Abstract
Active food packaging containing antimicrobial additive goes beyond traditional functions of packaging, once it can extend food shelf life maintaining its quality, safety and reducing postharvest losses by controlling food spoilage. Among several antimicrobial additives employed in polymeric films for packaging, metallic nanoparticles outstand due to its facility for synthesis, low-cost of production, and intense antimicrobial properties. In this work, extruded plain films of low-density polyethylene (LDPE) containing silver nanoparticles (AgNPs) embedded in SiO2 and TiO2 carriers (namely MS and MT, respectively) were produced and used as active packaging for maintaining the physicochemical and microbiological quality of carrots (Daucus Carota L. cv. Brasília). The neat (LDPE) and composite films containing MS and MT were characterized by scanning electron microscopy and permeability to oxygen and used for packaging fresh-cut sliced carrots stored at 10 °C for 10 days. After the storage time, the physicochemical properties of carrots were characterized, while the antimicrobial properties of films and AgNP migration were investigated. Our results revealed that both MT and MS packages showed antimicrobial activity even for films containing low concentration of AgNP. In addition, AgNP antimicrobial activity demonstrated to be carrier-dependent, once MT-LDPE showed improved performance compared to MS-LDPE. Regarding the physicochemical properties of packaged carrot, lower soluble solids and weight loss and higher levels of ascorbic acid were observed for carrots packaged with MT-LDPE films (compared to MS-LDPE), leading to a better postharvest quality conservation. Such differences observed in physicochemical properties of carrots are related to the distinct antimicrobial and film permeability properties for each composite film. In addition, under the conditions employed in this study, AgNP migration from the packages to fresh-cut carrot was not observed, which is highly desirable for food packaging safety, indicating the potential of such active packages for food preservation application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carrot (Daucus carota L.) consumption has increased in the last decades, especially due to its nutritional composition, including vitamin C and other phenolic compounds, that can benefit human health (Alasalvar et al. 2005; Klaiber et al. 2005; Kreutzmann et al. 2008). Although carrot stands as one of the most consumed vegetables in fresh-cut form (sliced, diced, grated) due to its versatility (Alasalvar et al. 2005). the susceptibility of fresh-cut carrot to microbial spoilage strongly limits its shelf life (Emmambux and Minnaar 2003).
In general, fresh-cut fruits and vegetables are a healthy source of food with nutritional and sensory characteristics equivalent to intact products although conveniently displayed to the consumer (Zhang et al. 2013; Luo et al. 2014). Washing, peeling, and cutting operations promote physiological, biochemical, and microbiological changes that accelerate the process of deterioration such as browning, discoloration, and generation of unpleasant odors and flavors. Such process can lead to the loss of nutritional value and firmness reducing the shelf life of the product (Emmambux and Minnaar 2003; Saltveit 2003; Ragaert et al. 2007; Mastromatteo et al. 2012). High respiration rate, water loss, and improper storage results in weight loss and metabolizing protein, which are favorable conditions for the proliferation of different microorganisms responsible for accelerating the deterioration process, which drastically decreases the quality and shelf life of various foods (Mohammed Fayaz et al. 2009).
The alternatives for enhancing food shelf life have gained enormous possibilities with nanotechnology, which enables, for instance, the fabrication of active food packaging combining properties of external barrier and antimicrobial agents. Such approach is capable of inhibiting or retarding microbial growth, and as a consequence, can slow down food deterioration providing extension of shelf-life (Azeredo 2013). Silver-based materials present intense biocidal effect for several species of bacteria, encouraging its application for packaging (Bosetti et al. 2002; Yang et al. 2009). In terms of safety regulation, the USA (EFSA 2005) sets a limit of silver ions of 0.05 mg of Ag/kg in food matrices (Fernández et al. 2009). However, the exposure risk of consumers relies on the migration of these nanoparticles from the packages to the food (Simon et al. 2008). Currently, only few experimental data about the migration of nanoparticles for foods are available in the literature (Simon et al. 2008; Cushen et al. 2014). In this context, the aim of this study was to produce plain films of low-density polyethylene (LDPE) containing silver nanoparticles (AgNPs) embedded in SiO2 and TiO2 carriers (namely silica (MS) and titanium dioxide (MT), respectively) and use them as active packaging for evaluating the physicochemical and microbiological quality of carrots (Daucus Carota L. cv. Brasília), as well as to investigate the risks of AgNP migration to carrots.
Materials and Methods
Film Processing
Two masterbatches based on LDPE containing AgNPs embedded in silica (SiO2) and in titanium dioxide (TiO2), named MS and MT, respectively, were employed. Every masterbatch (MS and MT) was mixed with pure LDPE pellets (PB 608, obtained from Quattor®) in plastic bags for 3 min, using mass percentage of 100:0, 50:50, and 2.5:97.5, according to the procedure previously described in Becaro et al. (2015a). The mixtures were extruded by a benchtop single-screw extruder (AX Plastic) using three heating zones, set with the following parameters: temperatures of 160 °C (zone 1), 145 °C (zone 2), and 130 °C (zone 3), and screw speed of 35 rpm for all materials. After the three heating zones reached equilibrium temperature, the compositions were extruded using a blown film die, and samples were collected as plain films with a thickness of 61 ± 11 μm and width of 18 cm, yielding the samples named MS 2.5, MS 50, MS 100 and MT 2.5, MT 50, MT 100.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) images allowed the identification of morphological features of the composite films produced, including the agglomerates present in the films. The samples were prepared by cryogenic fracture and imaged using a FEI microscope, Inspect S50 model and 25 kV, in backscattering mode. To analyze the chemical composition of the films, energy dispersive X-ray spectroscopy (EDS) measurements were carried out.
Permeability Analysis
The rate of oxygen permeability was determined by coulometric method, according to standard procedure described in D3985-05 (ASTM International 2005) using an Oxtran instrument (MOCON) Model 2/20, operating with pure oxygen as permanent gas. The test was carried out at 23 °C in dry a environment (dehumidified with silica gel) for 48 h. The effective permeation area of each specimen was determined as 100 cm2. The results were corrected to 1 atm of oxygen partial pressure gradient. Once TPO2 was determined, we calculated the coefficient of oxygen permeability (P) from the permeability rate, as follows:
where:
- P :
-
oxygen permeability coefficient (mL (CNTP) μmm2day−1 atm−1)
- TPO2 :
-
oxygen permeation rate (mL (CNTP) m2day−1)
- e :
-
average thickness of the specimen (μm)
- p :
-
partial pressure of oxygen in the gas permeating side of the diffusion cell (1 atm), once the partial pressure of O2 on the side of dragging gas (N2 + H2) is null.
Carrot Processing and Packaging Procedures
Carrots (Daucus Carota L. cv. Brasília) were purchased from a local commercial producer (Rio Claro city, São Paulo State, Brazil) and subjected to a selection regarding physical integrity, size, and color. The carrots were washed under running water and then immersed into a hypochlorite solution (200 mg L−1) for 10 min. After being peeled (using a manual peeler), the carrots were cut into slices of 3 cm of diameter, by using a food multiprocessor (Philco®, AllmOne PR2), and were were sequentially sanitized by immersion into Sumaveg® solution (chlorinated water dihydrate 20 mg L−1) for 3 min. The sliced carrots were then centrifuged using a domestic food centrifuge (Consul, Brazil) for 40 s, and each batch was then divided into 50-g portions. The carrot samples were manually packaged in plastic film bags (MS 2.5, MS 50, MS 100 and MT 2.5, MT 50, MT 100) and stored at 10 °C (±2 °C) and UR 88 % during 10 days. The plastic film bags were previously sanitized by immersion in 70 % ethanol and dried at room temperature (approximately 23 °C). Physico-chemical and microbiological analyses were performed at every 2 days.
Physico-Chemical Analyses
Soluble Solids
The concentration of soluble solids (SS) in homogenized carrot juice was determined with the aid of a digital refractometer Atago RX-5000cx bench. The results were expressed as degrees Brix (Adolfo Lutz Institute 2008).
Titratable Acidity
The content of titratable acidity (TA) was determined by using 10 mL of carrot juice diluted in 50 mL of distilled water by titration with NaOH 0.1 N. The results were expressed in grams of citric acid per 100 mL (Adolfo Lutz Institute 2008).
Ratio (SS/TA)
The ratio rate was determined by the ratio between the two variables (SS/TA).
pH
The pH values were determined using a benchtop potentiometer (Quimis Q400A) from the juice obtained from 50-g carrot (Adolfo Lutz Institute 2008).
Ascorbic Acid (AA)
The ascorbic acid content was determined by titration using a solution of 2.6 diclofenolindophenol (DCFI) at 0.1 % to obtain a clear permanent pink coloration, using 10 mL of carrot juice diluted in 50 mL of 0.5 % oxalic acid at 5 °C, according to Adolfo Lutz Institute (2008). The results were expressed in milligrams per 100 mL of juice.
Weight Loss
The percentage of weight loss was determined according to the following equation:
where: %ML(t) is the percent weight loss at time t; M 0 is the initial sample mass and M (t) is the sample mass at time t. The sample mass was determined by a digital precision balance (±0.01 g) from Marte AS 2000C. At each sampling time, the mass was measured three times using different samples.
Firmness Measurement
Firmness measurements were performed with the assistance of a digital bench texturometer TA.XT Plus Firmness Analyzer®. The compression force was applied by using a cylindrical flat probe of 2 mm (diameter), speed of 10 mm/s, and penetration distance of 2 mm. The mean value (and corresponding standard deviation) firmness was collected using three regions from the cortex of carrot samples (slices with 3 cm diameter), and the results were expressed in Newton (N).
Microbiological Analysis
Microbiological analyses were carried out in carrot samples of 50 g, which were washed in 1 % sterile peptone water with proportional volume, constituting the 10−1 dilution. The 10−2 and 10−3 dilutions were obtained by consecutive dilutions of sterile peptone water (1 %). After the incubation time for each microorganism, the counting of the colonies was carried out. The arithmetic mean of triplicate values was multiplied by the respective dilution. The results were expressed by colony-forming unit per milliliter of product (Downes and Ito, 2001). The following analyses were performed:
-
(a)
For total count of yeasts and molds, 3M Petrifilm plates, approved and stamped by the Association Of Analytical Communities (AOAC 2002) (method 997.02), were employed. The plates were inoculated with 1 mL of each dilution in duplicate and then incubated at 25 °C for 3 to 5 days.
-
(b)
For total count of psychrotrophic and mesophilic aerobic microorganisms, 3M Petrifilm plates, approved and stamped by the AOAC (method 990.12), were employed. The plates were inoculated with 1 mL of each diluted solution in triplicate. After plating, the plaques were incubated at 7 °C for 24 h. For total count of aerobic mesophilic, the plates were incubated at 35 °C for 48 h.
-
(c)
For total count of coliforms, we used 3M Petrifilm plates, approved and stamped by the AOAC (method 991.14). The plates were inoculated with 1 mL of each dilution in triplicate. The plates were then incubated at 35 °C for 24 h. In case of positive result for total coliforms, the same plates were incubated again at 45 °C for 24 h for the detection of Escherichia coli.
Migration Test
For the AgNP migration test, carrot cubes (samples of 50 g) packaged in plastic films with distinct AgNP concentrations (MS 2.5, MS 50, MS 100 and MT 2.5, MT 50, MT 100) were stored (in triplicate) for 10 days at 10 °C (±2 °C) and RH of 88 %. After the storage period, 5–8 g of carrot samples were removed and placed in a digester tube, where 5 mL of a mixture of nitric acid and perchloric acid (ratio 2:1) was added. The digester tube was heated up at 150 °C and kept at this temperature for about 1 h using a heating plate. The temperature was then increased up to 220 °C and kept for 2 h. The samples were then removed from the heating plate, and deionized water was added to digester tube up to 75 mL. The samples were subsequently homogenized, and the digestion was carried out with nitric acid and perchloric acid in ratio 5:2, at 150 °C for 2 h, for ICP-OES (inductively coupled plasma optical emission spectrometry) analysis using a Perkin Elmer equipment. The following instrumental parameters were employed for the ICP analysis: power of 1400 W; nebulizer gas flow of 0.8 L/min; auxiliary gas flow rate of 0.2 L/min; flame set in the axial position; and wavelength at 328,068 nm (model Optima 7000DV) (Kimura and Miller 1957).
Statistical Analyses
The effect of silver nanoparticles addition on the film antimicrobial properties and carrots physicochemical variables was evaluated by multivariate data analysis, including principal component analysis (PCA), which is a statistical method that condenses relevant information into a smaller set of orthogonal variables called principal components (PCs), in an attempt to assess the discriminatory power of the original variables (Goyeneche et al. 2014). The principal components, whose eigenvalues were greater than unity (Kaiser 1958). were considered for the analysis. Each pair of PCs generates a two-dimensional representation of the original sample space, called biplot, which demonstrates the regions of maximum variability. The coefficients of the linear functions, which define the PCs, were used to interpret the data, using the sign and relative size of the coefficients as an indication of the weight to be assigned to each variable. Statistical analysis was performed using SAS (SAS version 9, SAS institute, Cary, NC, USA). Permeability test results were submitted to analysis of variance (ANOVA) and mean values compared by Duncan’s multiple range test at the 5 % significance level.
Results and Discussion
SEM Images
Figure 1 displays SEM images of the cross-section of samples of plastic films containing AgNP embedded in silica (MS) and titanium dioxide (MT) carriers. According to Fig. 1, SEM image of sample MS 100 displays bright areas, corresponding to Si, O, and Ag elements, while SEM images of MT 2.5, 50, and 97.5 display bright areas corresponding to Ti, O, and Ag elements, respectively, according to EDS analysis previously reported in literature (Becaro et al. 2015a). Red circles in SEM images indicate irregularities on the film surface, while the blue rectangles indicate the agglomerates of silicon in MS samples.
Permeability Tests
Figure 2 displays the results of the O2 permeability coefficient and standard deviation (bar). One notes that higher values of permeability coefficient (263 and 294 mL μm m−2 dia−1 atm−1, respectively) are displayed for samples MS 50 and MS 100, which are significantly different from the other films (p < 0.05). As demonstrated in a previous study by Becaro et al. (2015a) MT films present a higher degree of crystallinity, which can contribute to its lower permeability, once crystalline domains tend to decrease the gas diffusion path, contributing to decrease permeability rate (Weinkauf and Paul 1990; Olkhov et al. 2003; Siracusa 2012). In addition, the presence of irregularities onto the film surface, displayed by SEM analysis (Fig. 1) could also contribute to increase permeability, which was more present in sample MS 100. The higher degree of crystallinity for sample MT can be a consequence of TiO2 nanoparticles (carriers) in film composition, which presence tends to increase the degree of crystallinity of nanocomposites (Nasiri et al. 2012; Ali and Noori, 2014).
Physico-Chemical Parameters
The soluble solid (SS) values oscillated within the range 5.19–8.27 °Brix, according to Fig. 3. In general, fresh-cut carrots packaged by LDPE and MS films tended to present similar values of SS, which are slightly higher than the values found for carrots packaged by MT films. The lower SS values for fresh-cut carrots packaged by MT films can be a consequence of the lower permeability of MT films, which decreased the metabolic activity of the carrot tissue, leading also to a decrease in weight loss (see also in Fig. 7).
Citric acid, the organic acid presented in high content in carrots, can be determined by titratable acidity analyses (TA). Figure 4 demonstrates that for all the samples, only small oscillations in TA values (close to 0.04 g citric acid 100 mL−1) could be observed during storage time. Also, pH values of samples were similar throughout the storage time, close to 6.0 (Mastromatteo et al. 2012, Lima et al. 2001).
SS/TA ratio is related to flavor in fruits (Chitarra and Chitarra 2005) and is an indicative of fruit ripeness level. Figure 5 shows that carrots packaged by LDPE and MS films tend to present a constant ratio along the storage days, while those packaged by MT films tend to present a subtle decay on ratio as the storage days pass by. This behavior tended to follow the behavior of SS variable (see Fig. 3).
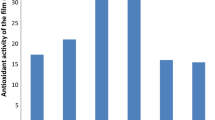
Results of ascorbic acid (AA) contents are represented in Fig. 6. A similar behavior for AA change was observed for all the samples (fresh-cut carrots packaged in LDPE, MS, and MT films). Carrot slices showed 5.34 mg 100 mL−1 of carrot juice (day 0) according to Fig. 6, which tended to decrease along the storage days.
Figure 7 shows that weight loss was observed for all the samples over the storage period. Sliced carrots packaged by MS films presented a more pronounced weight loss than carrots packaged by MT films. However, for carrots packaged by MT 2.5 film, it cannot be affirmed that significant changes occurred during the storage days, due to the high standard deviation. In addition, no significant differences for firmness was found for carrots packaged with the distinct films, as can be sen in Fig. 8.
In order to better understand the correlation among the physicochemical parameters of carrots packaged with distinct AgNP-containing films, the statistical technique Principal Component Analysis (Cabezas-Serrano et al. 2009; Radziejewska-Kubzdela et al. 2014; Goyeneche et al. 2014) was employed. In our study, the four principal components (PC1, PC2, PC3, and PC4) explain 84 % of the total variability (Table 1), where PC1, PC2, PC3, and PC4 explain 27.7, 25.0, 19.8, and 11.5 % of the variabilities, respectively. Each PC identifies the variables more strongly related to the fresh-cut carrots quality parameters and how they contribute to explain the total variability (Radziejewska-Kubzdela et al. 2014).
As can be seen in Fig. 9 (PCA plot of PC1 and PC2)., PC1 was positively correlated with compositional factor, SS, ratio, pH, and weight loss. On the other hand, this component was negatively correlated with TA, AA, aerobic mesophilic count (AM), coliform count (TC), and firmness. In order of significance, the attributes that are more related to this component are ratio (0.96), TA (−0.87), and SS (0.67). These are the main parameters that express the quality of fresh-cut products and estimate the shelf life of products. PC1 reveals a contrast between SS and Ratio (correlations of 0.67 and 0.96, respectively) versus TA (correlation 0.87). PC2 was positively correlated with microbiological properties (AM and TC) and negativity correlated with all other variables. Bacterial growth has the opposite behavior to quality parameters.
PC1 + PC2 contains 52.7 % of the total variability of the set of original data regarding physicochemical and microbiological parameters. The graph shows that compositional factors are positively correlated with pH, SS, and ratio (on the positive side of PC1) and negativity correlated with TA and AA. The attributes that are more related to PC2 were TC (0.76) AM (0.75), pH (0.57) and AA (−.56). Probably, pH and AA contents allowed an ideal environment to the growth of microorganisms (Hong et al. 2010). Film MSs (Fig. 9) were positively correlated with PC1 and can be characterized by fresh carrots presenting higher ratio, SS, and weight loss.
Microbiological Analyses
The present data provides information about the antibacterial capability of films containing AgNP used to package fresh-cut carrots storaged for 10 days at 10 °C. As mentioned, colony-forming units (CFUs) were determined by standard methodologies. Antimicrobial activity on fresh-cut carrots of MS and MT films are displayed in Figs. 10 and 11. The variation in mesophilic aerobic count (MA) in fresh-cut carrots varied from 8.35 × 103 to 4.45 × 108 CFU mL−1 (Fig. 10). The total TC for fresh-cut carrots ranged from 1.33 × 105 to 4.45 × 108 CFU mL−1 (Fig. 10).
When comparing only MS samples, it was observed that carrots packaged by MS 2.5 showed the lowest MA during days of storage, (Fig. 10a). The same behavior was found for MT films, where MT 2.5 presented a significant inhibitory effect on MA growth (Fig. 10b). Until the eighth day, MT 2.5 showed a good antimicrobial capacity on fresh-cut carrots, although on the last day of storage this capacity was decreased, probably because the MA population was high enough to impair superficial contact between AgNP and bacteria colony. Although the ideal growth temperature of MA microflora occurs at 35 °C, these microorganisms are able to grow at low temperatures with a reduced growth rate. Moreover, the MA count values shown by sliced carrots packaged by 2.5 films are within the acceptable limit for consumer consumption of uncooked foods (Solberg et al. 1990).
However, for samples containing a higher concentrations of the masterbatch MS, a different behavior was observed. For carrots packaged by film MS 100, analysis revealed the highest microbial growth during the days of analysis (Fig. 10a). This result probably arises due to its higher O2 permeability coefficient (see Fig. 2), which provides a more suitable environment for microbial growth. The weight loss data found by carrots packaged by MS 100 (see Fig. 7) corroborates these results. MT 50 and MT 100 fresh-cut carrots showed similar performances (Fig. 10b), revealing that higher concentration of masterbatches with silver nanoparticles does not interfere significantly on the antimicrobial action on fresh-cut carrots.
Figure 11 shows that antimicrobial activity of films against coliforms remained low during the second day of storage but began to increase from the third day on. Similarly to AM counting, MS 100 carrots showed higher count to TC, exceeding 108 CFC mL−1, due to its higher O2 permeability, as discussed previously. MS 2.5 carrots exhibited higher antimicrobial activity compared with MS 50 and MS 100 samples during the test days. On the other hand, carrots packed by film MT showed lower count to TC, not exceeding 1.5 × 107 CFC mL−1. Comparatively, MT 2.5 exhibed the best antimicrobial activity, (when comparing MT samples), with 8.3 × 103 UFC mL−1 on second day of storage. Such behavior can be related to the nanoparticles dispersion into the films, which in low concentrations can be more dispersed, becoming more effective regarding its antimicrobial activities.
MS 100 carrots showed an increase in bacterial growth for both AM and TC over the days of storage, achieving 4.5 × 108 and 4.45 × 108 CFU mL−1, respectively. It complies with SS results once carrots packaged by MS 100 showed lower values of degrees Brix than LDPE, MS 2.5 and MS 50 carrots. The main energy source to microorganism growth is sugar, thus a decrease on SS levels during the storage can indicate that microorganisms are developing into the medium (Lima et al. 2003). It is also important to emphasize that the presence of E. coli and mold and yeast population was not verified in these test conditions throughout the storage time.
Films with low masterbatch concentrations (2.5 %), showed the best results for the microbiological tests. It is important to mention that a lower AgNP concentration can also enhance the dispersion of masterbatches within the polymer matrix favoring the surface contact of AgNP with carrots (Fernández et al. 2010). Another important point is that the low concentrations of carriers for MS 2.5 and MT 2.5 yielded similar and lower permeability coefficients compared to MS 50 and MS 100, which can also have contributed to the antimicrobial properties.
Migration Test
Regarding ICP analysis of carrot samples packaged in films containing AgNP, no silver traces were found, which are a indicative that, up to the detection limit of the technique employed (10 ppb), no migration of AgNP occurred. The non-migration of AgNP is highly desirable for food packaging application, avoiding, for instance, toxic effects for humans and animals (Becaro et al. 2015b; Griffitt et al. 2012). However, the non-migration observed could also be caused by some factors which include relative short contact time between the carrots and the films and the detection limit of equipment (10 ppb) (Simon et al. 2008, Walczak et al. 2012).
Conclusion
Our results reveal that films containing AgNP were efficient as active packaging for sliced carrots, especially when AgNP was employed at low concentrations. Specifically, films with low concentration of AgNP (MS 2.5 and MT 2.5) showed higher antimicrobial activity probably due to the an optimized contact area between the AgNP and the microorganism. Films containing AgNP embedded in TiO2 carries showed lower permeability coefficient (although for low concentration of carriers, no significant difference was observed), which might have influenced the antimicrobial properties. For instance, carrots packaged with MT films presented lower mesophilic aerobic and total coliforms count, weight loss, indicating better physicochemical and microbiological properties. Besides, fresh-cut carrots packaged with MT films maintained ascorbic acid content of fresh-cut carrot. The presence of AgNP has not influenced significantly the pH and firmness values of packaged carrots. In addition, for the experimental conditions employed, AgNP migration from the films to the sliced carrots was not observed, which is highly desirable for food packaging applications.
References
Adolfo Lutz Institute. (2008). Métodos físico-químicos para análise de alimentos (4th ed.). São Paulo: Instituto Adolfo Lutz. 1020 p.
Alasalvar, C., Al-Farsi, M., Quantick, P. C., Shahidi, F., & Wiktorowicz, R. (2005). Effect of chill storage and modified atmosphere packaging (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots. Food Chemistry, 89, 69–76.
Ali, N. A., & Noori, F. T. M. (2014). Gas barrier properties of biodegradable polymer nanocomposites films. Chemistry and Materials Research, 6(1), 44–51.
AOAC. (2002). Official methods of analysis (17th ed.). Washington, DC: Association of Official Analytical Chemists International.
ASTM International (2005). ASTM D 3985–05: Standard test method for oxygen transmission rate through plastic film and sheeting using a coulometric sensor. Philadelphia 7p.
Azeredo, H. M. C. (2013). Antimicrobial nanostructures in food packaging. Trends in Food Science & Technology, 30(1), 56–69.
Becaro, A., Puti, F. C., Correa, D. S., Paris, E. C., Marconcini, J. M., & Ferreira, M. D. (2015a). Polyethylene films containing silver nanoparticles for applications in food packaging: characterization of physico-chemical and anti-microbial properties. Journal of Nanoscience and Nanotechnology, 15(3), 2148–2156.
Becaro, A. A., Jonsson, C. M., Puti, F. C., Siqueira, M. C., Mattoso, L. H. C., Correa, D. S., Ferreira, M. D. (2015b). Toxicity of PVA-stabilized silver nanoparticles to algae and microcrustaceans. Environmental Nanotechnology, Monitoring & Management, 3, 22–29. doi:10.1016/j.enmm.2014.11.002.
Bosetti, M., Masse, A., Tobin, E., & Cannas, M. (2002). Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomaterials, 23, 887–892.
Cabezas-Serrano, A. B., Amodio, M. L., Cornacchia, R., Rinaldi, R., & Colelli, G. (2009). Suitability of five different potato cultivars (Solanumtuberosum L.) to be processed as fresh-cut products. Postharvest Biology and Technology, 53, 138–144.
Chitarra, M. I. F., & Chitarra, A. B. (2005). Pós-colheita de frutos e hortaliças: fisiologia e manuseio (2nd ed.). Lavras: UFLA. 785 p.
Cushen, M., Kerry, J., Morris, M., Cruz-Romero, M., & Cummins, E. (2014). Evaluation and simulation of silver and copper nanoparticle migration from polyethylene nanocomposites to food and an associated exposure assessment. Journal of Agricultural and Food Chemistry, 62(6), 1403–1411.
Downes, F. P., & Ito, K. (Eds.). (2001). Compendium of methods for the microbiological examination of foods (4th ed.). Washington: APHA. 676 p.
EFSA (2005). Opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. http://www.efsa.eu.int/science/sc_commitee/sc_opinions/1201_en.html.
Emmambux, N. M., & Minnaar, A. (2003). The effect of edible coatings and polymeric packaging films on the quality of minimally processed carrots. Journal of Science of Food and Agriculture, 83(10), 1065–1071.
Fernández, A., Soriano, E., Lopez Carballo, G., Picouet, P., Lloret, E., Gavara, R., et al. (2009). Preservation of aseptic conditions in absorbent pads by using silver nano-technology. Food Research International, 42, 1105–1112.
Fernández, A., Picouet, P., & Lloret, E. (2010). Cellulose-silver nanoparticle hybrid materials to control spoilage-related microflora in absorbent pads located in trays of fresh-cut melon. International Journal of Food Microbiology, 142, 222–228.
Goyeneche, R., Roura, S., & Di Scala, K. (2014). Principal component and hierarchical cluster analysis to select hurdle technologies for minimal processed radishes. LWT - Food Science and Technology, 57, 522–529.
Griffitt, R. J., Brown-Peterson, N. J., Savin, D. A., Manning, C. S., Boube, I., Ryan, R., Brouwer, M. (2012). Effects of chronic nanoparticulate silver exposure to adult and juvenile sheepshead minnows (Cyprinodon variegatus). Environmental Toxicology and Chemistry, 31, 160–217
Hong, H., Qiu, J., & Liang, Y. (2010). Environmental factors influencing the distribution of total and fecal coliform bacteria in six water storage reservoirs in the Pearl River Delta Region, China. Journal of Environmental Sciences, 22(5), 663–668.
Kaiser, H. F. (1958). The varimax criterion for analytic rotation in factor analysis. Psychometrika Urbana, 23(3), 187–200.
Kimura, F. T., & Miller, V. (1957). Chromic oxide measurement. Improved determination of chromic oxide in cow feed and feces. Journal of Agricultural and Food Chemistry, 5, 216.
Klaiber, R. G., Baur, S., Koblo, A., & Carle, R. (2005). Influence of washing treatment and storage atmosphere on phenylalanine ammonia-lyase activity and phenolic acid content of minimally processed carrot sticks. Journal of Agricultural and Food Chemistry, 53(4), 1065–1072.
Kreutzmann, S., Christensen, L. P., & Edelenbos, M. (2008). Investigation of bitterness in carrots (Daucuscarota L.) based on quantitative chemical and sensory analyses. LWT--Food Science and Technology, 41(2), 193–205.
Lima, K. S. C., Grossi, J. L. S., Lima, A. L. S., Alves, P. F. M. P., Coneglian, R. C. C., Godoy, R. L. O., & Sabaa-Srur, A. U. O. (2001). Efeito da irradiação ionizante γ na qualidade. Ciência e Tecnologia de Alimentos, 21(2), 202–208.
Lima, K. S. C., Lima, A. L. S., Luchese, R. H., Godoy, R. L. O., & Sabaa-Srur, A. U. O. (2003). Cenouras minimamente processadas em embalagens com atmosferas modificadas e tratadas com radiação gama: avaliação microbiológica, físico-química e química. Ciência e Tecnologia de Alimentos, 23(2), 240–250.
Luo, Z., Wang, Y., Jiang, L., & Xu, X. (2014). Effect of nano-CaCO3-LDPE packaging on quality and browning of fresh-cut yam. LWT - Food Science and Technology. doi:10.1016/j.lwt.2014.09.021.
Mastromatteo, M., Conte, A., & Del Nobile, M. A. (2012). Packaging strategies to prolong the shelf life of fresh carrots (Daucus carota L.). Innovative Food Science & Emerging Technologies, 13, 215–220.
Mohammed Fayaz, A., Balaji, K., Girilal, M., Kalaichelvan, P. T., & Venkatesan, R. (2009). Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. Journal of Agricultural and Food Chemistry, 57, 6246–6252.
Nasiri, A., Shariaty-Niasar, M., & Akbari, Z. (2012). Synthesis of LDPE/Nano TiO2 nanocomposite for packaging applications. International Journal of Nanoscience and Nanotechnology, 8(3), 165–170.
Olkhov, A. A., Vlasov, S. V., Iordanskii, A. L., Zaikov, G. E., & Lobo, V. M. M. (2003). Water transport, structure features and mechanical behavior of biodegradable PHB/PVA blends. Journal of Applied Polymer Science, 90(6), 1471–1476.
Radziejewska-Kubzdela, E., Czapski, J., Czaczyk, K., & Biegańska-Marecik, R. (2014). The effect of pre-treatment and modified atmosphere packaging on contents of phenolic compounds and sensory and microbiological quality of shredded celeriac. Journal of the Science of Food and Agriculture, 94(6), 1140–1148.
Ragaert, P., Devlieghere, F., & Debevere, J. (2007). Role of microbiological and physio-logical spoilage mechanisms during storage of minimally processed vegetables. Postharvest Biology and Technology, 44, 185–194.
Saltveit, M. E. (2003). Fresh-cut vegetables. In J. A. Bartz & J. K. Brecht (Eds.), Postharvest physiology and pathology of vegetables (pp. 691–712). New York: Marcel Dekker.
Simon, P., Chaudhry, Q., & Bakos, D. (2008). Migration of engineered nanoparticles from polymer packaging to food—a physicochemical view. Journal of Food Nutrition Research, 47, 105–113.
Siracusa, V. (2012). Food packaging permeability behaviour: a report. International Journal of Polymer Science, 2012, 1–11.
Solberg, M., Buckalew, J. J., Chen, C. M., Schaffner, D. W., O’neil, K., McDowell, J., Post, L. S., & Borderck, M. (1990). Microbiological safety assurance system for foodservice facilities. Journal of Food Technology, 44, 68–73.
Walczak, A. P., Fokkink, R., Peters, R., Tromp, P., Herrera Rivera, Z. E., Rietjens, I. M., Hendriksen, P. J., & Bouwmeester, H. (2012). Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model. Nanotoxicology, 7, 1198–1210.
Weinkauf, D. H., & Paul, D. R. (1990). The effects of structural order on barrier properties. In W. J. Koros (Ed.), Barrier polymers and structures, chapter 3 (p. 60). Washington: American Chemical Society.
Yang, W., Shen, C., Ji, Q., An, H., Wang, J., Liu, Q., & Zhang, Z. (2009). Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology, 20, 85–102.
Zhang, S., Yu, Y., Xiao, C., Wang, X., & Tian, Y. (2013). Effect of carbon monoxide on browning of fresh-cut lotus root slice in relation to phenolic metabolism. LWT - Food Science and Technology, 53, 555–559.
Acknowledgments
The authors are grateful to CNPq, FAPESP, CAPES, MCTI, and Embrapa from Brazil for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becaro, A.A., Puti, F.C., Panosso, A.R. et al. Postharvest Quality of Fresh-Cut Carrots Packaged in Plastic Films Containing Silver Nanoparticles. Food Bioprocess Technol 9, 637–649 (2016). https://doi.org/10.1007/s11947-015-1656-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1656-z