Abstract
Antioxidant active packaging was prepared by coating a citrus extract on the surface of polyethylene terephthalate (PET) trays which had been either treated with an atmospheric pressure plasma jet or left untreated. The surface characteristics of the packaging were examined, as were its stability and antioxidant efficacy following storage for up to 24 weeks under the following three storage conditions: room temperature, 0 % relative humidity (RH) or 50 °C. Plasma pretreatment increased coating density, thickness and roughness, and oxygenated functional groups at the polymer surface, whereas water contact angle decreased. Trays stored at room temperature did not lose their antioxidant efficacy over 24 weeks and plasma pretreatment enhanced the efficacy from week 8 onwards. Gravimetric analysis of the coating revealed a loss of antioxidant compounds only after 16 weeks. Trays stored at 0 % RH lost coating from week 1 onwards, with lower loss in plasma pretreated trays, while loss of coating was highest at 50 °C, with lower loss in plasma pretreated trays only after 24 weeks. Overall, the surface characteristics of the antioxidant active packaging were modified by plasma pretreatment of the PET surface, with some improvement in antioxidant efficacy, and the efficacy of the packaging in delaying oxidative deterioration in cooked meats was retained during storage at ambient temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The shelf life of lipid-containing foods, such as meat and meat products, is reduced by degradative processes like lipid oxidation that adversely affect organoleptic properties (Ladikos and Lougovois 1990; Ruban 2009). Among the different strategies that can be adopted to increase shelf life, the use of active packaging has received considerable attention in recent years (Suppakul, et al. 2003; Kerry et al. 2006).
Active packaging systems can be classified as either scavenging or releasing systems (De Kruijf et al. 2002). The former prevent food degradation and preserve organoleptic properties by adsorbing compounds like oxygen, moisture or taints, while the latter release compounds such as water, antioxidants or preservatives into the food (Vermeiren et al. 1999). Polyolefin films containing substances with antimicrobial or antioxidant properties have been developed by incorporating the compounds into the resin before the formation of the plastic film (Chen et al. 2012; Jin et al. 2009) or in the food contact layer of multilayer packaging materials, so that they can migrate into the food (van Aardt et al. 2007; Wessling et al. 2000).
Despite the availability of synthetic compounds with antioxidant activity, natural extracts from herbs, fruits, plants and spices have been widely studied as alternative additives for the preservation of food. Specifically, the addition of rosemary, oregano, olive, grape seed or citrus extracts has been shown to retard oxidation processes in meat (Murphy et al. 1998; Georgantelis et al. 2007; DeJong and Lanari 2009; Contini et al. 2012). The use of natural extracts for the development of active packaging is therefore of interest.
Innovative approaches for surface modification of plastic materials offer the potential to significantly extend the range of new packaging methods for food preservation. By incorporating specific functional groups, the polymer surface can be altered to improve important characteristics such as wettability, sealability, printability and adhesion. Cold plasma treatments result in a surface modification that presents advantages over other surface treatment techniques, due to its speed of treatment, in addition to its ability to involve a very limited surface layer. A plasma is a partially ionized gas that consists of excited and ionized particles, photons and radicals. It is produced by means of an electrical discharge; it induces chemical reactions on the treated surface through complex mechanisms where active species in the plasma play a predominant role (Borcia et al. 2004a, b). As such, the gas used for plasma treatment largely affects not only the nature of the modifications to the polymer structure (Placinta et al. 1997) but also the cost of the process.
Due to their ease of application, there is considerable interest in the use of atmospheric pressure plasma jets (APPJs) for the activation of polymers (Foest et al. 2007; Bismarck et al. 2008; Sun et al. 2010). The PlasmaTreat system is an example of an APPJ which generates plasma in air and, due to its low cost and high efficiency, has been used extensively to activate polymers prior to adhesive bonding (Lommatzsch et al. 2007; Dowling et al. 2011). In this study, the use of this air plasma system as a pretreatment of polyethylene terephthalate (PET) food trays, prior to the application of a citrus extract, is investigated. The objective is to evaluate the antioxidant performance of the natural extract on both plasma activated and unactivated PET. To date, no plasma treatments have been reported in the preparation of an active packaging with antioxidant properties.
A previous study showed the effectiveness of an active packaging prepared by coating a citrus extract on the surface of PET trays in reducing lipid oxidation in cooked turkey meat (Contini et al. 2012). The aim of the present study was twofold. Firstly, the study assessed the effects of plasma treatment on the morphology and retention characteristics of the citrus extract coating on the PET surface and on the antioxidant efficacy of the citrus-coated active packaging in reducing lipid oxidation in cooked meat. Secondly, we evaluated the stability of the citrus extract coating under different environmental conditions, by storing the citrus-coated trays for up to 24 weeks at room temperature, 0 % relative humidity (RH) or 50 °C, and measuring their efficacy in reducing lipid oxidation in cooked meat.
Materials and Methods
Reagents
A commercially available natural citrus extract in powder form containing carboxylic acids and flavanones was provided by Citrox Biosciences, Kimbolton, Cambridgeshire, England. Hydrochloric acid (37 % w/w), 2-thiobarbituric acid (98 %), 1,1,3,3-tetraethoxypropane (≥96 %) and acetic acid (≥99 %) were purchased from Sigma-Aldrich Ltd., Dublin, Ireland. Recycled PET trays (100 × 150 × 25 mm) were supplied by Holfeld Plastics Wicklow, Ireland. Low-density polyvinylchloride (PVC) catering film (thickness, 7.0 μm; O2 transmission, 2,000 cm3 m−2 D−1 bar−1) was supplied by Western Plastic Ltd., Galway, Ireland.
Preparation of the Active Packaging
Plasma activated PET trays (PET-PA) were prepared by activating the surface of uncoated PET trays with an APPJ plasma system (PlasmaTreat GmbH, Steinhagen, Germany) as described previously (Dowling et al. 2011). The plasma treatment was applied using compressed air as working gas with an inlet pressure at 300 kPa. Following an iterative study, the system parameters used to activate the PET samples were as follows: voltage of 5 kV, frequency of 20 kHz, plasma cycle time of 50 % and nozzle speed of 250 mm s−1. The distance between the nozzle and the PET tray samples was 15.5 mm and one pass of plasma was used to activate the PET surface. The plasma was ‘flushed’ through the jet nozzle mounted onto a cncGraf computer numerical control system (Boenigk Electronics, Bonn, Germany).
Citrus extract (10 % w/v methanol solution) was deposited on the inner surface of both the untreated (PET) and the plasma pre-treated (PET-PA) trays through a Mira Mist high-pressure nebulizer (Burgener Research Inc., Ontario, Canada), following a procedure described by Contini et al. (2012). The uncoated trays without (PET) and with (PET-PA) plasma pretreatment were used as controls in subsequent comparisons with citrus extract-coated trays, PET-CIT and PET-PA-CIT, respectively.
Surface Characterization of PET and Plasma Treated PET
X-ray Photoelectron Spectroscopy and Surface Chemical Composition
The analysis of the elemental composition of the PET and PET-PA was carried out using the X-ray photoelectron spectrometer (XPS) technique (Fadley 2010). This analysis was performed on square pieces (1 × 1 cm) cut from PET and PET-PA trays. The instrument used was a Kratos AXIS 165 with a monochromated Al Kα radiation at energy of 1486.6 eV. Survey spectra at pass energy of 160 eV were acquired before and after the plasma pretreatment of the PET surface. High-resolution spectra of C 1s and O 1s were recorded at the surface and binding energies were determined using C 1s peak at 284.8 eV as charge reference. For constructing and fitting the synthetic peaks of high resolution spectra, a mixed Gaussian–Lorentzian function with a Shirley-type background subtraction was used (Fadley 1978).
Surface Wettability
Surface wettability was examined by measuring the water contact angle (WCA) on square pieces (1 × 1 cm) cut from PET and PET-PA trays. The measurements were performed with the sessile drop technique at room temperature using the optical contact angle measuring instrument OCA 20 (Dataphysics Instruments GmbH, Filderstadt, Germany). The average value of six measurements was determined. Measurements were carried out within 1 h of plasma activation.
Morphology of Citrus Extract Coating
The surface roughness and film thickness of the citrus extract coatings were measured using an optical profilometer (Wyko, NT1100) operating in vertical scanning interferometry mode. The instrument calculates the average surface roughness (Ra) as the arithmetic mean of the departures (peaks and valleys) from the centerline over the sampling length. From the variations in coating thickness, an average thickness was estimated over the scanned surface at an instrumental resolution of approximately 5 nm. Each surface characterization measurement was repeated three times on each substrate for the statistical evaluation of the morphology parameters. Surface and cross-sectional images of the deposited coatings were obtained using a FEI Quanta 3D FEG DaulBeam™ (FEI Ltd, Hillsboro, OR, USA) focused ion beam/scanning electron microscope (SEM) system.
Assessment of Antioxidant Activity of Trays
Cooked turkey breast meat was used for the evaluation of the antioxidant activity of the trays. The meat was cooked and cut into slices using a procedure previously described by Contini et al. (2012). Nine meat slices (30 × 30 × 5.5 mm) per tray were placed in direct contact with tray surface; the tray was then immediately overwrapped with a PVC catering film and placed in refrigerator at 4 °C. After 0, 1 and 2 days of storage, the level of lipid oxidation was determined in three meat slices for each time using the thiobarbituric acid reactive substances (TBARS) distillation procedure described by Contini et al. (2012).
Evaluation of the Stability of the Active Packaging Under Different Storage Conditions
To assess the stability of the antioxidant activity, the PET-CIT and PET-PA-CIT trays were stored under three different environmental conditions. A set of trays were stored at room temperature (approximately 20 °C and RH of ∼50 %) and analysed immediately after preparation and then after 2, 4, 8, 16 and 24 weeks. Other sets of trays were placed in a desiccator (approximately 20 °C and RH of 0 %) to understand the effects of a dry environment on the antioxidant efficacy. A final set of trays was placed in an oven at 50 °C to determine the influence of high storage temperatures. The trays stored at 0 % RH and 50 °C were evaluated for their antioxidant efficacy only after 2 and 4 weeks. For each storage time considered, the antioxidant activity of PET-CIT trays and PET-PA-CIT trays was measured in triplicate and compared to that of the uncoated PET and PET-PA trays, respectively.
Weight Change of Citrus Extract Coatings on PET and Plasma-Treated PET Under Different Storage Conditions
Changes in weight of the coated PET during storage were determined gravimetrically using a Sartorius CP225D analytical balance with a resolution of 0.01 mg. Discs of 34 mm diameter were cut from uncoated PET trays using a stainless steel punch. The discs were submitted to four the different treatments described in “Preparation of Active Packaging” section: untreated (PET disc), plasma pretreated (PET-PA disc), citrus extract coated without plasma pretreatment (PET-CIT disc) and citrus extract coated after plasma pretreatment (PET-PA-CIT disc). The quantity of citrus extract coated on PET discs was calculated as the difference in weight before and after the coating procedure. The value obtained after three consecutive weighing differing by less than 0.2 mg was recorded as the initial weight of each disc (weight at time 0). For each treatment, three discs were then stored under the three different environmental conditions (room temperature, 0 % RH at room temperature, 50 °C). Changes in disc weight were monitored after 1, 2, 4, 8, 16 and 24 weeks following the same weighing procedure as at time 0. The weight change across the treatments and storage conditions was calculated as follows:
where W 0 = weight of discs at time 0, W t = weight of discs at time t, and W c = weight change of discs
The change in weight of citrus extract coating (W cc) from the different types of disc treatments, corrected for change in weight of the PET disc, was then calculated as follows:
The same calculation was applied to determine the weight change of citrus extract in PET-PA-CIT discs.
Statistical Analysis
Each experiment was conducted in triplicate and results were expressed as the mean ± standard deviation of the three replicates. Statistical analysis of the data was performed using one-way analysis of variance and Bonferroni’s test by SPSS (version 18) statistical software (IBM Inc. Chicago, IL, USA).
Results
Effect of Plasma Pretreatment on Chemical Composition and Wettability of the PET Surface
The chemical composition of the PET surface was determined by analysing the high-resolution XPS spectra. The deconvolution of the C 1s and O 1s peaks revealed the binding energies of carbon and oxygen, which are indicative of the nature of the chemical bonds of these two elements on the PET surface. For untreated PET, the C 1s spectrum was fitted with three peaks (Fig. 1a) at 284.8, 286.6 and 288.8 eV, which correspond to bonds typical of a benzene ring (C–C, C=C), methylene carbon singly bound to oxygen (–C–O–) and ester carbon atoms (O–C=O), respectively (Gupta et al. 2002; Jie-Rong et al. 1999). The additional small broad peak at approximately 292 eV corresponds to π–π* shake up satellites in phenyl groups (Placinta et al. 1997). The O 1s spectrum, on the other hand, showed two peaks at 531.9 and 533.5 eV (Table 1), which can be attributed to the carbonyl oxygen (O=C) and singly bonded oxygen atoms in the ester groups (O–C) (Girardeaux et al. 1996).
The XPS analysis of the PET-PA samples revealed significant modifications in the chemical structure of the PET surface induced by the plasma pretreatment (Fig. 1b). The decrease in C=C/C–C together with an increase in –C–O– bonds (Table 1) indicates the substitution of hydrogen of aromatic rings by hydroxyl groups (Riccardi et al. 2003). Another evident modification in the polymer structure was the appearance of a new carbon peak with a binding energy of 287.6 eV, suggesting the introduction of carbonyl groups (Girardeaux et al. 1996; Cueff et al. 1997; Choi et al. 2004) Besides, the broadening of the peaks after plasma treatment, expressed by the larger full width at half height maximum (Table 1), further suggests the presence of other species too close in binding energies to be discriminated from the existing peaks, such as hydroxyl and hydroperoxide groups at 286.4 eV and carboxylic groups at 288.8 eV (Cueff et al. 1997; Gupta et al. 2002). The existence of these new oxygen reactive sites in the plasma-treated polymer structure was also confirmed by an increase in the intensity of the two peaks obtained from the deconvolution of oxygen and by the increase in the O 1s/C 1s ratio from 0.27 to 0.59 (Table 1).
The changes in chemical composition with the introduction of these new chemical groups resulted in an increase of hydrophilicity of the polymer surface, as indicated by the results of WCA measurement, which showed a decrease in WCA from 84.0 ± 4.5° for PET (control) to 33.7 ± 3.6° for PET-PA samples.
Effect of Storage Conditions and Plasma Pretreatment on the Morphology of Citrus Extract Coating
The optical profilometer results revealed the morphology of the citrus extract coating on the PET surface, the effect of the plasma pretreatment and the effect of the three different storage conditions. The plasma pretreatment resulted in the adhesion of a significantly thicker citrus coating on the PET surface. In fact, the initial values of coating thickness were 1.70 ± 0.61 μm for the PET-CIT discs (Fig. 2a), while after treatment the PET-PA-CIT discs exhibited a thickness of 4.41 ± 0.42 μm (Fig. 2b). The citrus extract layer deposited using both processes was found to exhibit a decrease in thickness with storage time. The reduction in thickness was the lowest at 0 % RH storage conditions (Fig. 2a). The most pronounced loss in coating thickness was observed for storage at 50 °C, resulting in lower values compared to the other treatments at week 1 and week 2. The coating thickness on the PET-PA-CIT discs remained significantly higher (p < 0.01) than that on the PET-CIT discs for all storage conditions and times (p < 0.01) (Fig. 2a, b).
Coating thickness of a PET-CIT discs and b PET-PA-CIT discs stored at room temperature (open circle), discs stored at 0 % RH (open square), discs stored at 50 °C (open triangle). (a, b, c) Within each storage time, points with different letters are significantly different due to treatment (p < 0.01). (x, y, z) Within each treatment, points with different letters are significantly different due to storage time (p < 0.01)
The optical profilometry examination demonstrated that the PET-PA-CIT coatings exhibited significantly higher roughness (Ra) values (Fig. 3). The Ra for the PET-CIT discs was 1.13 ± 0.18 μm while that for the PET-PA-CIT discs was 3.34 ± 1.52 μm (Fig. 3a, b). The roughness values of PET-CIT discs remained significantly lower (p < 0.01) than those of the PET-PA-CIT discs for all the storage conditions and times. For both treatments, the roughness of samples stored at room temperature and 0 % RH were similar, while the decrease in roughness at 50 °C was much greater with significantly lower values at week 1 and week 2 for PET-CIT discs (p < 0.01) and at week 1, 2, 4 and week 8 for PET-PA-CIT discs (p < 0.01). The tribology results were further supported by SEM which showed a thicker, rougher coating on the plasma-pretreated PET surface, compared to the unactivated control (Fig. 4).
Coating roughness of a PET-CIT discs and b PET-PA-CIT discs stored at room temperature (open circle), discs stored at 0 % RH (open square), discs stored at 50 °C (open triangle). (a, b) Within each storage time, points with different letters are significantly different due to treatment (p < 0.01). (x, y, z) Within each treatment, points with different letters are significantly different due to storage time (p < 0.01)
Effect of Storage Conditions and Plasma Pretreatment on the Weight of Citrus Extract Coating
The initial amount of citrus extract coating deposited was calculated as 4.14 ± 0.72 mg for PET-CIT discs and 7.08 ± 0.06 mg for PET-PA-CIT discs. The weight change of the citrus extract coated on trays stored at room temperature, expressed as milligram of coating weight loss, showed a very low decreasing trend up to week 8 for both PET-CIT and PET-PA-CIT discs (Fig. 5a). A more pronounced weight loss was observed at week 16 and week 24, to reach maximum loss values of 1.25 ± 0.03 mg for PET-CIT discs and 1.29 ± 0.16 mg for PET-PA-CIT discs. This represented 30.17 ± 0.86 and 18.28 ± 2.58 % of the initial coating deposited on the PET-CIT and PET-PA-CIT trays, respectively. Differences between the two types of discs were not significant at any storage time. The results for the discs stored at 0 % RH revealed more pronounced differences in weight loss between PET-CIT and PET-PA-CIT discs. The untreated coated discs exhibited a significantly higher rate of weight loss (p < 0.01) from week 1 onwards (p < 0.01, except for week 16) compared with the discs that had been subjected to the plasma pretreatment. The total weight loss of 1.34 ± 0.06 mg, obtained at week 24 (Fig. 5b), represented 32.29 ± 1.05 % of the initial coating deposited. For the PET-PA-CIT discs, in contrast, the weight loss trend was more similar to that obtained at room temperature, with a slow weight loss during the first 8 weeks followed by a higher loss at week 16 and week 24, up to a final value of 0.86 ± 0.02 mg (12.10 ± 1.22 % of the initial coating deposited). Storage at 50 °C had a much higher impact on the loss of coating weight for both types of discs, resulting in a decrease of coating weight after 1 week of 1.65 ± 0.06 and 1.97 ± 0.10 for PET-CIT and PET-PA-CIT discs, respectively. Hence, the loss in weight after 1 week at 50 °C was significantly higher (p < 0.01) than the maximum weight loss for the other storage conditions during the whole experimental period (Fig. 5c). After the rapid and pronounced initial decrease, the coating weight remained stable and similar for the two treatments until week 16. For the plasma-treated discs, no further weight loss was detected at week 24, whereas the untreated discs showed a loss of coating of 4.15 ± 0.413 mg by the end of storage period, a value significantly higher than that observed for the PET-PA-CIT discs (p < 0.01).
Coating weight loss of citrus extract from PET-CIT discs (filled circle) and PET-PA-CIT discs (open circle). The discs were stored a at room temperature; b at 0 % RH and c at 50 °C for periods of up to 24 weeks. (a, b) Within each storage time, points with different letters are significantly different due to treatment (p < 0.01). (x, y, z) Within each treatment, points with different letters are significantly different due to storage time (p < 0.01)
Effect of Storage Conditions and Plasma Pretreatment on the Antioxidant Activity of the Active Packaging
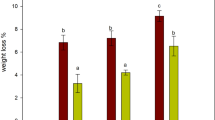
The antioxidant activity of the Citrus extract coating was evaluated by the percent reduction in TBARS values measured in the meat stored on PET-CIT and PET-PA-CIT trays compared to meat stored on uncoated PET and PET-PA trays, respectively. The trays stored at room temperature showed a reduction in lipid oxidation at week 0 of 36.7 ± 11.8 and 49.1 ± 11.1 % for PET-CIT and PET-PA-CIT trays, respectively (Fig. 5). During the experiment, the reduction of TBARS values remained almost unchanged in time, with the exception of the meat measured at week 8, for which the antioxidant effects were higher for both treatments. This likely relates to the lower initial oxidation of the meat used at week 8 compared to the other times periods (Table 2). A possible explanation could be a shorter cooking time due to the smaller size of the turkey breast used at week 8, which may have resulted in lower initial oxidation levels (Peiretti et al. 2012). Throughout the 24 weeks of the study, the oxidation levels in the meat stored on both the PET-CIT and PET-PA-CIT trays were significantly lower (p < 0.01) than those of the corresponding controls (PET and PET-PA, respectively) (Table 2), indicating that the antioxidant activity of the packaging was stable over 24 weeks of storage. The reduction in TBARS values in meat samples stored on PET-PA-CIT trays was more pronounced than for those stored on PET-CIT trays at all weeks, but the differences between the two treatments were statistically significant only from week 8 onwards (p < 0.01).
The antioxidant activity for the trays stored at 0 % RH was slightly lower compared to storage at room temperature, with percent reduction in lipid oxidation values of 21.99 ± 10.8 % for PET-CIT trays and 38.7 ± 8.0 % for PET-PA-CIT trays at week 4 (Fig. 6). The differences between the two treatments (PET-CIT vs PET-PA-CIT), however, were not statistically significant. Moreover, all the trays stored at 0 % RH maintained their antioxidant efficiency, as revealed by the significant lower TBARS values (p < 0.05) than those of the controls (Table 2). The loss of antioxidant efficiency with storage was most pronounced for the trays stored at 50 °C, with TBARS reductions of 8.71 ± 15.5 and 27.3 ± 20.3 % for the PET-CIT and PET-PA-CIT trays at week 4, respectively (Fig. 6). Under these storage conditions, the PET-CIT trays lost their antioxidant activity even after 2 weeks of storage, as indicated by the lack of a significant difference compared to uncoated control (Table 2). The results also show a large variability, as indicated by the high error bars. This is likely due to the degradation of the antioxidant coating with increasing temperature, reducing the interaction between the active substances and the meat. The results for trays stored at 50 °C further confirmed the enhancement of the stability of the citrus extract coating provided by the plasma pretreatment, expressed by the significantly lower TBARS values (p < 0.05) compared to the control (Table 2).
Percentage reduction in TBARS values of cooked turkey meat after 2 days storage at 4 °C on PET-CIT trays (black columns) and PET-PA-CIT trays (grey columns) previously stored for periods of up to 24 weeks at room temperature. (a, b) Within each storage time, bars with different letters are significantly different due to treatment (p < 0.01). (y, z) Within each treatment, bars with different letters are significantly different due to storage time (p < 0.01)
Discussion
The initial weight of citrus extract coatings on the surface of PET-CIT and PET-PA-CIT discs (4.14 ± 0.72 vs 7.08 ± 0.06 mg) clearly suggests that the modification of the PET surface induced by the plasma pretreatment was effective in increasing the amount of the extract coating adhering to the polymer surface. This effect was confirmed by the higher coating thickness value of 4.41 ± 0.42 μm for PET-PA-CIT discs compared to 1.70 ± 0.61 μm for PET-CIT discs. Other studies on the effect of plasma treatments of PET polymers have also shown an increase of PET surface roughness due to the removal of additives, processing aids or fragments of the polymer by etching effects (Gupta et al. 2002; Yang et al. 2009). Therefore, the higher polymer surface available for the adhesion of the citrus extract constituents due to the increased PET roughness could in part explain the higher amount of coating found in the PET-PA-CIT discs.
The adhesion of the coating on the PET surface is also determined by the chemical affinity between citrus extract components and the polymer. In our previous study, the citrus extract coating appeared hydrophilic with a WCA of 22 ± 0.6° (Contini et al. 2012) due possibly to the flavanones contained in the extract which are rich in oxygenated functional groups such as hydroxyl and keto groups (Yanishlieva et al. 2006; Belitz et al. 2009), while citric and salicylic acid contain carboxyl groups. Therefore, during the deposition of the extract, polar interactions could take place between its components to form a layer on the polymer surface. The establishment of polar interactions between the citrus extract coating and the oxygenated functional groups of the plasma-pretreated PET surface could further enhance the stability of the antioxidant coating. In that respect, the higher hydrophilicity of the polymer surface after plasma pretreatment, indicated by the decrease in WCA value, could have contributed to the enhancement in citrus extract deposition. In fact, dipole interactions and hydrogen bonds could take place between the carbonyl, hydroxyl, hydroperoxide and carboxyl groups, formed on the PET surface after plasma treatment, as confirmed by XPS, and the antioxidant molecules. Besides, reactions between hydroperoxides on the plasma-pretreated PET surface and carboxyl groups of citric and salicylic acid could induce the formation of covalent bonds, in a similar way to that observed in grafting processes of acrylic acid on PET polymers (Ying et al. 2003).
The higher roughness of the citrus extract coating observed in the PET-PA-CIT discs compared to PET-CIT discs (3.34 ± 1.52 vs 1.13 ± 0.18 μm) could be a consequence of the higher amount of citrus extract coated on the discs surface, amplifying a ‘cluttered’ disposition of the antioxidant molecules during the coating process. Nevertheless, it could also be a consequence of the higher surface roughness of the polymer induced by the plasma pretreatment that was reflected in the coating morphology.
The measurement of the weight loss of the coated discs stored at room temperature indicated that the antioxidant coating exhibited a high degree of stability up to week 8, with a final loss of 30.17 ± 0.86 % of the initial coating weight in the case of PET-CIT. This weight loss however did not have a significant effect on the antioxidant properties of the active packaging, which remained remarkably constant throughout the storage period. This suggests that either the components lost did not contribute to the antioxidant effect or the amount lost was not sufficient to reduce the efficacy of the packaging. In addition, the decrease in coating thickness, observed during the first 4 weeks of storage (Fig. 2a) resulted from a rearrangement of the molecule disposition on the surface without a loss of components. Modifications in surface roughness were minor with a slight decrease in accordance with the loss of coating components (Fig. 3a). The effect of plasma pretreatment enhanced the adhesion of the antioxidant substances, resulting in a final loss in active substances of only 18.28 ± 2.58 % of the initial value. This may explain the significantly higher reduction of TBARS values compared to PET-CIT trays from week 8 onwards (Fig. 6).
The results for storage at 0 % RH suggest an influence of the air humidity on the stability of the citrus extract coating, with a higher weight loss throughout the whole storage period, compared to the results observed for the discs stored at room temperature (Fig. 5b). The beneficial effect of plasma pretreatment on the stability of the antioxidant coating was further confirmed by the lower coating weight loss observed in PET-PA-CIT discs. It is interesting to note that, even if the weight loss observed at 0 % RH at week 4 was lower than that observed at room temperature at week 24 (Fig. 5a, b), the TBARS reduction obtained at week 4 (Fig. 7) was lower than that obtained at week 24 for the trays stored at room temperature (Fig. 6). This may indicate that the citrus extract constituents lost at 0 % RH were different from those lost at room temperature. Another possible explanation is that the lower decrease in film thickness compared to that observed at room temperature (Fig. 2a) may suggest a different rearrangement of the molecules of the coating induced by the lack of air humidity, which decreased the antioxidant effect.
Percentage reduction in TBARS values of cooked turkey meat after 2 days storage at 4 °C on PET-CIT trays (black column) and PET-PA-CIT trays (dark grey column) previously stored for up to 4 weeks at 0 % RH, and on PET-CIT trays (white column) and PET-PA-CIT trays (light grey column) previously stored for up to 4 weeks at 50 °C. (a) Within each storage time, bars with similar letters are not significantly different due to treatment (p < 0.05). (z) Within each treatment, bars with similar letters are not significantly different due to storage time (p < 0.05)
An immediate and noticeable decrease in all the physical parameters indicated a loss of the coating at 50 °C, which was also observed visually. Presumably, the higher temperature weakened the adhesion of the citrus extract components, with a loss of arguably the more volatile components of the coating. The degradation of the coating explains the loss of the antioxidant activity of the PET-CIT trays already apparent at week 2 (Fig. 7). Although the reduction of lipid oxidation decreased also for the PET-PA-CIT trays, the plasma pretreatment facilitated superior Citrus extract coating performance, with antioxidant activity maintained until week 4.
Conclusions
The study on the effect of plasma pretreatment and storage conditions on the effectiveness of PET packaging coated with citrus extract showed that the antioxidant effect of the active packaging exhibited good stability even after storage for a period of up to 24 weeks. The antioxidant performance was further enhanced by the plasma pretreatment of the trays, which resulted in the incorporation of oxygenated functional groups onto the plasma activated surface. Under given spray deposition conditions, significantly enhanced levels of citrus extract were obtained on the plasma-pretreated PET surface, possibly due to its more hydrophilic chemistry. This thicker and more adherent coating (based on weight loss studies) exhibited superior antioxidant effect, compared to the citrus coating deposited onto the unactivated polymer. The storage of the PET at 0 % RH and particularly at 50 °C accelerated the degradation of the coating, reducing the antioxidant properties of the packaging, although the plasma pretreatment still enhanced stability. These promising results indicate the suitability of the active packaging with plasma pretreatment for commercial use because of its potential to preserve meat products from oxidative degradation. Further studies on the optimization of the coating procedure could enhance control of the coating deposition and its antioxidant efficacy. In addition, from an economic perspective, the value of a packaging depends on the balance between the benefits of its use and the costs of its production, and this requires further investigation.
References
Belitz,H-D, Grosch,W & Schieberle, P (2009) Fruits and fruit products. In, Food chemistry, (pp 807–861). Berlin: Springer.
Bismarck, A, Brostow, W, Chiu, R, Lobland H-E-H & Ho K-K-C (2008) Effects of surface plasma treatment on tribology of thermoplastic polymers. Polymer Engineering and Science, 48: 1971–1976.
Borcia, C., Anderson, C.-A., & Brown, N.-M.-D. (2004a). The surface oxidation of selected polymers using at atmospheric pressure air dielectric barrier discharge. Part I. Journal of Applied Surface Science, 221, 203–214.
Borcia, C., Anderson, C.-A., & Brown, N.-M.-D. (2004b). The surface oxidation of selected polymers using at atmospheric pressure air dielectric barrier discharge. Part II. Journal of Applied Surface Science, 225, 186–197.
Chen, X., Lee, D.-S., Zhu, X., & Yam, K.-L. (2012). Release kinetics of tocopherol and quercetin from binary antioxidant controlled-release packaging films. Journal of Agricultural and Food Chemistry, 60, 3492–3497.
Choi, H.-S., Kim, Y.-S., Zhang, Y., Tang, S., Myung, S.-W., & Shin, B.-C. (2004). Plasma-induced graft co-polymerization of acrylic acid onto the polyurethane surface. Surface & Coating Technology, 182, 55–64.
Contini, C., Katsikogianni, M.-G., O’Neill, F.-T., O’Sullivan, M., Dowling, D.-P., & Monahan, F.-J. (2012). PET trays coated with citrus extract exhibit antioxidant activity with cooked turkey meat. Food Science and Technology, 47, 471–477.
Cueff, R., Band, G., Benmalek, M., Besse, J.-P., Butruille, J.-R., & Jaquet, M. (1997). X-ray photoelectron spectroscopy studies of plasma-modified PET surface and alumina/PET interface. Journal of Applied Polymer Science, 115, 292–298.
DeJong, S., & Lanari, M.-C. (2009). Extracts of olive polyphenols improve lipid stability in cooked beef and pork: contribution of individual phenolics to the antioxidant activity of the extract. Food Chemistry, 116, 892–897.
Dowling, D.-P., O’Neill, F.-T., Langlais, S.-J., & Law, V.-J. (2011). Influence of dc pulsed atmospheric pressure plasma jet processing conditions on polymer activation. Plasma Processes and Polymers, 8(8), 718–727.
Fadley, C.-S. (1978). Basic concepts of X-ray photoelectron spectroscopy. In C. R. Brundle & A. D. Baker (Eds.), Electron spectroscopy: theory, techniques and applications (pp. 2–145). London: Academic.
Fadley, C.-S. (2010). X-ray photoelectron spectroscopy: progress and perspectives. Journal of Electron Spectroscopy and Related Phenomena, 178–179, 2–32.
De Kruijf, N., Van Beest, M., Rijk, R., Sipiläinen-Malm, T., Paseiro Losada, P., & De Meulenaer, B. (2002). Active and intelligent packaging: applications and regulatory aspects. Food Additives and Contaminants, 19, 144–162.
Foest, R., Bindemann, T., Brandenburg, R., Kindel, E., Lange, H., Stieber, M., & Weltmann, K.-D. (2007). On the vacuum ultraviolet radiation of a miniaturized non-thermal atmospheric pressure plasma jet. Plasma Processes and Polymers, 4, 460–464.
Georgantelis, D., Ambrosiadis, I., Katikou, P., Blekas, G., & Georgakis, S.-A. (2007). Effect of rosemary extract, chitosan and tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C. Meat Science, 7, 172–181.
Girardeaux, C., Zammatteo, N., Art, M., Gillon, B., Pireaux, J.-J., & Caudano, R. (1996). Animation of poly(ethylene terephthalate) polymer surface for biomedical application. Plasmas and Polymers, 1(4), 327–346.
Gupta, B., Plummer, C., Bisson, I., Frey, P., & Hilborn, J. (2002). Plasma-induced graft polymerization of acrylic acid onto poly (ethylene terephthalate) film: characterization and human smooth muscle cell growth on grafted film. Biomaterials, 23(3), 863–871.
Jie-Rong, C., Xue-Yan, W., & Tomiji, W. (1999). Wettability of poly(ethylene terephthalate) film treated with low-temperature plasma and their surface analysis by ESCA. Journal of Applied Surface Science, 72(10), 1327–1334.
Jin, X., Zumbrunnen, D.-A., Balasubramanian, A., & Yam, K. (2009). Tailored additive release rates in extruded plastic films produced with smart blending machines. Journal of Plastic Film and Sheeting, 425, 115–140.
Kerry, J.-P., O’Grady, M.-N., & Hogan, S.-A. (2006). Past, current and potential utilization of active and intelligent packaging systems for meat and muscle-based products: a review. Meat Science, 74, 113–130.
Ladikos, D., & Lougovois, V. (1990). Lipid oxidation in muscle food: a review. Food Chemistry, 35, 295–314.
Lommatzsch, U., Pasedag, D., Baalmann, A., & Ellinghorst, G. (2007). Atmospheric pressure plasma jet treatment of polyethylene surfaces for adhesion improvement. Plasma Processes and Polymers, 4, 1041–1045.
Murphy, A., Kerry, J.-P., Buckley, J., & Gray, I. (1998). The antioxidative properties of rosemary oleoresin and inhibition of off-flavours in precooked roast beef slices. Journal of Science and Food Agriculture, 77, 235–243.
Peiretti, P.-G., Medana, C., Visentin, S., Dal Bello, F., & Meineri, G. (2012). Effect of cooking method on carnosine and its homologues, pentosidine and thiobarbituric acid reactive substance contents in beef and turkey meat. Food Chemistry, 132, 80–85.
Placinta, G., Arefi-khonsari, F., Gheorghiu, M., Amouroux, J., & Popa, G. (1997). Surface properties and the stability of poly(ethylene terephthalate) films treated in plasmas of helium-oxygen mixtures. Journal of Applied Polymer Science, 66, 1367–1375.
Riccardi, C., Barni, R., Selli, E., Mazzone, G., Massafranca, M.-R., Marcandalli, B., & Poletti, G. (2003). Surface modification of poly (ethylene terephthalate) fibers induced by radio frequency air plasma treatment. Journal of Applied Surface Science, 211, 386–397.
Ruban, S.-W. (2009). Lipid peroxidation in muscle food—an overview. Global Veterinaria, 3(6), 509–513.
Sun, J., Yao, L., Gao, Z., Peng, S., Wang, C., & Qiu, Y. (2010). Surface modification of PET films by atmospheric pressure plasma-induced acrylic acid inverse emulsion graft polymetization. Surface & Coating Technology, 204, 4101–4106.
Suppakul, P., Miltz, J., Sonneveld, K., & Bigger, S.-W. (2003). Active packaging technologies with an emphasis on antimicrobial packaging and its applications. Journal of Food Science, 68(2), 408–420.
van Aardt, M., Duncan, S.-E., Marcy, J.-E., Long, T.-E., O’Keefe, S. F., & Sims, S.-R. (2007). Release of antioxidants from poly(lactide-co-glycolide) films into dry milk products and food simulating liquids. International Journal of Food Science and Technology, 42(11), 1327–1337.
Vermeiren,L, Devlieghere,F, Debevere, J, Smolander, M, Hurme, E, Ahvenainen, R, Van Beest M-D & Rijk, M-A-H (1999) An in-depth review of technologies, legislation, market and consumer needs and trends in active and intelligent packaging in relation to current European food packaging regulations. Literature review of FAIR-project Actipak (CT 98–4170), October, 55p.
Wessling, C., Nielsen, T., & Giacin, J.-R. (2000). Antioxidant ability of BHT- and α-tocopherol-impregnated LDPE film in packaging of oatmeal. Journal of the Science of Food and Agriculture, 81, 194–20.
Yang, L., Chen, J., Guo, Y., & Zhang, Z. (2009). Surface modification of a biomedical polyethylene terephthalate (PET) by air plasma. Applied Surface Science, 255, 4446–4451.
Yanishlieva, N.-V., Marinova, E., & Pokorný, J. (2006). Natural antioxidants from herbs and spices. European Journal of Lipid Science and Technology, 108, 776–793.
Ying, L., Yin, C., Zhuo, R.-X., Leong, K.-W., Mao, H.-Q., Kang, T.-E., & Neoh, K.-G. (2003). Immobilization of galactose ligands on acrylic acid graft-copolymerized poly(ethylene terephthalate) film and its application to hepatocyte culture. Biomacromolecules, 4, 157–165.
Acknowledgments
This work was undertaken within the Precision Strategic Research Cluster supported by Science Foundation Ireland grant 08/SRC/I1411.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Contini, C., Katsikogianni, M.G., O’Neill, F.T. et al. Storage Stability of an Antioxidant Active Packaging Coated with Citrus Extract Following a Plasma Jet Pretreatment. Food Bioprocess Technol 7, 2228–2240 (2014). https://doi.org/10.1007/s11947-013-1210-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1210-9