Abstract
Osmotic dehydration of yacon (Smallanthus sonchifolius) was carried out in varying temperatures (30 and 50 °C) and glycerol or sorbitol concentration (30, 50, and 70 %). The solution of Fick’s law for unsteady state mass transfer in a plane sheet configuration was used to calculate the effective diffusivities of water. Peleg’s model was used to predict the equilibrium condition, which was shown to be appropriate for water loss and solute uptake. It was found to have two rate periods of dehydration. For the above conditions of osmotic dehydration, the effective diffusivity of water was found to be in the range of 5.82 ± 0.68 × 10−10 to 2.15 ± 0.61 × 10−10 m2/s in first period and 1.60 ± 0.28 × 10−10 to 1.29 ± 0.24 × 10−10 m2/s in second period for glycerol tests and, for sorbitol, was 3.82 ± 0.17 × 10−10 to 1.54 ± 0.50 × 10−10 m2/s for the first period and 1.73 ± 0.04 × 10−10 to 1.33 ± 0.06 × 10−10 m2/s for the second. The greatest reduction in water activity was achieved when 70 % of glycerol was used at 50 °C (final a w 0.704 ± 0.010). The treatments with 70 % of solution concentration at 30 °C were repeated, and by adding 20 g/L of calcium lactate in the osmotic solution, it resulted in higher calcium content, strengthening the cell wall for both solutes. Microstructure of the yacon samples (fresh, blanched, and in different conditions of osmotic dehydration) was examined by scanning electron microscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yacon is a native root crop from the Andean region (South America) which interest has increased recently because it is a plant with the largest content of fructooligosaccharides (FOS). FOS are a type of sugar that has a lower caloric value than other sugar types (approximately 25 to 35 % of the calories of normal carbohydrates). The consumption of FOS is also known to promote better health of the intestinal tract (Manrique et al. 2005). Due to its high amount of water, usually 86–90 % of fresh weight of yacon (Habib et al. 2011; Goto et al. 1995), its shelf life under ambient conditions is approximately 1 week. In this context, it is important to apply some technique to increase its shelf life.
Osmotic dehydration (OD) is one alternative to reduce post-harvest losses, and it is a common process for producing dried fruits, which can be directly consumed or used as an ingredient in cakes, pastries, and many others (Mercali et al. 2011). OD is a dewatering process in which cellular materials (mostly fruits and vegetables) are immersed in a hypertonic solution, and water is removed from inside the cells by osmosis. The driving force for the diffusion of water from the tissue into the solution is provided by the higher osmotic pressure or chemical potential of the hypertonic solution (Rastogi and Raghavarao 2004; Rastogi et al. 2002). In addition to dewatering, solutes from the osmotic solution penetrate into the product, and plant solutes (organic acids and sugars) are removed, and considerable shrinkage occurs (Rastogi and Raghavarao 2004; Aguilera and Stanley 1999). The rate of diffusion of water from any material tissues depends upon factors such as temperature and concentration of the osmotic solution, the size and geometry of the material, the solution to material mass ratio, and the level of agitation of the solution. A number of publications have described the influence of these variables on mass transfer rates during osmotic dehydration (Angilelli et al. 2014; Silva et al. 2014b; Mercali et al. 2011; Dionello et al. 2007; Rastogi and Raghavarao 2004; Shi and Le Maguer 2002). Silva et al. (2014b) also stated that the characteristics of the osmotic agent used, such as its molecular weight and ionic behavior, strongly affect dehydration, both water loss and solute gain.
The osmotic agent must have high water solubility, low cost, and positive effect on the sensory properties and must ensure the stability of the final product (Dionello et al. 2007). The most used solutes are sucrose (in fruits and vegetables) and sodium chloride (in meats, fishes, and vegetables), but there are studies in the literature that use alternative osmotic agents. Researchers used sorbitol in the dehydration of apricots (Toğrul and İspir 2008), strawberries (Rizzolo et al. 2007), and green pepper (Ozdemir et al. 2008), among other fruits and vegetables. Scientific works of OD with glycerol include chestnuts (Moreira et al. 2007), grapes (Rózek et al. 2009), Andes berries, and tamarillos (Osorio et al. 2007).
Glycerol and sorbitol are classified as food additives by the Codex Alimentarius (2012). Glycerol is used to improve the texture of foods (plasticizer), as a thickener, and has the advantage of being a microbiological protectant (Codex Alimentarius 2012; Moreira et al. 2007); its caloric value is 4.3 kcal/g, and it is a by-product of biodiesel production. So, its use in osmotic dehydration in industrial scale would be an alternative to decrease the surplus generated. Sorbitol is the polyol mostly found in nature, and its main feature is the sweetening power (up to 70 % of the sweetness of sucrose); it also has chemical, thermal, and bacteriological stability. Sorbitol has low calorie (2.4 kcal/g), is non-cariogenic, and reduces water activity. According to Patel and Goyal (2012), sorbitol is also prebiotic, with proven health-promoting properties, for example reduction of serum cholesterol.
The use of a ternary system (water/sugar/salt) in the osmotic dehydration of plants has been studied by some researchers, and the results have shown higher rates of water loss by addition of salt, even in solutions with lower concentrations of solutes (Mercali et al. 2011). In the present work, aiming to maintain the healthy appeal of the product, calcium lactate was employed as a ternary agent instead of sodium chloride. Several studies have been conducted with dosages up to 2 % calcium lactate (rated as a food additive by Codex Alimentarius 2012), including apples (Castelló et al. 2009), mangos (Torres et al. 2006), and cherry tomatoes (Heredia et al. 2007).
This work aims to study the osmotic dehydration of yacon, through the use of solutes that can be consumed by people with diabetes, keeping their prebiotic characteristics with low energy.
Materials and Methods
Osmotic Dehydration
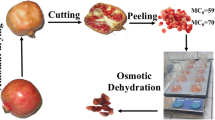
Yacon (Smallanthus sonchifolius) roots were cultivated in the state of São Paulo, Brazil, and obtained in the Supply Centers of the State of Rio Grande do Sul (CEASA) in the city of Porto Alegre, RS, Brazil. They have been selected considering the absence of visual damage and were stored under refrigeration (8 ± 2 °C) until use, which was less than 24 h from time of purchase.
The roots were washed and cut into discs of 5 ± 0.05 cm in diameter and 0.5 ± 0.01 cm in thickness (diameter/thickness >10, characterizing a plane sheet) and weighted. Steam blanching (100 °C) was carried out for 4 min, followed by cooling in an ice bath for 3 min, according to the best condition found by Fante et al. (2013), aiming for FOS preservation. After cooling, the discs were dried on paper towels and weighed.
Osmotic solutions were prepared with distilled water, glycerol, and sorbitol in the concentrations of 30, 50, and 70 %, respectively. These solutes were chosen in a previous work (Brochier et al. 2014). Osmotic dehydration was carried out in an acrylic tank with a coil connected to a thermostatic bath to maintain a constant temperature. The experiments were performed at temperatures of 30 ± 2 and 50 ± 2 °C. Concentration and temperature are important parameters that influence mass transfer, so the worked ranges were chosen after a review of published studies (Castelló et al. 2009; Toğrul and İspir 2008; Osorio et al. 2007; Rizzolo et al. 2007).
A sample-to-solution ratio of 1:20 (w/w, according to Heredia et al. 2007; Rastogi and Raghavarao 1997) was kept in order to guarantee that the concentration of the osmotic media could always be considered constant. The osmotic medium was agitated continuously with a mechanical agitator (Fisatom, model 713; São Paulo, SP, Brazil) at 1000 rpm to ensure the specified concentration on the surface of the yacon discs.
The osmotic dehydration process was performed in a period of 6 h under constant conditions of temperature and agitation. The process time was pre-established in previous experiments and taking into account some works which studied OD for 6 h or less. Rizzolo et al. (2007) worked with strawberries until 6 h of OD process, and other authors used even less time, e.g., Mercali et al. (2010) who studied the osmotic dehydration of blueberries for 5 h and Rastogi and Raghavarao (1997) osmodehydrated carrots for 4 h. Samples were withdrawn from the osmotic solution at determined intervals of time and then drained and dried with a filter paper to remove adhering solution.
The treatments performed with 70 % of solute concentration at 30 °C were also realized including 20 g/L of calcium lactate to the osmotic solution. All the tests were carried out in duplicate.
Analytical Determinations
The analytical determinations were made for fresh and osmotically treated samples. The moisture was measured using an AOAC 930.04 method (1990); soluble solids were measured with a bench type refractometer (model 32-G, Carl Zeiss) using the AOAC 932.12 method (2002), and water activity was directly measured at 25 °C in a thermoconstanter electric hygrometer, LabMaster-aw device (Novasina, Switzerland), using the AOAC 978.18 method (1990). Calcium was analyzed in the fresh and blanched samples treated with 20 g/L calcium lactate. The determinations were carried out using atomic absorption spectrometer with flame with the introduction of a sample solution using pneumatic nebulization, following an adaptation of the AOAC method 975.03 (2006). All analyses were performed in triplicate.
The mass loss (ML), water loss (WL), and solids gain (SG) were calculated according to Eqs. (1), (2), and (3).
where m 0 and m t are the masses of the samples and Xw 0 and Xw t are the moisture content (wet basis) at baseline and at time t, respectively.
Scanning electron microscopy (SEM) was applied to the fresh, blanched, and osmotically dehydrated yacon samples. Approximately 10 mg of the sample was placed on a double-faced adhesive tape fixed to a stub following an adaptation of the method used by Pan et al. (2008). The scanning electron microscope (Hitachi TM3000) was operated at Analy mode (greater electron emission). The images of the samples were obtained at ×200 magnification.
Kinetic Model
The estimation of moisture content when the equilibrium conditions were achieved was made by using Peleg’s (1988) model, shown in Eq. (4):
where X t and X 0 are the moisture content (dry basis) of a sample at dehydration time t and at the beginning, respectively; k 1 and k 2 are the model parameters.
The X t values were expressed according to Eq. (5).
where m Tt is the total mass of samples at the time and m S0 and m St are the mass of solids at baseline and at time t, respectively.
The constant k 2 is related to water concentration at equilibrium conditions, according to Eq. (6)
where X ∞ is the moisture content (dry basis) at equilibrium; this value is necessary to calculate the effective mass diffusivity.
The used model, based on Fick’s unsteady state law of diffusion, determines the amount of water leaving the yacon discs as a function of time. The solution is valid for the following initial and boundary conditions:
where x (m) is the characteristic dimension of the material (half plate thickness) and t (s) is the immersion time. X 0 represents the initial moisture content (dry basis), and when t > 0, on the surface of the sheet (x = L), X ∞ is the moisture content at equilibrium (dry basis). This condition is achieved due to the high convective mass transfer coefficient (k c →∞) obtained through the maintenance of a constant and intense agitation; the problem is symmetrical considering the central plane of the sheet. All results were taken into account the gain of solutes, which modify the dry basis.
The following assumptions were used in the development of the model: samples of yacon are plane sheets (diameter ten times greater than thickness), initial water concentration in the yacon is uniform, the process is isothermal, there is only the exit of water from yacon to solution (other mass transfers in this direction do not occur), shrinkage is neglected, external resistance to mass transfer is negligible, and constant diffusion coefficient conditions were provided.
According to Crank (1975), the dimensionless moisture content obtained by the solution of Fick’s second law for diffusion in a plane sheet, assuming uniform initial distribution and a specified surface concentration as a function of time (t), is given by Eq. (7)
where M t /M ∞ denotes the dimensionless values of water loss, M t denotes the total amount of diffusing water which has left the sheet at time t, M ∞ denotes the corresponding amount of water after infinite time, and Fo is the Fourier number for diffusion, defined as
where D e (m2/s) is the effective mass diffusivity. Equation (7) is the result of the integration of the solution over axial distance, from −L to +L (the thickness of the sheet is 2L).
Values of the Fo were obtained by nonlinear regression analysis from Eq. (7), taking into account the first four terms of the series according to Rastogi and Raghavarao (1997) and Mercali et al. (2011). The Fourier numbers were plotted against the immersion time (using the Excel Solver 2007 version program, according to Eq. (8)), and the effective mass diffusivity values were taken from the slopes of these plots (Rastogi and Raghavarao 1997). For regression analysis and estimation of effective diffusivity, Excel 2007 version was used.
Statistical Analysis
ANOVA was used for the statistical analyses, and the treatments were compared by way of Tukey’s means multiple comparison test. The SAS 9.3 program was used for the statistical tests.
For each treatment, the goodness of fit was determined using the root mean square error (RMSE, Eq. (9)) according to Schmidt et al. (2009)
Results and Discussion
Characterization
Samples of fresh yacon used in the experiments had a moisture content of 88.16 ± 1.60 %, values according to Manrique et al. (2005) who reported that about 85 to 90 % of the fresh weight of yacon consists of water and to Scher et al. (2009) who found moisture of 88.69 ± 0.10 %. The water activity of the fresh yacon was high, showing values of 0.977 ± 0.003, typical of fresh products, but lower than the values 0.994 ± 0.001 found by Scher et al. (2009) and 0.991 by Michels (2005).
The soluble solids of fresh yacon were 11.6 ± 1.1 °Brix which is in agreement with the values reported by Manrique et al. (2005) from 8 to 12 °Brix. The fresh samples showed 81.31 ± 5.12 mg calcium/100 g of dry matter (d.m.) of yacon, a result in accordance with values reported by Ojansivu et al. (2011).
Mass Loss
Figure 1 shows the mass loss over the yacon OD time for both solutes. It was possible to verify the initial increase of mass loss (%) of the samples over time for soaking solutions of glycerol and sorbitol, in the process of osmotic dehydration of yacon. Mass losses were higher in the first hour, due to the greater concentration gradient between the osmotic solution and yacon in the beginning of the OD process. According to Raoult-Wack (1994), under typical conditions used for dehydrating fruits and vegetables at atmospheric pressure, the mass transfer occurs significantly only during the first 2 h of process. Then, mass transfer rates become progressively slower until water loss stops, whereas solute gain continues to increase steadily.
Mass loss (%) of yacon in glycerol (straight line) and sorbitol (broken lines) solutions over osmotic dehydration time. Osmotic solution concentration and temperature are as follows: without calcium lactate, empty circle 30 % at 30 °C, filled circle 30 % at 50 °C, empty square 50 % at 30 °C, filled square 50 % at 50 °C, empty triangle 70 % at 30 °C, and filled triangle 70 % at 50 °C and, with calcium lactate, multiplication sign Ca 70 % at 30 °C
The mass loss curves behavior was similar to that found by Moreira et al. (2007) using glycerol solutions in OD of chestnuts. After 1 h of the osmodehydration process, the mass loss rate of yacon treated with glycerol solution decreased due to lower water removal in relation to the solute uptake. The mass loss rate of yacon treated with sorbitol solution decrease after 2 h of process. According to El-Aouar et al. (2006), the water loss is usually attributed to the influence of natural tissue membranes as well as the diffusive properties of water and solutes, as a function of their respective molar masses (MMs). In this case, sorbitol (MM = 182.17 kg/kmol) has lower permeability in the food matrix than glycerol (MM = 92.09 kg/kmol).
Basically, three periods of mass loss rate were identified for both solutes. Initially, a quick mass loss is caused by the major water removal rates of the food, based on the difference in osmotic pressure between the solution and yacon, and according to Rastogi et al. (2002) and Saurel et al. (1994), the mass transfer rate during osmotic dehydration also depends on the temperature of the solution, nature and molecular weight of the dehydration agent, concentration of the solution, ratio between solution and food, geometry, cellular structure of the food, and processing time. In the sequence, there is a constant period, or at a lower rate (mostly seen in treatments with more severe conditions), where the water removal rates may be equivalent to the input of solute rates, finally, there is an inversion of the slope relative to the first time, because there is a mass gain associated with the higher rate of solid gain in relation to the countercurrent water from yacon. According to Toğrul and İspir (2008), in the dynamic period, the mass transfer rates are increased or decreased until equilibrium is reached. Equilibrium is the end of osmotic process, i.e., the net rate of mass transport is zero. However, equilibrium state was not achieved in this study as well as in the work of Mercali et al. (2010) in which the authors studied the mass transfer during 5 h of osmotic dehydration of blueberry with sucrose solutions.
For both solutes, Fig. 1 also shows higher rates as the osmotic solution concentration increases; the treatment with calcium lactate (multiplication sign) showed no difference among the test with the same conditions without the salt. Table 1 shows the ANOVA for osmodehydrated yacon mass loss. ANOVA showed that concentration and temperature and their interaction had significant effect (p < 0.05) on the mass loss during osmotic dehydration.
Moisture Content
Figure 2 shows the values of the dry basis moisture content normalized with the initial content X 0 (X t /X 0) versus osmotic dehydration process time. It is possible to observe that the moisture of the samples decreased along the osmotic process for both solutes (final X t /X 0 values varied from 0.276 ± 0.010 until 0.082 ± 0.013 to glycerol and from 0.225 ± 0.010 until 0.067 ± 0.003 to sorbitol).
Moisture content variation (dimensionless) in dry basis over immersion time using glycerol (straight line) and sorbitol (broken lines) solutions in osmotic dehydration of yacon. Osmotic solution concentration and temperature are as follows: without calcium lactate, empty circle 30 % at 30 °C, filled circle 30 % at 50 °C, empty square 50 % at 30 °C, filled square 50 % at 50 °C, empty triangle 70 % at 30 °C, and filled triangle 70 % at 50 °C and, with calcium lactate, multiplication sign Ca 70 % at 30 °C
Except for process carried out at 30 % of glycerol solution at 30 °C, treatments showed no difference among them after 2 h of the process. This indicates that it is not necessary to increase the temperature from 30 to 50 °C when the concentrations of osmotic agent are higher than 50 % according to Tukey’s test (p > 0.05). For glycerol solution, only concentration and the interaction between concentration and temperature showed significant changes (p < 0.05) on moisture contents. Table 2 shows the ANOVA for osmodehydrated yacon moisture content.
The increase in the concentration of osmotic solution enhanced the dehydration, by increasing the osmotic pressure of surrounding solution which, in turn, became higher than the osmotic pressure of cellular solution. The same behavior was achieved by Bellary et al. (2011) with coconut slices, Mercali et al. (2011) who studied OD of bananas, and El-Aouar et al. (2006) with papaya slices.
Concerning the use of calcium lactate, it is possible to see in Fig. 2 that the results are not significantly different from the treatment without this salt (multiplication sign and empty triangle) for the first 2 h; after 4 h, it is observed that the addition of salt as a ternary agent decreased the dehydration. The results of the mass transfer kinetics indicated that the addition of calcium decreased water loss. The same was observed by Castelló et al. (2009) in osmotic dehydration of apple slices in a 50 °Brix glucose solution at 30 °C with 0, 1, and 2 % (m/m) calcium lactate. The researchers observed that, with the addition of calcium, more time was needed to reach the same level of dehydration; calcium interactions in the tissue cause a reduction of the effective mass diffusivity of water. This is also reported by other authors (Vieira 2010; Barrera et al. 2004). This reduction can be explained not only by the calcium cross-linking effects in the cellular matrix, which affects the water permeability of the cell structures (Azcón-Bieto and Talón 2000), but also by the ion influence on the active water transport through the cell membrane (Tyerman et al. 1999; Johansson et al. 1996). The effect may also be a consequence of the strengthening of the cellular structure, which implies more difficulties for shrinking, reducing the water loss, and increasing the solute gain.
In general, after 4 h of osmotic dehydration with both osmotic agents, there was no significant change in the amount of water removed (p > 0.05), so OD time could be reduced. This behavior indicates that these solutes are around the equilibrium conditions since the moisture content was found to be fairly constant in the final times of OD; this is in agreement with Moreira et al. (2007) who found that a water loss rate is high at the beginning and starts to decrease as the time passes when the system is approaching the pseudoequilibrium condition. Moisture contents at equilibrium conditions were determined using Peleg’s equations (1988) and are given in Table 3. Peleg’s equation proved to be suitable for modeling water removal, as the determination coefficients were above 0.979 and the RMSE values were low for all treatments. With increasing temperature, there was a significant decrease in equilibrium moisture content, and the values were significantly lower at 70 %; this effect was also observed by Mercali et al. (2011) with bananas and Bellary et al. (2011) with coconut slices.
Panels a and b in Fig. 3 show the water loss and solid gain versus time for the treatments with glycerol and sorbitol solutions, respectively. As can be seen, the samples lost more water than gained solutes. In the case of water loss, in most treatments, high rates of mass transfer were evident in the first 2 h and then the system tended to reach equilibrium. However, to the solid gain, it was observed that the root still had the ability to gain solutes for the time employed, since the tendency to form an asymptote (typical from systems approaching equilibrium) was not observed.
Water loss (straight line) and solids gain (broken lines) versus time for glycerol (a) and sorbitol (b). Osmotic solution concentration and temperature are as follows: without calcium lactate, empty circle 30 % at 30 °C, filled circle 30 % at 50 °C, empty square 50 % at 30 °C, filled square 50 % at 50 °C, empty triangle 70 % at 30 °C, and filled triangle 70 % at 50 °C and, with calcium lactate, multiplication sign Ca 70 % at 30 °C
Effective Mass Diffusivities of Water
The methodology used by Mercali et al. (2011) and Rastogi and Raghavarao (1997) to determine the effective mass diffusivity was used in the present work. The plot of Fourier numbers against immersion time for water loss showed two different periods of dehydration; so, there are two linear curves and two effective mass diffusivities of water.
In conventional materials, it is generally assumed that the water diffusion occurs at a constant rate under the influence of a uniform moisture gradient. Nevertheless, this does not appear to be true in biological materials, especially after the initial stages of the process, as the physical structure of the material begins to change as the osmotic dehydration continues. A nonuniform moisture gradient is developed over the course of osmotic treatment and the effective mass diffusivity changes with position and time of dehydration. As can be seen in Fig. 2, the slopes of the curves are greater in the beginning of the dehydration process showing faster dehydration and, thus, higher effective mass diffusivity of water. In fruits, the mass diffusivity generally shows a decreasing trend over time because of the shrinkage phenomenon. Fruits with a preserved cell structure are more susceptible to shrinkage due to the occurrence of plasmolysis upon osmodehydration (Mercali et al. 2011; Rastogi et al. 2000). According to Singh et al. (2007), progressive solid uptake might have been resulted in the formation of high-solid subsurface layer, which interfered with the concentration gradient across the product–solution interface and acted as a barrier against removal of water and uptake of solids. Souraki et al. (2014) had shown that the values of effective diffusivities with considering shrinkage are lower than those without considering this phenomenon, and this fact demonstrates that Fick’s second law of diffusion without considering shrinkage overestimates the transference of mass by diffusion.
Table 3 also shows the results for effective mass diffusivity of water. It can be seen that the values are in the range of 5.82 ± 0.68 × 10−10 to 2.15 ± 0.61 × 10−10 m2/s in the first period and 1.60 ± 0.28 × 10−10 to 1.29 ± 0.24 × 10−10 m2/s in the second period using glycerol as a solute. When sorbitol was used, the mass diffusivity varied from 3.82 ± 0.17 × 10−10 to 1.54 ± 0.50 × 10−10 m2/s in the first period and from 1.73 ± 0.04 × 10−10 to 1.33 ± 0.06 × 10−10 m2/s in the second period. One possible explanation for these values of glycerol is that once it has lower molar mass than sorbitol, it has higher mobility through the tissue. The mass diffusivity values were higher than those found by Maldonado et al. (2008) of 3.20 × 10−10 m2/s at the first period and 1.84 × 10−11 m2/s at the second period when yacon was osmotically dehydrated at 25 °C and 40 % of sucrose concentration. These differences can be explained by the use of different solutes (smaller molecules diffuse more easily than larger ones), temperatures (the mass transfer tends to be higher at higher temperatures), concentration and water activity of the osmotic solution and yacon cultivar, and maturation degree (changes regarding to the matrix of root). Raoult-Wack et al. (1991) reported that with the use of different solutes, water loss increases and solute gain decreases with increasing molecular weight of the solute. Yao and Le Maguer (1997) explained that at equilibrium, differences of molecular weight only affected the mass concentration in the extracellular volume and, as the molecular weight increased, the mass of solute in the extracellular volume increased when the mole fraction was kept constant. In this way, they affirmed that, because more extracellular volume was occupied by solute and less volume was available for water, the tissue lost more water as the molecular weight increased. Therefore, the water loss at equilibrium was slightly larger for the solute with larger molecular weight.
The influence of temperature on the water effective diffusion can be due to the swelling and plasticizing of cell membranes, faster moisture diffusion within the product, and better mass (water) transfer on the surface due to lower viscosity of the osmotic medium (Li and Ramaswamy 2006). The increases of temperature or concentration were not significantly different for the second period of both solutes, but for the first period, it was found that the increase in temperature caused a significant increase in effective diffusivity. These results are in agreement with fundamental theories which state that mass diffusivity strongly depends on the temperature and pressure and on the components involved. High temperatures cause an increase in membrane permeability (which promotes swelling and plasticization of the cell membranes) and a reduction in the solution viscosity, reducing external resistance to mass transfer. These both phenomena make water and solute transport easier. However, temperatures above 60 °C reduce the final product quality, changing the structure of cell membranes, resulting in loss of selectivity, and leading to greater solute incorporation into the fruit. In addition, high temperatures may induce significant changes in texture and nutritional composition of the food as, among others, a consequence of the losses of nutrients to osmotic solution (Mercali et al. 2011; Torreggiani 1993).
Water Activity
Water activity (a w) is intrinsically related to the preservation of food, and according to Moraga et al. (2011), in the range of high water activity, a small decrease of a w implies an important decrease in the relative rate of all deteriorative reactions and microbial growth. Figure 4 shows the water activity plotted against osmotic dehydration process time. As can be seen, the a w(t)/a w(0) values decreased with time and higher temperature and osmotic solution concentration due to greater water removal and solute incorporation during the dehydration process. Best isolated results (0.704 ± 0.010) were achieved by glycerol treatment using 70 % solute concentration at 50 °C. The greatest reduction of water activity when glycerol was used supports the idea that water was connected to glycerol decreasing the yacon free water, i.e., more extracellular volume was occupied by glycerol and less volume was available for water. According to Vermeulen et al. (2014), our samples can be considered as intermediate moisture foods (IMF), and they are, in general, microbiologically stable products once IMF are characterized by a moisture content of around 15–50 % and, while there is no consensus on the range of water activity in intermediate moisture foods, Chirife and Buera (1994) consider an appropriate variation between 0.65 and 0.90 for this type of food. In addition, Zhengyong et al. (2008) mentioned that most microorganisms would not grow in foods with a w below 0.6. In the same conditions of the best glycerol result, the sorbitol-treated samples presented 0.865 ± 0.002 which, in agreement with Aguilera and Stanley (1999), is in the lower limit for growth of pathogenic bacteria (0.91) and yeasts (≈0.87), but superior to the limit for microbial growth.
Variation of water activity (dimensionless) with the immersion time using glycerol (straight line) and sorbitol (broken lines) solutions in osmotic dehydration of yacon. Osmotic solution concentration and temperature are as follows: without calcium lactate, empty circle 30 % at 30 °C, filled circle 30 % at 50 °C, empty square 50 % at 30 °C, filled square 50 % at 50 °C, empty triangle 70 % at 30 °C, and filled triangle 70 % at 50 °C and, with calcium lactate, multiplication sign Ca 70 % at 30 °C
Table 4 shows the ANOVA for osmodehydrated yacon water activity reduction. As can be seen, the response was dependent on the concentration and temperature of the osmotic solution, in addition to the combined effect of these parameters for both solutes (p < 0.05).
The use of calcium lactate did not improve a w results and, as can be seen in Table 3, did not significantly affect the results of the equilibrium moisture content and the effective diffusivity of water to the same condition of temperature and concentration for both solutes. Similar behavior was observed by Vieira (2010) using 0.6 and 1.2 % of calcium lactate in sucrose solutions in the osmotic dehydration of guava slices, once the salt inclusion reduced the water loss of the fruits. This shows that the salt did not improve the mass transfer during the OD process, although it may have improved the tissue structure. This result can be explained in terms of the ability of calcium to increase the stiffness and fragility of the cellular network (Gras et al. 2003), thus reducing the ability of the cells to deform and relax. This will also reduce the flux of water and soluble solids initiated by the deformation–relaxation phenomena (Barrera et al. 2009).
Kotovicz et al. (2013) studied OD coated with an edible sodium alginate and uncoated yacon in fructose solution. They observed that the water activity reduction is more significant in the uncoated sample and at the higher osmotic solution concentration. According to Lenart (1996), water activity of osmotically dehydrated fruits and vegetables, in laboratory scale or in industrial conditions, is close to 0.90, which is the reason that such products require a subsequent process as freezing, freeze-drying, vacuum drying, or air-drying. The water activity level presented by the material after dehydration, if combined with other preservation techniques such as the use of chemical preservatives, pH reduction, and/or further drying, could promote greater product stability (Fennema 2010).
Soluble Solids
At the end of 6 h of immersion, the samples treated with 70 % polyol solution at 50 °C presented significantly highest final soluble solids (SSs) if compared to the other treatments, 53.1 ± 0.78 °Brix for glycerol and 60.9 ± 0.07 °Brix for sorbitol, and higher values can be attributed to sorbitol solution due to this initial soluble solids of 65.8 °Brix against 56.3 °Brix of glycerol solution. Figure 5 shows the SS variation of the yacon samples over time of OD process. As expected, the SS content increased with solution concentration and operation time for all experiments. The greater glycerol increase (SS t /SS 0) was for the 70 % solution at 30 °C treatment showing an increase of 5.79 ± 0.36 relative to the initial sample; when calcium lactate was used, values grown to 6.33 ± 0.11. The yacon treated with 70 % sorbitol at 30 °C had the highest increases in soluble solids (SS t /SS 0 = 6.76 ± 0.38 without calcium and decreased to 6.49 ± 0.63 when calcium lactate was used). Silva et al. (2014b) also found that the addition of calcium to the osmotic solution reduced the water content of pineapple and solute incorporation rate, inhibiting sucrose impregnation and increasing process efficiency. Dionello et al. (2007) observed an increase of three to four times compared to the initial content of total soluble solids in the osmotic dehydration of pineapple in 0.40 to 0.47 g/mL invert sugar and sucrose solutions at 40 and 50 °C.
Variation of soluble solids (dimensionless) with the immersion time using glycerol (straight line) and sorbitol (broken lines) solutions in osmotic dehydration of yacon. Osmotic solution concentration and temperature are as follows: without calcium lactate, empty circle 30 % at 30 °C, filled circle 30 % at 50 °C, empty square 50 % at 30 °C, filled square 50 % at 50 °C, empty triangle 70 % at 30 °C, and filled triangle 70 % at 50 °C and, with calcium lactate, multiplication sign Ca 70 % at 30 °C
One possible source of error in these results was the fact that the osmotic solution was reused for the duplicate of each experiment. As known, besides water, soluble solids of yacon can migrate for the osmotic solution, interfering with the result.
Table 5 shows the ANOVA for osmodehydrated yacon-soluble solid variation. Concentration and temperature of the osmotic solution showed significant effect (p < 0.05) on the soluble solid increase. Only for the glycerol solution, the interaction terms of the process parameters had negligible effects on soluble solid gain (p > 0.05), compared to the linear terms of process variables.
Osmotic drying also promotes osmotic solution enrichment of certain water-soluble natural components, such as bioactive components, extracted from yacon, besides to have prebiotic component. Rosa and Giroux (2001) reported that during osmotic dehydration, a large amount of water is transferred from the food to the osmotic solution which results in a dilution of the osmotic solution, an increase of the solution mass, as well as a decrease in the dewatering potential. Commonly, osmotic solutions are normally not reused and this solution can be considered as an industrial waste that greatly increases the cost of the osmodehydrated products; for this reason, to recycle it for new food formulation seems to be an attractive alternative or the possibility of reusing the osmotic solution in successive dehydration cycles (Garcı́a-Martı́nez et al. 2002). According to Rastogi et al. (2005), in order to make osmotic dehydration a feasible operation in food processing, concentration of spent osmotic solution involving evaporation, addition of solute, membrane concentration, or cryoconcentration is necessary.
Calcium Content
It has been found that the addition of calcium lactate to the osmotic solution caused the incorporation of Ca2+ in the treated samples, resulting in a statistically significant increase (p < 0.05) in the calcium content in the samples from all treatments in which the salt was used, reaching values about 6.5 times higher than those of the fresh root. Analyzing separately the osmodehydrated samples, it was found that the sample treated with glycerol showed a higher increase of calcium (514.63 ± 75.79 mg calcium/100 g d.m. of yacon) when compared with the sample treated with sorbitol (395.57 ± 28.74 mg calcium/100 g d.m. of yacon) (p < 0.05). Silva et al. (2014a) showed that both calcium lactate and sucrose concentration affected calcium and sucrose gain during osmotic dehydration of pineapple.
Scanning Electronic Microscopy
The scanning electron microscopy technique was used to observe the changes in the structure of the samples after the osmotic treatment. Figure 6a shows the image of yacon fresh tissue, where there are presence of elongated and polyhedral turgid cells, defined as the cell wall (very clear due to the cut made in the sample of yacon, indicated by continuous arrows), and presence of intercellular spaces (circled). The plasmalemma (indicated by dashed arrows) appears to be associated to the cell wall. Plasmalemma could be differentiated in some cells where the cell wall was detached during the cut of the sample. After blanching (Fig. 6b), it is possible to see loss of turgor and partial loss of the polyhedral shape due to the reduction of cell size. According to Aguilera and Stanley (1999), turgor is lost when fruits or vegetables are deprived of water or when they cease to respire. The application of blanching obviously damaged the cell wall structure, leaving the cells more permeable. This effect was responsible for the subsequent reduction in water content. Rastogi and Niranjan (1998) observed the same behavior when they pretreated pineapple at high pressure to osmotic dehydration.
Yacon SEM images. a Fresh. b Blanched. c Osmodehydrated by 70 % glycerol solution at 50 °C. d Osmodehydrated by 70 % sorbitol solution at 50 °C. e Tissue with calcium lactate from treatment with glycerol. f Tissue with calcium lactate from treatment with sorbitol. cw cell wall, is intercellular space, p plasmalemma
Panels c and d in Fig. 6 are very similar, once they show the osmotic solutions filling the yacon matrix. It was found that the osmotic treatment, both with glycerol and sorbitol, without addition of calcium resulted in heavy damage to the cell structure of yacon. The tissue showed strong plasmolysis caused by the loss of water from the cytoplasm beyond deformed and collapsed cells, and the nonturgid aspect of the cells was clearly observed. A qualitative relationship could clearly be established between the concentration profiles and the degree of cellular disturbance in osmosed tissue. Micrographs of osmosed yacon tissue (Fig. 6c, d) show clearly different degrees of cellular alteration and collapse (Salvatori et al. 1998). A similar result was observed by Vieira (2010) who found a severe structural damage caused by osmotic dehydration with sucrose solutions in guavas. The author observed an intense cell plasmolysis and large cell collapse.
In Fig. 6e, f, it is possible to observe calcium lactate adhered to the surface of tissue, being more evident the presence of salt in the sample treated with glycerol, which small and large particles of calcium lactate (white dots) can be checked. The addition of calcium to osmotic solutions reinforces the structure of cell matrix once calcium is supposed to be placed into the cell walls of the product improving the preservation of cell structure and strengthening the cell wall for both solutes compared to Fig. 6c and d. The beneficial effect of the addition of calcium salts against damage caused by osmotic dehydration of the cell structure was also observed with guavas in the work of Vieira (2010).
Conclusions
The present work studied the osmotic dehydration of yacon in solutions of glycerol and sorbitol at different concentrations and temperatures. Both concentration and temperature showed significant effect on the mass loss, the moisture and water activity reduction, and the soluble solid increase of the yacon samples.
The water diffusion showed two periods among the immersion time. On the other hand, different stages of mass loss were identified for these solutes which have been observed to have higher rates of water removal in the beginning of the process.
Although the results of water loss and soluble solids for solutes glycerol and sorbitol were similar, glycerol allowed greater reduction of water activity, being more suitable for the conservation of yacon. The use of calcium lactate did not cause further dehydration of the root but showed strength on tissue structural yacon according to the micrographs.
Beside the advantage of osmotic dehydration in incorporated prebiotic solutes, some inconveniences were observed, such as long process time, and the final product had an intermediate water activity, so the product should then be submitted to another drying process such as hot air, vacuum, or microwaves in order to obtain the more shelf life stable product.
References
Aguilera, J. M., & Stanley, D. W. (1999). Microstructural principles of food processing and engineering (2nd edn.). Gaithersburg, Maryland: Aspen.
Angilelli, K. G., Orives, J. R., Silva, H. C., Coppo, R. L., Moreira, I., & Borsato, D. (2014). Multicomponent diffusion during osmotic dehydration process in melon pieces: influence of film coefficient. Journal of Food Processing and Preservation. doi:10.1111/jfpp.12236.
AOAC (1990, 2002, 2006). Official methods of analysis. Washington, DC: Association of Official Analytical Chemists.
Azcón-Bieto, J., & Talón, M. (2000). Fundamentos de fisiología vegetal. Spain: Mac Graw-Hill Interamerica y Universitat de Barcelona, (Chapter 14).
Barrera, C., Betoret, N., & Fito, P. (2004). Ca2+ and Fe2+ influence on the osmotic dehydration kinetics of apple slices (Var Granny Smith). Journal of Food Engineering, 65, 9–14.
Barrera, C., Betoret, N., Corell, P., & Fito, P. (2009). Effect of osmotic dehydration on the stabilization of calcium-fortified apple slices (var. Granny Smith): influence of operating variables on process kinetics and compositional changes. Journal of Food Engineering, 92, 416–424.
Bellary, A. N., Sowbhagya, H. B., & Rastogi, N. K. (2011). Osmotic dehydration assisted impregnation of curcuminoids in coconut slices. Journal of Food Engineering, 105, 453–459.
Brochier, B., Marczak, L. D. F., & Noreña, C. P. Z. (2014). Use of different & kinds of solutes alternative to sucrose in osmotic dehydration of yacon. Brazilian Archives of Biology and Technology. doi:10.1590/S1516-8913201400035.
Castelló, M. L., Igual, M., Fito, P. J., & Chiralt, A. (2009). Influence of osmotic dehydration on texture, respiration and microbial stability of apple slices (Var. Granny Smith). Journal of Food Engineering, 91, 1–9.
Chirife, J., & Buera, M. D. P. (1994). Water activity, glass transition and microbial stability in concentrated/semimols food systems. Journal of Food Science, 59(5), 925–927.
Codex Alimentarius (2012). Food standards: GSFA online. FAO/WHO, USA. www.codexalimentarius.net/gsfaonline/index.html. Accessed 25 Aug 2012.
Crank, J. (1975). The mathematics of diffusion (2nd ed.). Oxford: Clarendon.
Dionello, R. G., Berbert, P. A., Molina, M. A. B., Viana, A. P., Carlesso, V. O., & Queiroz, V. A. V. (2007). Desidratação por imersão-impregnação de abacaxi em soluções de sacarose e em xarope de açúcar invertido. Ciência e Tecnologia de Alimentos, 27(4), 701–709.
El-Aouar, A. A., Azoubel, P. M., Barbosa, J. L., Jr., & Murr, F. E. X. (2006). Influence of the osmotic agent on the osmotic dehydration of papaya (Carica papaya L.). Journal of Food Engineering, 75, 267–274.
Fante, L., Scher, C. F., Noreña, C. P. Z., & Rios, A. O. (2013). Study of enzyme inactivation using steam in yacon (Smallanthus sonchifolius) roots. Journal of Food Processing and Preservation, 37, 16–24.
Fennema, O. R. (2010). Química de alimentos (4th ed.). Porto Alegre: Artmed.
Garcı́a-Martı́nez, E., Martı́nez-Monzó, J., Camacho, M. M., & Martı́nez-Navarrete, N. (2002). Characterisation of reused osmotic solution as ingredient in new product formulation. Food Research International, 35, 307–313.
Goto, K., Fukai, K., Hikida, J., Nanjo, F., & Hara, Y. (1995). Isolation and structural analysis of oligosaccharides from yacon (Polymnia sonchifolia). Bioscience, Biotechnology, and Biochemistry, 59, 2346–2347.
Gras, M. L., Vidal, D., Betoret, N., Chiralt, A., & Fito, P. (2003). Calcium fortification of vegetables by vacuum impregnation. Interactions with cellular matrix. Journal of Food Engineering, 56, 279–284.
Habib, N. C., Honoré, S. M., Genta, S. B., & Sánchez, S. S. (2011). Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats: biochemical approach. Chemico-Biological Interactions, 194, 31–39.
Heredia, A., Barrera, C., & Andrés, A. (2007). Drying of cherry tomato by a combination of different dehydration techniques. Comparison of kinetics and other related properties. Journal of Food Engineering, 80, 111–118.
Johansson, I., Larsson, C., Ek, B., & Kjellbom, P. (1996). The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. The Plant Cell, 8, 1181–1191.
Kotovicz, V., Ellendersen, L. S. N., Clarindo, M. M., & Masson, M. L. (2013). Influence of process conditions on the kinetics of the osmotic dehydration of yacon (Polymnia sonchifolia) in fructose solution. Journal of Food Processing and Preservation. doi:10.1111/jfpp.12064.
Lenart, A. (1996). Osmo-convective drying of fruits and vegetables: technology and application. Drying Technology, 14, 391–413.
Li, H., & Ramaswamy, H. S. (2006). Osmotic dehydration of apple cylinders: III. Continuous medium flow microwave heating conditions. Drying Technology, 24, 643–651.
Maldonado, S., Santapaola, J. E., Singh, J., Torrez, M., & Garay, A. (2008). Cinética de la transferencia de masa durante la deshidratación osmótica de yacón (Smallanthus sonchifolius). Ciência e Tecnologia de Alimentos, 28, 251–256.
Manrique, I., Párraga, A., & Hermann, M. (2005). Jarabe de yacon: Principios y Procesamiento. Series: Conservación y uso de la biodiversidad de raíces y tubérculos andinos: Una década de investigación para el desarrolo (1993–2003). 8A. International Potato Center Univesidad Nacional Alcides Carrión. Erbacher Foundation. Lima: Swiss Agency for Development and Cooperation.
Mercali, G. D., Kechinski, C. P., Coelho, J. A., Tessaro, I. C., & Marczak, L. D. F. (2010). Estudo da transferência de massa durante a desidratação osmótica de mirtilo. Brazilian Journal of Food Technology, 13, 91–97.
Mercali, G. D., Marczak, L. D. F., Tessaro, I. C., & Noreña, C. P. Z. (2011). Evaluation of water, sucrose and NaCl effective diffusivities during osmotic dehydration of banana (Musa sapientum, shum.). LWT - Food Science and Technology, 44, 82–91.
Michels, I. (2005). Aspectos tecnológicos do processamento mínimo de tubérculos de yacon (Polymnia sonchifolia) armazenados em embalagens com atmosfera modificada, Dissertação de Mestrado. Curitiba: Universidade Federal do Paraná.
Moraga, M. J., Moraga, G., & Martínez-Navarrete, N. (2011). Effect of the re-use of the osmotic solution on the stability of osmodehydro-refrigerated grapefruit. LWT - Food Science and Technology, 44, 35–41.
Moreira, R., Chenlo, F., Torres, M. D., & Vázquez, G. (2007). Effect of stirring in the osmotic dehydration of chestnut using glycerol solutions. LWT - Food Science and Technology, 40, 1507–1514.
Ojansivu, I., Ferreira, C. L., & Salminen, S. (2011). Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends in Food Science & Technology, 22, 40–46.
Osorio, C., Franco, M. S., Castaño, M. P., González-Miret, M. L., Heredia, F. J., & Morales, A. L. (2007). Colour and flavour changes during osmotic dehydration of fruits. Innovative Food Science and Emerging Technologies, 8, 353–359.
Ozdemir, M., Ozen, B. F., Dock, L. L., & Floros, J. D. (2008). Optimization of osmotic dehydration of diced green peppers by response surface methodology. LWT - Food Science and Technology, 41, 2044–2050.
Pan, Z., Shih, C., McHugh, T. H., & Hirschberg, E. (2008). Study of banana dehydration using sequential infrared radiation heating and freeze-drying. LWT - Food Science and Technology, 41, 1944–1951.
Patel, S., & Goyal, A. (2012). The current trends and future perspectives of prebiotics research: a review. 3 Biotech, 2, 115–125.
Peleg, M. (1988). An empirical model for the description of moisture sorption curves. Journal of Food Science, 53, 1216–1219.
Raoult-Wack, A. L. (1994). Recent advances in the osmotic dehydration of foods. Trends in Food Science & Technology, 5, 255–260.
Raoult-Wack, A. L., Guilbert, S., Le Maguer, M., & Rios, G. (1991). Simultaneous water and solute transport in shrinking media. Part I. Application to dewatering and impregnation soaking process analysis (osmotic dehydration). Drying Technology, 9, 589–612.
Rastogi, N. K., & Niranjan, K. (1998). Enhanced mass transfer during osmotic dehydration of high pressure treated pineapple. Journal of Food Science, 63, 508–511.
Rastogi, N. K., & Raghavarao, K. S. M. S. (1997). Water and solute diffusion coefficients of carrot as a function of temperature and concentration during osmotic dehydration. Journal of Food Engineering, 34, 429–440.
Rastogi, N. K., & Raghavarao, K. S. M. S. (2004). Mass transfer during osmotic dehydration of pineapple: considering Fickian diffusion in cubical configuration. LWT - Food Science and Technology, 37, 43–47.
Rastogi, N. K., Angersbach, A., & Knorr, D. (2000). Valuation of mass transfer mechanisms during osmotic treatment of plant materials. Journal of Food Science, 65, 1016–1021.
Rastogi, N. K., Raghavarao, K. S. M. S., Niranjan, K., & Knorr, D. (2002). Recent developments in osmotic dehydration: methods to enhance mass transfer. Trends in Food Science and Technology, 13, 48–59.
Rastogi, N. K., Raghavarao, K. S. M. S., & Niranjan, K. (2005). Emerging technologies for food processing. (Chapter 9: Developments in osmotic dehydration). pp. 222–249.
Rizzolo, A., Gerli, F., Prinzivalli, C., Buratti, S., & Torreggiani, D. (2007). Headspace volatile compounds during osmotic dehydration of strawberries (cv Camarosa): influence of osmotic solution composition and processing time. LWT - Food Science and Technology, 40, 529–535.
Rosa, M. D., & Giroux, F. (2001). Osmotic treatments (OT) and problems related to the solution management. Journal of Food Engineering, 49, 223–236.
Rózek, A., Achaerandio, I., Güell, C., López, F., & Ferrando, M. (2009). Grape phenolic impregnation by osmotic treatment: influence of osmotic agent on mass transfer and product characteristics. Journal of Food Engineering, 94, 59–68.
Salvatori, D., Andrés, A., Albors, A., Chiralt, A., & Fito, P. (1998). Structural and compositional profiles in osmotically dehydrated apple. Journal of Food Science, 63, 606–610.
Saurel, R., Raoult-Wack, A. L., Rios, G., & Guilbert, S. (1994). Mass transfer phenomena during osmotic dehydration of apple I. Fresh plant tissue. International Journal of Food Science and Technology, 29, 531–542.
Scher, C. F., Rios, A. O., & Noreña, C. P. Z. (2009). Hot air drying of yacon (Smallanthus sonchifolius) and its effect on sugar concentrations. International Journal of Food Science and Technology, 44, 2169–2175.
Schmidt, F. C., Carciofi, B. A. M., & Laurindo, J. B. (2009). Application of diffusive and empirical models to hydration, dehydration and salt gain during osmotic treatment of chicken breast cuts. Journal of Food Engineering, 91, 553–559.
Shi, J., & Le Maguer, M. (2002). Osmotic dehydration of foods: mass transfer and modeling aspects. Food Reviews International, 18(4), 305–335.
Silva, K., Fernandes, M. A., & Mauro, M. A. (2014a). Osmotic dehydration of pineapple with impregnation of sucrose, calcium, and ascorbic acid. Food and Bioprocess Technology, 7, 385–397.
Silva, K., Fernandes, M. A., & Mauro, M. A. (2014b). Effect of calcium on the osmotic dehydration kinetics and quality of pineapple. Journal of Food Engineering, 134, 37–44.
Singh, B., Kumar, A., & Gupta, A. K. (2007). Study of mass transfer kinetics and effective diffusivity during osmotic dehydration of carrot cubes. Journal of Food Engineering, 79, 471–480.
Souraki, B. A., Ghavami, M., & Tondro, H. (2014). Correction of moisture and sucrose effective diffusivities for shrinkage during osmotic dehydration of apple in sucrose solution. Food and Bioproducts Processing, 92, 1–8.
Toğrul, I. T., & İspir, A. (2008). Equilibrium distribution coefficients during osmotic dehydration of apricot. Food and Bioproducts Processing, 86, 254–267.
Torreggiani, D. (1993). Osmotic dehydration in fruit and vegetable processing. Food Research International, 26, 59–68.
Torres, J. D., Talens, P., Escriche, I., & Chiralt, A. (2006). Influence of process conditions on mechanical properties of osmotically dehydrated mango. Journal of Food Engineering, 74, 240–246.
Tyerman, S. D., Bohnert, H. J., Maurel, C., Steudle, E., & Smith, J. A. C. (1999). Plant aquaporins, their molecular biology, biophysics and significance for plant water relations. Journal of Experimental Botany, 50, 1055–1071.
Vermeulen, A., Marvig, C. L., Daelman, J., Xhaferi, R., Nielsen, D. S., & Devlieghere, F. (2014). Strategies to increase the stability of intermediate moisture foods towards Zygosaccharomyces rouxii: the effect of temperature, ethanol, pH and water activity, with or without the influence of organic acids. Food Microbiology. doi:10.1016/j.fm.2014.01.003.
Vieira, G. S. (2010). Otimização do processo de desidratação osmótica de goiaba e avaliação do uso de pulso de vácuo e sais de cálcio. Msc Thesis. Campinas, Brazil: Department of Food Engineering, Campinas State University.
Yao, Z., & Le Maguer, M. (1997). Mathematical modelling and simulation of mass transfer in osmotic dehydration processes. Part III: parametric study. Journal of Food Engineering, 32, 33–46.
Zhengyong, Y., Sousa-Gallagher, M. J., & Oliveira, F. A. R. (2008). Sorption isotherms and moisture sorption hysteresis of intermediate moisture content banana. Journal of Food Engineering, 86, 342–348.
Acknowledgments
The authors acknowledge the financial support received from Coordenadoria de Aperfeiçoamento de Pessoal para o Ensino Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and the product donation from Corn Products do Brasil.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Brochier, B., Marczak, L.D.F. & Noreña, C.P.Z. Osmotic Dehydration of Yacon Using Glycerol and Sorbitol as Solutes: Water Effective Diffusivity Evaluation. Food Bioprocess Technol 8, 623–636 (2015). https://doi.org/10.1007/s11947-014-1432-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-014-1432-5