Abstract
The properties of buffalo and bovine milk differ and the procedures developed to make bovine yoghurt may require optimisation for the production of buffalo yoghurt. This study aimed to apply cryo-scanning electron microscopy and confocal laser scanning microscopy to determine the optimal temperature for processing buffalo yoghurt. Milk was fermented at three different temperatures (37, 40 and 43 °C), stored for 28 days and the yoghurt microstructure, physicochemical and rheological properties assessed. Yoghurt fermented at 37 °C had a compact microstructure and the probiotic Lactobacillus acidophilus La-5 was more viable on storage. In contrast, yoghurt produced from a faster fermentation at 43 °C was firmer with a more porous microstructure that exhibited a higher degree of syneresis. The rheological properties during storage including the thixotropy, consistency coefficient and flow behaviour index were not significantly affected by temperature nor were the concentration of lactose, ionic calcium or titratable acidity. This study shows how changes to processing can be used to alter the microstructure of buffalo products and suggests that a decrease in fermentation temperature could be used to improve the quality of buffalo yoghurt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Buffalo milk is significantly different to bovine milk in both chemical composition and physicochemical properties. These differences lead to advantages but also disadvantages during milk processing. Benefits include a higher yield of cheese, cream, ghee and butter (Menard et al. 2010), faster separation of cream, as well as easier churning and reduced fat loss during butter production (Sahai 1996). Yoghurt production is simpler without the need for fortification with milk powder or the addition of thickeners or stabilisers (Addeo et al. 2007). Drawbacks include an acceleration of the Maillard browning reaction during pasteurisation or sterilisation (Sahai 1996) and a higher buffering capacity that results in slower acidification and longer fermentation during the production of cheese and yoghurt (Ahmad et al. 2008). The larger fat globules and the higher fat content in buffalo milk also lead to a more porous yoghurt microstructure that has a high degree of syneresis; a major defect that requires the optimisation of processing conditions (Nguyen et al. 2013).
Much is known about the factors that affect gel formation and syneresis for bovine yoghurt, although the effect of these variables on buffalo yoghurt is not well understood. The fermentation temperature, starter culture type, starter culture concentration and milk base used all affect yoghurt production from bovine milk (Abbasi et al. 2009; Folkenberg et al. 2004; Lucey et al. 1998a, b; McClements 2007; Sodini et al. 2004; Xu et al. 1992). Among these, fermentation temperature is considered most significant, due to the significant impact of temperature on gel formation and acidification rate (Lee and Lucey 2003, 2004; Sodini et al. 2004; Wu et al. 2009; Purwandari et al. 2007; Tamime and Robinson 2007; Laligant et al. 2003). A fermentation temperature of 37–45 °C is typically selected for the production of bovine yoghurt to achieve optimal growth of the mixed bacterial starter cultures, such as Streptococcus thermophilus and Lactobacillus bulgaricus (Purwandari et al. 2007; Sfakianakis and Tzia 2011; Paseephol et al. 2008). While faster fermentation at a higher fermentation temperature may be advantageous in industrial production, it can lead to several defects, such as an increase in whey separation (Lee and Lucey 2003, 2004; Purwandari et al. 2007), a decrease in gel firmness, viscosity and smoothness, a decrease in desirable sensory properties (Tamime and Robinson 2007; Wu et al. 2009), and a weaker protein network with a coarser microstructure (Lee and Lucey 2004; Lucey and Singh 1997). Conversely, the physical and sensory properties of bovine yoghurt can be improved when a lower fermentation temperature of 32–39 °C is employed (Sodini et al. 2004; Martin et al. 1999), although these improvements come at the cost of increased production time.
Research on buffalo yoghurt, especially on factors affecting the syneresis such as the fermentation temperature, is still limited. Buffalo yoghurt has long been produced in countries such as India and Pakistan using traditional processing technology (Ahmad et al. 2008) but the only readily accessible prior study is by Shiby and Mishra (2008), who examined the simultaneous effects of fermentation temperature, starter culture and milk total solids concentration on the rate of acidification, firmness and syneresis of buffalo yoghurt. Unfortunately, the use of a mesophilic lactic acid bacteria that necessitates overnight incubation limits the application of this study to commercial yoghurt production where short incubations are preferred (Chandan and O'Rell 2006; Tamime and Robinson 2007). Most other studies have focused on the fortification of buffalo yoghurt (Kumar and Mishra 2003; Ghadge 2008) or yoghurt production from a mixture of buffalo and other milk types (Bezerra et al. 2012). Further, buffalo yoghurt is usually prepared with unhomogenised and unstandardised milk (Tamime and Robinson 2007; Addeo et al. 2007), which is different to the normal case for bovine yoghurt. These attributes together with the markedly different chemical composition and properties of buffalo milk affect the processing and quality of the resulting dairy products. Consequently, the impact of fermentation temperature on buffalo yoghurt may be substantially different. Additionally, the fermentation required for buffalo yoghurt takes longer than for bovine yoghurt. For this reason, it is important to determine an optimum fermentation temperature for this product that will balance the extended production time and the quality of the yoghurt. This forms the focus of the present paper.
This study aims to investigate the effect of three fermentation temperatures (37, 40 and 43 °C) on the properties of probiotic buffalo yoghurt during fermentation and cold storage. The second aim is to apply microscopy tools known to preserve the microstructure of hydrated gels to investigate the microstructure of buffalo yoghurt as a function of fermentation temperature.
Materials and Methods
Yoghurt Preparation
Buffalo yoghurt was produced from raw buffalo milk provided by a local dairy farmer in Shaw River (Victoria, Australia) with chemical composition of 4.1 ± 0.4 (% w/w) protein, 7.9 ± 0.3 (% w/w) fat, 5.0 ± 0.2 (% w/w) lactose and 17.1 ± 0.4 (% w/w) total solids. Four litres of the buffalo milk were batch-pasteurised (85 °C, 30 min) using a water bath (Qualtex, Watson Victor Ltd., Australia). The pasteurised milk was cooled to three different fermentation temperatures of 37, 40 or 43 °C before inoculation with 0.062 g L−1 freeze dried direct vat starter culture ABT-5 containing probiotic Lactobacillus acidophilus La-5, Bifidobacterium lactis Bb-12 and S. thermophilus (CHR-Hansen, Bayswater, Victoria, Australia). The inoculated milk was distributed into several plastic containers and fermented at 37, 40 or 43 °C in three water baths containing water that had been tempered to the appropriate fermentation temperature. The fermentation was terminated when the milk reduced to a pH of 4.5. Two trials of yoghurt were produced for each fermentation temperature on different days.
Chemical Analysis
Measurement of pH and Ionic Calcium Concentration
The changes in pH during fermentation were measured using an electrode pH meter (Orion 720A plus, Orion Pacific Pty Ltd., Victoria, Australia) while the changes in ionic calcium concentration were determined using an Orion 93–20 calcium half-cell electrode in conjunction with an Orion 90–02 Ag/AgCl double junction reference electrode (Orion Pacific, Victoria, Australia) as previously described (Nguyen et al. 2013). Three independent samples per trial were used for the measurement of pH and ionic calcium concentration at each time point during the fermentation. Two trials of yoghurt production were carried out for each fermentation temperature.
Determination of Fat, Protein, Lactose, Total Solids and Titratable Acidity
The concentration of milk fat and protein was determined following the methods previously described by Atwood and Hartmann (1992), Pesce and Strande (1973) and modified by Nguyen et al. (2013) using a spectrophotometer (Fluostar Optima, BMG labtech, Ortenberg, Germany). Concentration of lactose was analysed following a previous method (Gosling et al. 2009) using an HPLC Shimadzu Prominence system (NSW, Australia) equipped with a RID-10A refractive index detector and a 300 × 7.8 mm Rezex RCM-Monosaccharide Ca2+ column (Phenomenex, NSW, Australia). Total solids and titratable acidity were determined using the methods of Association of Official Analytical Chemists (AOAC 2006). Three independent samples per trial were used for the analysis of lactose and titratable acidity at each time point during the fermentation while fat, protein and total solids content of the milk used for yoghurt production were analysed in triplicates in each trial. Two trials of yoghurt production were carried out for each fermentation temperature.
Syneresis Determination
Syneresis of yoghurt was determined following the method described previously (Purwandari et al. 2007) using a bench-top centrifuge (Eppendorf 5810R, VIC, Australia). Syneresis was expressed as a weight percentage of the whey separated from the gel over the initial weight of the gel. Three independent samples per trial were used for the determination of syneresis at each time point during storage. Two trials of yoghurt production were carried out for each fermentation temperature.
Texture Analysis
The texture of yoghurt was analysed following a previously described method (Nguyen et al. 2013) using a TA.XT-2 texture analyser (Stable Microsystems, Surrey, England) equipped with a 2-kg load cell and a 10-mm-diameter cylindrical probe. The contact area was set at 1 mm2 and the contact force set at 5 g. The instrument speed was set at 1 mm s−1. The compression distance, the distance of penetration from the surface of the sample, was set at 20 mm. Data were recorded at a rate of 200 points per second. Three independent samples per trial were used for the determination of texture at each time point during storage. Two trials of yoghurt production were carried out for each fermentation temperature.
Rheological Analysis
Rheological Properties (Storage Modulus G′) During Fermentation
Rheological properties during fermentation were determined using a controlled strain rheometer (Advanced Rheometrics Expansion System, TA Instruments, New Castle, USA) equipped with a cup 34 mm in diameter and a six-blade vane fixture 32 mm in diameter and 33 mm in height as previously described (Nguyen et al. 2013). A total of two rheological analyses were performed for each temperature treatment.
Rheological Properties (Thixotropy, Flow Behaviour Index and Consistency Index) During Storage
The rheological properties of yoghurt during storage were investigated following a previously described method (Purwandari and Vasiljevic 2009) using a controlled stress rheometer (AR-G2, TA instruments Ltd., New Castle, USA) fitted with a cone plate (40 mm diameter/4° angle). Three independent samples per trial were used for the rheological analysis at each time point during storage. Two trials of yoghurt production were carried out for each fermentation temperature.
Microstructural Analysis Using Confocal Laser Scanning Microscopy Cryo-Scanning Electron Microscopy (Cryo-SEM)
The confocal laser scanning microscopy (CLSM) analysis was carried out using an inverted confocal scanning laser microscope (Leica TCS SP2; Leica Microsystems, Heidelberg, Baden-Wurttemberg, Germany) with sample preparation for CLSM analysis described in details in our previous work (Nguyen et al. 2013). The cryo-scanning electron microscopy (cryo-SEM) analysis was performed using a field-emission scanning electron microscope (Quanta, Fei Company, Hillsboro, Oregon, USA) as previously described by Ong et al. (2011). Two images were taken for each yoghurt sample in each trial. Two trials of yoghurt were carried for each fermentation temperature and hence, a total of four images were collected for each sample and a typical image is presented.
Microbiological Analysis
The growth and viability of bacteria during fermentation and storage were assessed using the pour plate technique and different selective media as previously described (Nguyen et al. 2013). Two plates with 25–250 colonies were selected for manual counting per trial for each yoghurt sample. Two trials of yoghurt production were carried out for each fermentation temperature.
Statistical Analysis
Data were analysed using statistical Minitab software (V16, Minitab Inc., Stage College, PA, USA). Two-way and one-way analysis of variance (ANOVA) and Fisher’s paired comparison were used to assess the differences between means, with a significance level of P = 0.05.
Results and Discussion
The effect of fermentation temperature on the properties of buffalo yoghurt was assessed using pasteurised, unhomogenised, unstandardised milk and commercial starter cultures to reflect industrially relevant conditions used for yoghurt production in Australia.
The Effect of Fermentation Temperature on Gel Development
During fermentation, the bacteria in the starter culture consume lactose and produce acid, leading to an increase in H+ concentration and titratable acidity (Fig. 1a and b) and a decrease in lactose concentration (Fig. 1c). As the pH of the milk decreases towards the isoelectric point, the colloidal calcium phosphate present within the casein micelles dissociates and causes an increase in the concentration of ionic calcium (Fig. 1d). These biochemical changes were negligible during the first 150 min of the fermentation, with significant changes occurring later in the fermentation after the lag phase of bacterial growth.
Changes in the concentration of dissociated H+ ion (a), titratable acidity (b), lactose (c), ionic calcium (d) and storage modulus G′ (e) during the fermentation of buffalo yoghurt fermented at 37 °C (filled black circle), 40 °C (filled grey circle) or 43 °C (open circle). Each data point is the average of six replicates (n = 6) in a–d and two replicates (n = 2) in e. The error bars are the standard deviation of the mean. The inset in e corresponds to the data between 140 and 260 min of fermentation time
The decreasing negative charge of the casein micelles also results in a decrease in the electrostatic repulsion and an increase in the hydrophobic interactions between casein molecules. These factors facilitate the formation of a casein network which in turn leads to an increase in the storage modulus G′ (Fig. 1e). The increase in the storage modulus G′ is minimal until it reaches the gelation point, defined as the time when the storage modulus G′ exceeded 1 Pa (Lee and Lucey 2003).
The fermentation time, defined as the time for milk samples to reach an H+ concentration of ~3.2 × 10−5 M (equivalent to pH 4.5), increased from 360 to 420 and then 510 min, for samples incubated at 43, 40 and 37 °C respectively (Fig. 1a). The gelation time was also shortest for yoghurt formed at 43 °C compared to 40 or 37 °C (inset of Fig. 1e). The shortest gelation and fermentation time observed for samples at 43 °C are consistent with the fastest acidification rate under these conditions, as indicated by the steepest gradients in the plots of H+ concentration versus fermentation time (Fig. 1a). This observation is similar to that reported in previous studies for bovine yoghurt (Purwandari et al. 2007; Lee and Lucey 2003; Laligant et al. 2003). For example, Lee and Lucey (2003) found a decrease in fermentation and gelation time from 790 and 389 min to 490 and 180 min when the fermentation temperature decreased from 46 to 34 °C.
While the rate of change in these parameters varies with the fermentation temperature, at the end of the fermentation time, no significant difference is observed in titratable acidity or the concentration of lactose between treatments (P > 0.05). The ionic calcium concentration in samples fermented at 40 or 37 °C was slightly lower than at 43 °C (P < 0.05) possibly due to the slower rate of fermentation. These results suggest there was no significant difference in the overall metabolism of the lactic bacteria as a function of temperature under the conditions used in this study.
At the end of the fermentation (pH ~4.5, also indicated by the black arrows in Fig. 1e), the G′ value was lowest at the highest fermentation temperature (43 °C). This result is consistent with the previous work of Lee and Lucey (2003) who reported a lower storage modulus G′ when bovine yoghurt was fermented at a higher temperature. An increase in fermentation temperature results in a faster acidification rate but allows less time for the interaction between protein particles leading to the formation of a less branched network that decreases the storage modulus G′ (Fig. 1e). Furthermore, according to Lee and Lucey (2004), the greater mobility of the protein molecules at the high fermentation temperature may also contribute to an increased protein network rearrangement. This possibly results in a less stable and weaker gel network, indicated by the lower storage modulus G′.
The concentration of lactose at the end of fermentation of buffalo yoghurt in our study was lower than in previous studies for buffalo yoghurt, 3.9 ± 0.2 (% w/v) vs. 4.7–5.0 (% w/v) (Shiby and Mishra 2008) while the titratable acidity was higher, 1.1 ± 0.1 (% lactic acid equivalents) vs. 0.7–0.9 (% lactic acid equivalents) (Shiby and Mishra 2008; Yadav et al. 2007; Nahar et al. 2007). This is likely due to the lower pH used to define the end of fermentation in our study compared to others (pH 4.50–4.54 vs. pH 4.80–4.90) (Bezerra et al. 2012; Yadav et al. 2007), consistent with the requirement for yoghurt production in Australia (Australia and New Zealand Food Standards 2006).
The Effect of Fermentation Temperature on the Firmness of Buffalo Yoghurt
The yoghurt gel firmness was affected by fermentation temperature (P < 0.05) and was higher for yoghurt fermented at 43 °C compared to 37 °C as shown in Fig. 2a. Our result is in contradiction to a number of other studies using bovine milk who reported that a lower fermentation temperature leads to a stronger gel network (Lee and Lucey 2003, 2004; Anema 2008). In these cases, it was argued that the lower temperature allows more interaction and cross-links within proteins in the gel leading to the formation of a network that is more branched and homogenous in structure.
The gel firmness of the buffalo yoghurt in this study increased significantly with storage time by 40 to 50 % for all treatments. This behaviour is consistent with that observed for bovine yoghurt (Saccaro et al. 2009; Salvador and Fiszman 2004), but not in other reports for buffalo yoghurt where the gel firmness decreased (Yadav et al. 2007). This inconsistency in previous studies is possibly due to the different starter cultures used. Starter cultures such as B. lactis have been observed to contribute to the increase in gel firmness in bovine yoghurt (Saccaro et al. 2009), possibly due to its capacity to produce exopolysaccharide (EPS) including l-rhamnopyranose, d-glucopyranose, d-galactopyranose and d-galactofuranose (Hidalgo-Cantabrana et al. 2013; Leivers et al. 2011) which may interact with the protein network, resulting in yoghurt with an improved texture (Zhang and Zhang 2012). This bacteria strain was also present in the starter culture used in our study and hence, the gel firmness was also expected to increase during cold storage.
The Effect of Fermentation Temperature on the Syneresis of Buffalo Yoghurt
Syneresis is a major physical defect in yoghurt and is determined by the amount of whey that separates from the yoghurt over time. The centrifugation method was used in this study to facilitate the collection and assessment of the whey expelled.
The fermentation temperature had a significant effect on the syneresis of buffalo yoghurt (P < 0.05) (Fig. 2b). A significantly greater mass of whey was expelled from buffalo yoghurt samples fermented at 43 °C compared to those fermented at 40 °C or 37 °C (P < 0.05), while no difference was observed between buffalo yoghurt fermented at these two lower temperatures (P > 0.05). Syneresis was also affected by storage time (P < 0.05); prolonged storage time increased syneresis for samples fermented at 43 °C. The greater expulsion of whey from buffalo yoghurt samples fermented at this temperature is possibly linked to the more rapid acidification occurring. A higher acidification rate may result in a less developed protein network with fewer protein cross-links leading to a weaker gel that is more susceptible to syneresis (Lee and Lucey 2003; Purwandari et al. 2007; Wu et al. 2009). Furthermore, the increased hydrophobic interactions at an increased fermentation temperature could also lead to the contraction of the protein strands resulting in a weaker network containing thinner protein strands as reported in the study of Lee and Lucey (2004).
While there was a significant decrease in syneresis when the fermentation temperature was lowered from 43 to 40 °C, there was no significant change when this was further decreased to 37 °C. This result is of practical importance as it indicates that fermentation at 40 °C could allow for a relatively short production time while maintaining a level of syneresis similar to that at 37 °C. However, the lower syneresis level observed here for buffalo yoghurt samples fermented at reduced fermentation temperature was still approximately eight times higher than that observed for bovine yoghurt in our previous work (Nguyen et al. 2013). This result shows that more parameters other than the fermentation temperature need to be investigated to improve the quality of buffalo yoghurt.
The Effect of Fermentation Temperature on the Rheological Properties of Buffalo Yoghurt
The storage time was found to have a significant effect (P < 0.05) on the rheological properties of buffalo yoghurt, whereas the effect of fermentation temperature was minimal (P > 0.05) (Fig. 3). Three measures were used to assess the buffalo yoghurt rheological properties. These were (i) the thixotropy, which is defined as the difference in the energy required to recover the original structure after deformation, (ii) the consistency coefficient K, which indicates the viscosity of the fluids, and (iii) the flow behaviour index n, which measures the deviation degree from a Newtonian fluid.
Changes in the thixotropy (a), consistency coefficient (b) and flow behaviour index (c) during cold storage of buffalo yoghurt fermented at 37 °C (filled black circle), 40 °C (filled grey circle) or 43 °C (open circle). Each data point is the average of six replicates (n = 6) and the error bars are the standard deviation of the mean
During the 28 days of storage, the thixotropy and consistency coefficient K increased for all samples, while the flow behaviour index n decreased. These measures indicate that the buffalo yoghurt was more susceptible to structural breakdown under external force and less capacity to recover to its original structure after storage. No significant differences were observed in the thixotropy and consistency coefficient K within buffalo yoghurt fermented at different temperatures (P > 0.05) (Fig. 3a, b). A subtle difference was observed in the flow behaviour index n of buffalo yoghurt fermented at different temperatures, but only on the first day of storage (P < 0.05) (Fig. 3c).
Our results differ to the findings of previous studies of bovine yoghurt where rheological properties were significantly affected by the fermentation temperature. Haque et al. (2001) observed a considerable increase in the viscosity of bovine yoghurt fermented at higher temperatures (46 vs. 37 °C) as measured by funnel flow. Purwandari et al. (2007) found the highest thixotropy of bovine yoghurt at the intermediate temperature (37 °C vs. 30 °C or 42 °C) after 30 days of storage. The differences in the response of the rheological properties of buffalo and bovine yoghurt to the changes in fermentation temperature is likely due to the differences in the properties of the two milk types. The minimal effect of fermentation temperature on the rheological properties of buffalo yoghurt indicates that procedures that have long been established to produce bovine yoghurt might not be appropriate for buffalo yoghurt, and such protocols therefore require optimisation prior to application for buffalo yoghurt production.
It is also noted that the rheological properties observed here for buffalo yoghurt were considerably different to bovine yoghurt made with homogenised milk reported in our previous study (Nguyen et al. 2013). For example, the thixotropy values measured at the first day of storage for buffalo yoghurt fermented at 43, 40 or 37 °C were 1,686 ± 517 (Pa sn), 1,508 ± 381 (Pa sn) and 1,767 ± 207 (Pa sn) respectively, significantly higher than that of 479 ± 35 (Pa sn) observed for the bovine yoghurt. The flow behaviour index n at the first day of storage of buffalo yoghurt fermented at 43, 40 or 37 °C were 0.07 ± 0.06, 0.16 ± 0.07, 0.16 ± 0.06 respectively, significantly lower compared to 0.42 ± 0.03 for the bovine yoghurt. This comparison gave an indication that the use of homogenised milk for buffalo yoghurt production may improve the viscosity and reduce the thixotropy value, hence giving yoghurt structures that recover better upon deformation.
The Effect of Fermentation Temperature on the Microstructure of Buffalo Yoghurt
Both fermentation temperature and storage were found to significantly affect the microstructure of buffalo yoghurt as assessed by CLSM and cryo-SEM (Fig. 4). While large serum pores (black areas) could be observed in all yoghurt samples regardless of the fermentation temperature, these were more numerous within the gel network of yoghurt fermented at 43 °C. This difference was particularly apparent after the yoghurt samples were stored for 28 days (Fig. 4i vs. a, e; Fig. 4k vs. c, g). The protein network of buffalo yoghurt fermented at 43 °C appeared less dense compared to other treatments (Fig. 4j vs. b, f; Fig. 4l vs. d, h). This observation correlates well with the observation that buffalo yoghurt fermented at a higher temperature or left in storage is more susceptible to whey separation (Fig. 2b), more sensitive to the external force indicated by the flow behaviour index n (Fig. 3c) and less able to recover to the original structure after deformation, as indicated by the thixotropy value (Fig. 3a). The protein strands at day 28 of the storage are denser than day 1 (Fig. 4d vs. b; Fig. 4h vs. f; Fig. 4l vs. j), probably due to the fusion of the protein aggregates or the further development of the network during the cold storage. This observation is consistent with the increase in gel firmness of buffalo yoghurt with storage (Fig. 2a)
Microstructure of buffalo yoghurt fermented at 37 °C (a–d), 40 °C (e–h) o 43 °C (i–l) at day 1 (two left column images) and day 28 (two right column images) of storage as observed by CLSM and cryo-SEM. Nile Red-stained fat appears red, FCF-stained protein appears green, the black areas are serum pores and CLSM images were captured using a ×63 objective using a 1× digital zoom (first and third column images). Cryo-SEM images were captured using a solid-state detecstor at ×16,000 magnification (second and fourth column images). The scale bars are 10 μm (first and third column CLSM images) or 5 μm in length (second and fourth column cryo-SEM images)
These results are in agreement with the findings of Lee and Lucey (2003) who observed the larger pores within a less dense protein network in bovine yoghurt fermented at a higher temperature (46 °C vs. 40 °C). These authors suggest that hydrophobic interaction within the casein particles increase with temperature, leading to a decrease in the voluminosity and the contact area between casein particles within the protein network, resulting in a yoghurt microstructure with larger serum pores and thinner protein strands.
The Effect of Fermentation Temperature on the Bacterial Growth During Fermentation and Viability of Bacteria During Storage
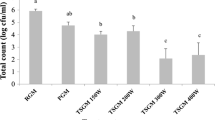
Similarities were observed in the growth and viability of starter culture bacteria during fermentation and storage regardless of the fermentation temperature. During fermentation, the number of all three bacterial starter strains increased significantly (P < 0.05) from a similar level of bacterial inoculation with the fastest growth rate observed for S. thermophilus, followed by L. acidophilus La-5 and the lowest rate in B. lactis Bb-12 (Fig. 5). During storage, the number of viable probiotic L. acidophilus La-5 and B. lactis Bb-12 bacteria reduced significantly (P < 0.05) with the more profound decrease observed in La-5, while the viability of S. thermophilus remained unchanged (P > 0.05).
Bacterial growth and viability during the fermentation and storage of buffalo yoghurt fermented at 37 °C (filled black circle), 40 °C (filled grey circle) or 43 °C (open circle). Each data point is the average of six replicates (n = 6) and the error bars are the standard deviation of the mean. Storage commenced after 6, 7 and 8.5 h for buffalo yoghurt fermented at 37, 40 and 43 °C respectively
The superior growth and viability of S. thermophilus and the slower growth and poorer viability of L. acidophilus La-5 have been reported in previous studies of bovine yoghurt (Damin et al. 2008; Oliveira et al. 2001; Dave and Shah 1997). Briefly, this phenomenon is thought to arise due to the higher proteolytic activity of S. thermophilus compared to L. acidophilus and the susceptibility of the latter strain to acidic and cold conditions during storage (Ozer and Kirmaci 2010; Marafon et al. 2011; Gilliland and Lara 1988).
The fermentation temperature did not significantly affect the growth of bacteria but altered the bacterial viability during storage (P > 0.05). During storage, B. lactis Bb-12 survived in greater number in the buffalo yoghurt fermented at 43 °C and 40 °C (Fig. 5b) while L. acidophilus La-5 survived better in buffalo yoghurt fermented at 37 °C (Fig. 5c).
Several studies reported on the optimum growth of L. acidophilus at 37 °C (Baati et al. 2004; Bozanic et al. 2008; Shafiee et al. 2010) while there are no studies to date on the effect of fermentation temperature on the survival of this probiotic bacteria in yoghurt during cold storage. It is, however, interesting to note that a relationship exists between the fermentation temperature and the survival rate of L. acidophilus La-5 during frozen stage or cryo-storage at −20 °C that is often used for the preservation of the starter culture (Murga et al. 2000; Wang et al. 2005a, b). Wang et al. (2005a) found that L. acidophilus was more cryo-resistant when it was cultured at low temperatures between 30 and 37 °C compared to 42 °C. L. acidophilus survival after freeze-thawing also increased systematically from 14 to 67 % when it was fermented at 25 °C compared to 40 °C. This improved resistance is thought to be due to the increase in the ratio of unsaturated fatty acids, the high concentration of 19-carbon cyclopropane membrane fatty acid (cyc C19:0) and the upregulation of specific proteins that help the bacterial cells to adapt to freezing (Murga et al. 2000; Wang et al. 2005a, b). These adaptations may also assist L. acidophilus during cold storage, leading to the higher numbers of survival of this bacteria strain in the yoghurt samples fermented at 37 °C observed here.
Conclusion
The fermentation temperature used to produce buffalo yoghurt significantly affected the yoghurt quality, altering the physical appearance and yoghurt microstructure. The faster fermentation at a higher fermentation temperature of 43 °C increased the ionic calcium concentration and the gel firmness of buffalo yoghurt but altered the protein network leading to larger and more numerous serum pores within the microstructure resulting in higher syneresis. The rheological properties, lactose and titratable acidity were not affected by fermentation temperature but the viability of the probiotic L. acidophilus La-5 bacteria was significantly reduced with increased fermentation temperature. In contrast, fermentation at the lower temperatures of 37 or 40 °C was longer, leading to a more consistent product and these temperatures are recommended to improve the microstructure and syneresis of buffalo yoghurt. The gel firmness, thixotropy and consistency coefficient increased while the flow behaviour index decreased on storage for all treatments. The high level of syneresis observed here relative to bovine yoghurt, even for those samples fermented at lower fermentation temperatures, suggests that further work is required to optimise the processing conditions for buffalo yoghurt. In particular, while this yoghurt is typically made from unhomogenised milk, it may be useful to examine whether homogenisation might be used to improve the yoghurt structure. This study also confirms that while there are many similarities between buffalo and bovine yoghurt, studies specific to buffalo products are required to optimise production.
References
Abbasi, H., Mousavi, M. E., Ehsani, M. R., Emamdjomea, Z., Vaziri, M., Rahimi, J., et al. (2009). Influence of starter culture type and incubation temperatures on rheology and microstructure of low fat set yoghurt. International Journal of Dairy Technology, 62(4), 549–555.
Addeo, F., Alloisio, V., & Chianese, L. (2007). Tradition and innovation in the water buffalo dairy products. Italian Journal of Animal Science, 6, 51–57.
Ahmad, S., Gaucher, I., Rousseau, F., Beaucher, E., Piot, M., Grongnet, J. F., et al. (2008). Effects of acidification on physico-chemical characteristics of buffalo milk: a comparison with cow's milk. Food Chemistry, 106(1), 11–17.
Anema, S. G. (2008). Effect of temperature and rate of acidification on the rheological properties of acid skim milk gels. Journal of Food Processing and Preservation., 32(6), 1016–1033.
AOAC. (2006). Official methods of analysis. Washington: Association of Official Analytical Chemists.
Atwood, C. S., & Hartmann, P. E. (1992). Collection of fore and hind milk from the sow and the changes in milk-composition during suckling. Journal of Dairy Research, 59(3), 287–298.
Australia and New Zealand Food Standards (2006). Fermented milk Products-F2011C00622- Standard 2.5.3.
Baati, L., Roux, G., Dahhou, B., & Uribelarrea, J. L. (2004). Unstructured modelling growth of Lactobacillus acidophilus as a function of the temperature. Mathematics and computers in simulation., 65(1–2), 137–145.
Bezerra, M. F., Souza, D. F. S., & Correia, R. T. P. (2012). Acidification kinetics, physicochemical properties and sensory attributes of yoghurts prepared from mixtures of goat and buffalo milks. International Journal of Dairy Technology, 65(3), 437–443.
Bozanic, R., Brletic, S., & Lovkovic, S. (2008). Influence of temperature and sugar addition on soymilk fermentation by probiotic bacteria. Mljekarstvo, 58(1), 61–68.
Chandan, R. C., & O'Rell, K. R. (2006). Principles of yoghurt processing. In R. C. Chandan, C. H. White, A. Kilara, & Y. H. Hui (Eds.), Manufacturing yoghurt and fermented milks (pp. 195–210). Oxford, UK: Wiley-Blackwell.
Damin, M. R., Minowa, E., Alcantara, M. R., & Oliveira, M. N. (2008). Effect of cold storage on culture viability and some rheological properties of fermented milk prepared with yoghurt and probiotic bacteria. Journal of Texture Studies, 39(1), 40–55.
Dave, R. I., & Shah, N. P. (1997). Effect of level of starter culture on viability of yoghurt and probiotic bacteria in yoghurts. Food Australia., 49(4), 164–168.
Folkenberg, D. M., Dejmek, P., Skriver, A., Guldager, H. S., & Ipsen, R. (2004). Sensory and rheological screening of exopolysaccharide producing strains of bacterial yoghurt cultures. International Dairy Journal, 16(2), 111–118.
Ghadge, P. N. (2008). Effect of fortification on the physico-chemical and sensory properties of Buffalo milk yoghurt. Electronic journal of environmental, agricultural and food chemistry., 7(5), 2890–2899.
Gilliland, S. E., & Lara, R. C. (1988). Influence of storage at freezing and subsequent refrigeration temperatures on beta-galactosidase activity of Lactobacillus acidophilus. Applied and environmental microbiology., 54(4), 898–902.
Gosling, A., Alftren, J., Stevens, G. W., Barber, A. R., Kentish, S. E., & Gras, S. L. (2009). Facile pretreatment of Bacillus circulans beta-galactosidase increases the yield of galactosyl oligosaccharides in milk and lactose reaction systems. Journal of Agricultural and Food Chemistry, 57(24), 11570–11574.
Haque, A., Richardson, R. K., & Morris, E. R. (2001). Effect of fermentation temperature on the rheology of set and stirred yogurt. Food Hydrocolloids, 15, 593–602.
Hidalgo-Cantabrana, C., Sanchez, B., Moine, D., Berger, B., de Los Reyes-Gavilán, C. G., Sánchez, B., et al. (2013). Insights into the ropy phenotype of the exopolysaccharide-producing strain Bifidobacterium animalis subsp. lactis A1dOxR. Applied and environmental microbiology, 79(12), 3870–3874.
Kumar, P., & Mishra, H. N. (2003). Effect of mango pulp and soymilk fortification on the texture profile of set yoghurt made from buffalo milk. Journal of Texture Studies, 34(3), 249–269.
Laligant, A., Famelart, M. H., Paquet, D., & Brule, G. (2003). Fermentation by lactic bacteria at two temperatures of pre-heated reconstituted milk. II—dynamic approach of the gel construction. Le Lait, 83(4), 307–320.
Lee, W. J., & Lucey, J. A. (2003). Rheological properties, whey separation, and microstructure in set-style yoghurt: effects of heating temperature and incubation temperature. Journal of Texture Studies, 34(5–6), 515–536.
Lee, W. J., & Lucey, J. A. (2004). Structure and physical properties of yoghurt gels: effect of inoculation rate and incubation temperature. Journal of Dairy Science, 87(10), 3153–3164.
Leivers, S., Hidalgo-Cantabrana, C., Robinson, G., Margolles, A., Ruas-Madiedo, P., & Laws, A. P. (2011). Structure of the high molecular weight exopolysaccharide produced by Bifidobacterium animalis subsp lactis IPLA-R1 and sequence analysis of its putative eps cluster. Carbohydrate Research, 346(17), 2710–2717.
Lucey, J., Tamehana, M., Singh, H., & Munro, P. (1998a). A comparison of the formation, rheological properties and microstructure of acid skim milk gels made with a bacterial culture or glucono-delta-lactone. Food Research International, 31(2), 147–155.
Lucey, J. A., & Singh, H. (1997). Formation and physical properties of acid milk gels: a review. Food Research International, 30(7), 529–542.
Lucey, J. A., Tamehana, M., Singh, H., & Munro, P. A. (1998b). Effect of interactions between denatured whey proteins and casein micelles on the formation and rheological properties of acid skim milk gels. Journal of Dairy Research, 65(4), 555–567.
Marafon, A. P., Sumi, A., Alcantara, M. R., Tamime, A. Y., & de Oliveira, M. N. (2011). Optimization of the rheological properties of probiotic yoghurts supplemented with milk proteins. Lwt-Food Science and Technology., 44(2), 511–519.
Martin, N. C., Skokanova, J., Latrille, E., Beal, C., & Corrieu, G. (1999). Influence of fermentation and storage conditions on the sensory properties of plain low fat stirred yoghurts. Journal of Sensory Studies, 14(2), 139–160.
McClements, D. J. (2007). Understanding and controlling the microstructure of complex foods. Understanding and controlling the microstructure of complex foods. Cambridge, England: Woodhead.
Menard, O., Ahmad, S., Rousseau, F., Briard-Bion, V., Gaucheron, F., & Lopez, C. (2010). Buffalo vs. cow milk fat globules: size distribution, zeta-potential, compositions in total fatty acids and in polar lipids from the milk fat globule membrane. Food Chemistry, 120(2), 544–551.
Murga, M. L. F., Cabrera, G. M., de Valdez, G. F., Disalvo, A., & Seldes, A. M. (2000). Influence of growth temperature on cryotolerance and lipid composition of Lactobacillus acidophilus. Journal of Applied Microbiology, 88(2), 342–348.
Nahar, A., Amin, M. A., Alam, S. M. K., Wadud, A., & Islam, M. N. (2007). A comparative study on the quality of Dahi (yoghurt) prepared from cow, goat and buffalo milk. International Journal of Dairy Science., 2(3), 260–267.
Nguyen, H. T. H., Ong, L., Lefevre, C., Kentish, S. E., & Gras, S. L. (2013). The microstructure and physicochemical properties of probiotic buffalo yoghurt during fermentation and storage: a comparison with bovine yoghurt. Food and Bioprocess Technology. doi:10.1007/s/1947-013.1082z.
Oliveira, M. N., Sodini, I., Remeuf, F., & Corrieu, G. (2001). Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. International Dairy Journal, 11(11–12), 935–942.
Ong, L., Dagastine, R. R., Kentish, S. E., & Gras, S. L. (2011). Microstructure of milk gel and cheese curd observed using cryo scanning electron microscopy and confocal microscopy. Lwt-Food Science and Technology., 44(5), 1291–1302.
Ozer, B. H., & Kirmaci, H. A. (2010). Functional milks and dairy beverages. International Journal of Dairy Technology, 63(1), 1–15.
Paseephol, T., Small, D. M., & Sherkat, F. (2008). Rheology and texture of set yogurt as affected by inulin addition. Journal of Texture Studies, 39(6), 617–634.
Pesce, M. A., & Strande, C. S. (1973). New micromethod for determination of protein in cerebrospinal-fluid and urine. Clinical Chemistry, 19(11), 1265–1267.
Purwandari, U., Shah, N. P., & Vasiljevic, T. (2007). Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. International Dairy Journal, 17(11), 1344–1352.
Purwandari, U., & Vasiljevic, T. (2009). Rheological properties of fermented milk produced by a single exopolysaccharide producing Streptococcus thermophilus strain in the presence of added calcium and sucrose. International Journal of Dairy Technology, 62(3), 411–421.
Saccaro, D. M., Tamime, A. Y., Pilleggi, A., & Oliveira, M. N. (2009). The viability of three probiotic organisms grown with yoghurt starter cultures during storage for 21 days at 4 degrees C. International Journal of Dairy Technology, 62(3), 397–404.
Sahai, D. (1996). Buffalo milk: chemistry and processing technology (p. 276). New Delhi, India: Karnal.
Salvador, A., & Fiszman, S. M. (2004). Textural and sensory characteristics of whole and skimmed flavored set-type yoghurt during long storage. Journal of Dairy Science, 87(12), 4033–4041.
Sfakianakis, P. & Tzia, C (2011). Yoghurt from ultrasound treated milk: monitoring of fermentation process and evaluation of product quality characteristics. In: Taoukis PS, Stoforos NG, Karathanos VT & Saravacos GD (eds) The 11th International Congress on Engineering and Food (ICEF11): Food process engineering in a changing world. Comosware, Athens, Greece.
Shafiee, G., Mortazavian, A. M., Mohammadifar, M. A., Koushki, M. R., Mohammadi, A., & Mohammadi, R. (2010). Combined effects of dry matter content, incubation temperature and final pH of fermentation on biochemical and microbiological characteristics of probiotic fermented milk. African Journal of Microbiology Research, 4(12), 1265–1274.
Shiby, V. K., & Mishra, H. N. (2008). Modelling of acidification kinetics and textural properties in dahi (Indian yoghurt) made from buffalo milk using response surface methodology. International Journal of Dairy Technology, 61(3), 284–289.
Sodini, I., Remeuf, F., Haddad, S., & Corrieu, G. (2004). The relative effect of milk base, starter, and process on yogurt texture: a review. Critical Reviews in Food Science and Nutrition, 44(2), 113–137.
Tamime, A. Y., & Robinson, R. K. (2007). Background to manufacturing practice. Yoghurt: science and technology (Vol. 140). Cambridge: Woodhead.
Wang, Y., Corrieu, G., & Béal, C. (2005a). Fermentation pH and temperature influence the cryotolerance of Lactobacillus acidophilus RD758. Journal of Dairy Science, 88(1), 21–29.
Wang, Y., Delettre, M., Guillot, A., Corrieu, G., & Beal, C. (2005b). Influence of cooling temperature and duration on cold adaptation of Lactobacillus acidophilus RD758. Cryobiology, 50(3), 294–307.
Wu, S., Li, D., Li, S. J., Bhandari, B., Yang, B. L., Chen, X. D., et al. (2009). Effects of incubation temperature, starter culture level and total solids content on the rheological properties of yoghurt. International Journal of Food Engineering, 5(2), 1–17.
Xu, S. Y., Stanley, D. W., Goff, H. D., Davidson, V. J., & Lemaguer, M. (1992). Hydrocolloid milk gel formation and properties. Journal of Food Science, 57(1), 96–102.
Yadav, H., Jain, S., & Sinha, P. R. (2007). Evaluation of changes during storage of probiotic Dahi at 7 °C. International Journal of Dairy Technology, 60(3), 205–210.
Zhang, S. & Zhang, L. (2012). Effect of exopolysaccharide producing lactic acid bacterial on the gelation and texture properties of yogurt. In: Chen R, Sun D & Sung WP (eds) International Conference on Frontiers of Advanced Materials and Engineering Technology, vol 430–432. p^pp 890–893. Trans Tech Publication LTD, Zurich, Switzerland, Xiamen, China.
Acknowledgements
The authors would like to acknowledge the Australian Government for providing the Australian Postgraduate Award (International) (APA—International) scholarship, the Rural Industries Research and Development Cooperation (RIRDC) for financial support and Shaw River for kindly supplying the buffalo milk. The authors also thank the Particulate Fluids Processing Centre (PFPC) and the Bio21 Molecular Science and Technology Institute for access the equipment, Mr Roger Curtain for his help in operating the cryo-SEM and Dr Sandy Clarke (Department of Mathematics and Statistics, University of Melbourne) for her assistance in statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, H.T.H., Ong, L., Kentish, S.E. et al. The Effect of Fermentation Temperature on the Microstructure, Physicochemical and Rheological Properties of Probiotic Buffalo Yoghurt. Food Bioprocess Technol 7, 2538–2548 (2014). https://doi.org/10.1007/s11947-014-1278-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-014-1278-x