates significantly when the Re number rises. Besides, the volume fraction of nanoparticles affected this parameter. On average, the increase rates in heat transfer coefficient of 2 and 4 % Al2O3 nanofluids were 5.42 and 11.94 %, respectively; furthermore, 4 % Al2O3 had the highest overall heat transfer coefficient for all Re numbers. Albadr et al. (2013) reported that heat transfer capacities of 2 % Al2O3 nanofluids were much higher than those of the base fluid; as an example, 2 % nanofluids had an overall heat transfer coefficient of 700.242 W/m2 K at flow rate of 0.0125 L/s while this parameter was 339.15 W/m2 K for distilled water, nearly 1.75 times or 57 % higher. In the same way, Nasiri et al. (2011) expressed that for specific Peclet number, the convective heat transfer coefficient (h) of nanofluid (Al2O3/H2O) was higher than that of the base fluid, the difference of which was dependent on the concentration of Al2O3/H2O considerably. For example, at Peclet number about 24,000, the heat transfer coefficients were 2.5 and 26.8 % greater than those of the base fluid when the nanoparticle concentrations were 0.1 and 1.5 vol.%, respectively.
Wen and Ding (2004) surveyed alumina–water nanofluids under laminar flow and reported higher convective heat transfer coefficients of nanofluids by about 41–47 % with an increase in nanoparticle concentration from 0 to 1.6 % (vol), dependent on the exact Re number. Likewise, Palm et al. (2006) examined convective heat transfer in Al2O3–water nanofluids and reported an increase in heat transfer rates by about 24 % after an increase in nanoparticle concentration from 0 to 4 %. The increase in convective heat transfer of nanofluids is due to the increase in the thermal conductivity, intensification of turbulence or eddy, dispersion of suspended particles, suppression boundary layer growth, and their chaotic movements (Keblinski et al. 2002). Xuan and Li (2003) investigated Cu–water nanofluids under turbulent flow and attributed the increase in convective heat transfer of nanofluids to the increase in their thermal conductivity, chaotic movements, and suspension in nanofluid flow and represented new equations for the heat transfer of nanofluids.
Nusselt Numbers
Al2O3 nanofluids, compared with the base fluid of water, rated higher Nu numbers at all Re numbers (Fig. 3). The highest increase in Nu number of the 4 % nanofluid, compared with that of water, could be seen at a Re number of about 200 with nearly 13 % difference between their Nu numbers. Similarly, Putra et al. (2013) studied the thermal performance of Al2O3–water nanofluids at 1, 3, and 5 % volume concentrations and SnO2–water nanofluid at 1 % concentration in a microchannel heat exchanger and reported that nanofluids had higher Nusselt numbers than the base fluid of water at all concentrations with SnO2–water resulting in higher Nusselt numbers than alumina nanoparticles and 5 % resulting in higher Nu numbers than 3 and 1 %, respectively. Albadr et al. (2013) claimed that the Nu number was 587 for 2 % nanofluids at the flow rate of 0.0125 L/s whereas it was 367.759 for the base flow of water at the same flow rate, indicating 1.596 times or 62.6 % higher rate for the nanofluid compared with water.
Effectiveness
Figure 4 illustrates incorporating nanoparticles into the base fluid of water can augment effectiveness of the heat exchanger by 49 %. The difference in effectiveness between two nanoparticle concentrations of 2 and 4 % could be as high as 10 % at NTU of about 0.44. Previously, it was reported by Putra et al. (2013) that SnO2–water concentration of 1 % could improve effectiveness of a microchannel heat exchanger from 36 to 43 %, depending on the flow rate.
Pressure Drop
As it can be seen in the Fig. 5, pressure drop intensified as the Re number increased. Total pressure drop was higher for nanofluids in comparison with that of water. This increase was up to 28 % for 4 % nanoparticle concentration at 264 Re number, compared with water at a Re number of around 290. Besides, nanoparticle concentration affected pressure drop considerably since nanoparticles raise dynamic viscosity of base fluid and lead to higher pressure drops; besides, friction coefficient increases when volume fraction of nanoparticles increases (Albadr et al. 2013). Due to this reason and to compensate for the pressure drop during thermal processing of tomato juice, pumping speed of nanofluids (900 rpm) was higher than that of the food product (600 rpm).
The corresponding tendency was mentioned by Pandey and Nema (2012) that at a given coolant flow rate in a counter flow corrugated plate heat exchanger, pressure drop was least for water and it increased with a rise in Al2O3 concentration of the nanofluid. Similar pressure drops of 0.5–1.5 kW were reported by Putra et al. (2013) when they applied alumina–water nanofluids in microchannel heat exchangers to improve heat transfer of water base fluid. Mare et al. (2011) reported that at the same Reynolds numbers, the pressure drop for the nanofluid composed of aqueous alumina (diameter of 37 nm) and 1 wt% surfactant was up to three times superior to that of water.
Processing Time
Not only could nanofluids reach the maximum temperature (around 90 °C) sooner than base fluid when the heater was turned on but also tomato juices processed with nanofluids reached the maximal temperature (around 80 °C) much sooner than its counterparts when the pumps were turned on (Fig. 6). In fact, processing time was shortened when nanofluid, rather than water, was applied. Process duration for water and 2 and 4 % nanofluids was 54, 42, and 29 min, respectively (Fig. 7). So, thermal processing time of tomato juices was shorter for nanofluids, compared with water, by 22.23 and 46.29 %, respectively. Indeed, not only could 4 % nanofluid, compared with water, dwindle the time required to reach pasteurization temperature by half but also it reduced total processing time to half (Fig. 6).
The reason of reduction in processing time for nanofluids relies on their heat transfer properties. Heat transfer coefficient of nanofluids is dependent upon some factors including thermal conductivity, heat capacities of fluids and nanoparticles, viscosity of nanofluids, volume fraction of suspended particles, morphology, and dimension of particles. The increase in conductive heat transfer coefficient of nanofluids results in a rise in their thermal conductivity, turbulence intensification, stopping boundary layer growth, and distribution of suspended particles and their chaotic movements (Keblinski et al. 2002).
The introduced time reduction could bring about significant advantages for food quality. In detail, the degradation of a heat-labile nutrient such as vitamin C or thiamin is known to be dwindled when shorter times are applied; besides, heat-labile nutrients as well as sensory parameters including color, texture, and flavor follow similar patterns for specific food products (Richardson 2008). Similarly, in the case of lycopene in tomato products, Ajmera (2006) investigated the effects of different heating times (15, 30, 45 min) and temperatures (325, 350, and 375 °F) on lycopene content of tomato sauce. They concluded that heating for a short period of time at higher temperatures helps better retention of lycopene during home or industry processing compared with longer time at lower temperatures; however, the current research keeps maximum temperature comparable for each time duration, so the gaining would even be more considerable.
Energy Consumption
In this project, the thermal processing was divided into two stages to monitor energy consumption more precisely: In the first stage where temperature of fluids increased, heater was on and pumps were off; in the second stage, heat exchanges occurred between the product and heating fluids. In the latter stage, pumps of both heating fluids and food products were on and the heater was off. Total energy consumption of two stages was computed separately for thermal processing with water and 2 and 4 % nanofluids, and the results were represented in Fig. 7.
Decreasing processing time could help lower energy consumption as well as improve qualitative and nutritional indices. According to Fig. 7, total energy consumptions for thermal processing of tomato juices were 1.507, 1.117, and 0.772 kW for water and 2 and 4 % nanofluids, respectively. Indeed, energy saving rates for 2 and 4 % nanofluids improved by 22.3 and 48.76 %, respectively.
Conclusion
Four percent Al2O3 nanofluid had higher overall heat transfer coefficients than water at all Re numbers. The highest increase in Nu number of the 4 % nanofluid, compared with that of water, could be seen at a Re number of about 200 with nearly 13 % difference between their Nu numbers. Besides, nanoparticle concentration affected pressure drop considerably since nanoparticles raised dynamic viscosity of base fluid and led to higher pressure drops. The difference in effectiveness between two nanoparticle concentrations of 2 and 4 % could be as high as 10 % at NTU of about 0.44. Up to 46 and 49 % decrease in processing time and energy consumption was observed after incorporation of alumina nanoparticle into water. These findings demonstrated that nanofluid technology could be deployed for improving thermal processing of food products significantly and successfully. However, further investigations into reduction in processing time achieved by other types of nanofluids as well as improvement in food characteristics of nanofluid-treated products are still required.
Abbreviations
- A:
-
Heat transfer area (m2)
- Cp :
-
Specific heat (kJ kg−1 K−1)
- D:
-
Tube diameter (m)
- d:
-
Nanoparticle diameter (m)
- h:
-
Heat transfer coefficient (W m−2 K−1)
- k:
-
Thermal conductivity (W m−1 K−1)
- L:
-
Tube length (m)
- m:
-
Mass flow rate (kg s−1 )
- Nu:
-
Nusselt number
- Pe :
-
Peclet number
- Pr :
-
Prandtl number
- Q:
-
Heat quantity (W)
- Re:
-
Reynolds number
- T:
-
Temperature (K)
- U:
-
Overall heat transfer coefficient (W m−2 K−1)
- V:
-
Flow velocity (m s−1)
- ƒ:
-
Coefficient of friction
- ∆p:
-
Pressure drop (Pa)
- P:
-
Pump power (W)
- α :
-
Thermal diffusivity (m2 s−1)
- ρ :
-
Density (kg m −3 )
- η:
-
Kinematic viscosity (m2 s−1)
- φ :
-
Volume concentration (%)
- ∆Tlm :
-
Log mean temperature difference
- f:
-
Fluid
- in:
-
Inlet
- m:
-
Mean
- nf:
-
Nanofluid
- out:
-
Outlet
- p:
-
Particle
- w:
-
Wall
- i:
-
Inside
- o:
-
Outside
- H:
-
Hot
- C:
-
Cold
- min:
-
Minimum
- max:
-
Maximum
References
Adekunte, A. O., Tiwari, B. K., Cullen, P. J., Scannell, A. G. M., & O’Donnell, C. P. (2010). Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chemistry, 122(3), 500–507.
Ajmera, S. (2006). The effects of different cooling times and temperatures on tomato sauce lycopene content. MSc thesis: Bowling Green State University.
Albadr, J., Tayal, S., & Alasadi, M. (2013). Heat transfer through heat exchanger using Al2O3 nanofluid at different concentrations. Case Studies in Thermal Engineering, 1, 38–44.
Choi, S. U. S., Zhang, Z. G., Yu, W., Lockwood, F. E., & Grulke, E. A. (2001). Anomalous thermal conductivity enhancement in nanotube suspensions. Applied Physics Letters, 79(14), 2252–2254.
Cortes, C., Esteve, M. J., & Frigola, A. (2008). Color of orange juice treated by high intensity pulsed electric fields during refrigerated storage and comparison with pasteurized juice. Food Control, 19(2), 151–158.
Coulson, J. M., & Richardson, J. F. (1999). Chemical engineering design (third ed.pp. 635–702). London: Butterworth Heinemann.
Farajollahi, B., Etemad, S. G., & Hojjat, M. (2010). Heat transfer of nanofluids in a shell and tube heat exchanger. International Journal of Heat and Mass Transfer, 53, 12–17.
Food and Drug Administration (FDA). (2004). Guidance for industry: juice HACCP hazards and controls guidance, first ed.
Ho, C. J., Wei, L. C., & Li, Z. W. (2010). An experimental investigation of forced convective cooling performance of a microchannel heat sink with Al2O3/water nanofluid. Applied Thermal Engineering, 30, 96–103.
Jabbari, S. S., Jafari, S. M., Dehnad, D., & Shahidi, S.A. (2016). Effects of thermal processing by nanofluids on vitamin C, total phenolics and total soluble solids of tomato juice. Journal of Food Science and Technology, In Press.
Jafari, S. M., Azizi, D., Mirzaei, H., & Dehnad, D. (2016). Comparing quality characteristics of oven-dried and refractance window-dried kiwifruits. Journal of Food Processing and Preservation, 40(3), 362–372.
Jafari, S. M., Saremnejad, F., Dehnad, D., & Rashidi, A. M. (2016a). Evaluation of performance and thermophysical properties of alumina nanofluids as heating medium in a shell and tube exchanger. Journal of Food Process Engineering, Under Review.
Jafari, S. M., Saremnejad, F., Dehnad, D., & Rashidi, A. M. (2016b). Nano-fluid thermal processing of watermelon juice in a shell and tube heat exchanger and evaluating its qualitative properties. Innovative Food Science and Emerging Technologies, Under Review.
Keblinski, P., Phillpot, S. R., Choi, S. U. S., & Eastman, J. A. (2002). Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). International Journal of Heat and Mass Transfer, 45, 855–863.
Luciu, R. S., Mateescu, T., Cotorobai, V., & Mare, T. (2009). Nusselt number and convection heat transfer coefficient for a coaxial heat exchanger using Al2O3–water pH=5 Nano-fluid. Bulletin of the Polytechnic Institute of Jassy, Constructions, Architechture Section, 2, 71–80.
Mare, T., Halelfadl, S., Sow, O., Estelle, P., Duret, S., & Bazantay, F. (2011). Comparison of the thermal performances of two nanofluids at low temperature in a plate heat exchanger. Experimental Thermal and Fluid Science, 35(8), 1535–1543.
Nasiri, M., Etemad, S. G., & Bagheri, R. (2011). Experimental heat transfer of nanofluid through an annular duct. International Communications in Heat and Mass Transfer, 38(7), 958–963.
Nguyen, C. T., Roy, G., & Lajoie, P. R. (2005). Refroidissement des microprocesseurs a haute performance en utilisant des Nano-fluids. In Proceedings of Congres Francais de Thermique SFT, Université de Reims Champagne-Ardenne, France, pp. 483–488.
Pak, B. C., & Cho, Y. I. (1998). Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Experimental Heat Transfer, 11, 151–170.
Palm, S. J., Roy, G., & Nguyen, C. T. (2006). Heat transfer enhancement with the use of nanofluids in radial flow cooling systems considering temperature-dependent properties. Applied Thermal Engineering, 26, 2209–2218.
Pandey, S. D., & Nema, V. K. (2012). Experimental analysis of heat transfer and friction factor of nanofluid as a coolant in a corrugated plate heat exchanger. Experimental Thermal and Fluid Science, 38, 248–256.
Peyghambarzadeh, S. M., Hashemabadi, S. H., Hoseini, S. M., & Seifi Jamnani, M. (2011). Experimental study of heat transfer enhancement using water/ethylene glycol based nanofluids as a new coolant for car radiators. International Communications in Heat and Mass Transfer, 38, 1283–1290.
Pritiviraj, M., & Andrews, M. J. (1996). A numerical investigation of the 3-D flow in shell and tube heat exchangers, Proceedings of the ASME Heat Transfer Division, Vol. 4, ASM.
Putra, N., Septiadi, W. N., Julian, G., Maulana, A., & Irwansyah, R. (2013). An experimental study on thermal performance of Nano-fluids in microchannel heat exchanger. International Journal of Technology, 2, 167–177.
Richardson P. (2008). Pack processed foods improving quality, first ed., Woodhead Publishing, p.34.
Wen, D., & Ding, Y. (2004). Experimental investigation into convective heat transfer of nanofluids at the entrance region under laminar flow conditions. International Journal of Heat and Mass Transfer, 47, 5181–5188.
Worth, D., & Mascone, C. F. (1991). Heat transfer heads into the 21st century, Chemical Engineering Progress.
Xuan, Y., & Li, Q. (2003). Investigation on convective heat transfer and flow features of Nano-fluids. Journal of Heat Transfer, 125, 151–155.
Xuan, Y., & Roetzel, W. (2000). Conceptions for heat transfer correlation of Nano-fluid. International Journal of Heat and Mass Transfer, 43, 3701–3707.
Zeinali Heris, S., Noie, S. H., Talaii, E., & Sargolzaei, J. (2011). Numerical investigation of Al2O3/water nanofluid laminar convective heat transfer through triangular ducts. Nanoscale Research Letters, 6(1), 179–189.
Acknowledgment
The Iran National Science Foundation (INSF) and the Iran Nanotechnology Initiative Council (INIC) are appreciated for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jafari, S.M., Jabari, S.S., Dehnad, D. et al. Heat Transfer Enhancement in Thermal Processing of Tomato Juice by Application of Nanofluids. Food Bioprocess Technol 10, 307–316 (2017). https://doi.org/10.1007/s11947-016-1816-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-016-1816-9