Abstract
Amaranth nutritional value has been widely recognized, but the required conditions for its processing cannot be adapted to traditional technologies. For the proposal of alternative strategies, the changes of several components should be understood. Enriched starch and lipid–protein fractions of amaranth flour upon different milling treatments were obtained and characterized by attenuated total reflectance–Fourier transform-infrared spectroscopy. Starch- and lipid–protein-enriched amaranth fractions were obtained by abrasive milling; amaranth starch was isolated by wet-milling procedure, and flour samples were obtained from planetary ball milling. Changes on starch, protein, and lipids relative contents, on starch crystallinity and on lipids and protein stability after milling and 6-month storage, were evaluated. The Fourier transform-infrared (FT-IR) spectroscopy peaks of the main grain components were identified in the middle-infrared range. By calculating the ratios between height intensities of selected specific peaks, several characteristics of the samples could be explained: increased protein content and lipid proportion of the enriched fraction; decrease of the starch crystallinity degree by abrasive milling and especially by ball milling due to starch amorphization during these processes; and lipids modification in milled and in 6-month aged samples. FT-IR analysis can be considered a rapid, nondestructive, solvent-free, sensitive, and useful tool to investigate starch, lipid, and protein modifications provoked by processing and storage as well as to determine, based on intensity ratio, the relative proportion of grain components within amaranth milling fractions. The abrasive milling associated to planetary ball milling to obtain modified different fractions is presented as an interesting strategy for the processing of amaranth grain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amaranth is a pseudo cereal that shows rapid growth, with high tolerance to arid climate conditions and poor soils where traditional cereals cannot be grown. The main cultivars used are Amaranthus hipochondriacus, Amaranthus cruentus, and Amaranthus caudatus. Amaranth seeds contain a significant proportion of starch (62 %), protein (14–17 %), and fat (5–9 %) which make the flour a promising material for film production (Tapia-Blácido 2006). Amaranth has shown interesting functional properties compared to other widely used grains in starch production, regarding its water binding, swelling, and pasting properties (Calzetta Resio et al. 2006). By applying acid wet-milling procedure, Calzetta Resio (2007) has isolated amaranth starch at laboratory scale. However, the grain fractionation by wet or dry milling has not been performed at industrial scale due to several factors which involve the poor development of grain varieties suitable for mechanical harvesting (Peiretti 2009) and the lack of suitable milling method to overcome low fraction yields associated to grain size. Enriched amaranth fractions were obtained by Tosi et al. (2000) who developed a differential dry-milling technique to produce three fractions: a protein enriched with more than 40 % of protein, another rich in dietary fiber, and a starchy fraction containing about 79 % of starch. More recently, Roa-Acosta et al. (2011) have obtained enrichment fractions by abrasive milling of amaranth grain. Starch fraction (enrichment of 88 %) and lipid–protein-enriched fraction with 23 % of lipid content and protein content of 46 % were obtained. Such abrasive milling was presented as a pretreatment in wet-milling method to isolate amaranth starch.
Fourier transform-infrared (FT-IR) spectra analysis has being widely used to evaluate product composition and to determine chemical modifications induced by different food processing methods. This technique was used by Shrestha et al. (2010) to study changes in long- and short-range molecular order provoked by extrusion procedure in high-amylose maize starch. The formation of hydrogen bonds between the blend components was confirmed using FT-IR spectroscopy by Jiménez-Elizondo et al. (2009), who developed biodegradable films based on amaranth. Liu et al. (2011) have determined changes in crystalline/amorphous fractions of maize starch subjected to ball-milling treatment. Capron et al. (2007) studied the organization of various starch samples varying in molecular structure and moisture content by attenuated total reflectance–FT-IR (ATR-FT-IR) spectroscopy. These authors analyzed main changes related to the glass transition as function of moisture content and crystalline/amorphous ratio. During sesame protein isolation, FT-IR analysis revealed that heat treatment as well as use of chemical additives (NaCl and ammonium sulfate) at lower concentrations induced changes in protein conformation, whereas at higher concentrations of the latter additives, the protein conformation was more stable (Achouri et al. 2012).
The development of suitable fractionation technologies for the application of amaranth grains will provide benefits from nutritional and economical standpoints. The aim of this work was to analyze several amaranth milling treatments as innovative strategies for obtaining enriched starch and lipid–protein fractions and to characterize those fractions by using FT-IR. Changes on starch, protein, and lipid relative content, on starch conformation after abrasive, ball, and wet milling and on lipid stability after 6-month storage, were evaluated.
Materials and Methods
Material
Amaranth grain (A. cruentus) used in this study was harvested on the west of Buenos Aires. The grains were screened to remove foreign matter and stored in sealed containers at room temperature previous to their use. The moisture content of amaranth grain was 10.5 ± 0.1 g/100 g (determined by official method AOAC 2000, 925.09).
Milling Treatments
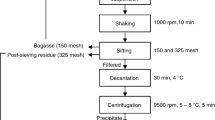
Figure 1 shows a flow chart with the milling procedures. Raw seeds [control samples (C)] were ball milled (C-BM) and also were used for starch isolation by wet milling (SWM). Abrasive milled was also performed from raw seeds, obtaining two fractions: lipid–protein-enriched fraction (LPEF) and starch-enriched fraction (SEF). SEF fraction was also subjected to ball milling at different times (obtaining SEF-BM1 and SEF-BM2). LPEF samples were aged to analyze lipid and protein changes. All milling procedures were performed by triplicate.
Amaranth milling diagram. Milling and analysis conditions are reported in “Materials and Methods” section
Abrasive Milling
Starch- and lipid–protein-enriched fractions were obtained by abrasive milling using a laboratory rice mill Suzuki MT-95 (Suzuki, San Pablo, Brazil), which separates automatically pearled amaranth (starchy fraction yield, 70 %) and bran (lipid–protein fraction yield, 28 %). After an optimization evaluation through an experimental design, the selected milling conditions involved 100 g of milling load and 90 min of milling time (Roa-Acosta et al. 2011). In order to observe the changes during long-time storage of enriched fractions, some LPEF samples (10 g) were stored at 8 °C during 6 months in sealed containers (aged LPEF).
Ball Milling
The SEF sample previously obtained by abrasive milling and control sample were milled in a planetary ball mill. A PM-100 planetary ball mill (Retsch, Haan Mettman, Germany) with one stainless steel milling cylinder (500 mL) was used. The amaranth samples and five times weight stainless steel balls (φ = 10 mm) were placed into the stainless steel container up to about one third of their capacity. The milling was rotated horizontally at constant milling speed of 350 rpm for 47 min obtaining SEF-BM1 and C-BM samples and for 180 min obtaining SEF-BM2 sample. The ball milling was changed in rotational direction in every 30 s. Such milling conditions were adopted based on literature findings (Martínez-Bustos et al. 2007; Zhang et al. 2010). Samples milled were sifted in a vibrating screen Zonytest (model EJR200; Buenos Aires, Argentina) through sieves no. 60 (250 μm), no. 80 (177 μm), and no. 200 (74 μm). The residue (74 μm) contained the flour and was selected for FT-IR analysis.
Wet Milling: Starch Conventional Isolation
Amaranth starch was isolated by acid wet-milling procedure developed by Calzetta Resio et al. (2006). Soaking was performed at 50 °C during 120 min, using a soaking solution of 0.55 g SO2/L (made with NaHSO3 from Sigma Chemical Co., St. Louis, USA) with a ratio of solution/grain of 3:1 (in milliliter per gram). The isolated starch (SWM) was dried in a vacuum oven at 40 °C for 48 h and stored at room temperature in a sealed container.
Attenuated Total Reflectance–Fourier Transform-Infrared Spectroscopy
FT-IR spectra were obtained in a FT-IR spectrometer model spectrum 400 (PerkinElmer, Inc., Shelton CT, USA) with a DTGS detector. All the samples were measured as obtained respectively by the milling treatment. Whole-seed control flour and SEF samples were prepared in a butt mill (Decalab Fbr®, Buenos Aires, Argentina) with 30 s of milling time in order to obtain a powder; this grinding mild condition was considered negligible regarding starch structure. All the powders were studied in a MIRacle single-reflection attenuated total reflectance (ATR) accessory (PIKE Technologies, Inc., Madison WI, USA) with a single-reflection diamond/ZnSe crystal at an incident angle of 45°. The spectrum of each sample was obtained by taking the average of 64 scans at a resolution of 4 cm−1, at 25 °C. The spectra were acquired between 600 and 4,000 cm−1. A strong apodization was used, with a magnitude phase correction. A flat tip was used to obtain an intimate contact between sample and crystal, without pressure control. A background spectrum was recorded in air (without sample) prior to each spectrum measurement. Spectral analysis was performed using the Spectrum software version 6.3.5 (PerkinElmer, Inc.). The average of the triplicates for each milled sample was reported. Since the used crystal showed a great absorbance (around 95 % of the incident light) in the spectral region between 1,800 and 2,500 cm−1, this region was subtracted. Baseline was corrected, and the spectra were normalized (between 0 and 1) for figure presentation. The spectra shown in Fig. 4 were previously normalized using max/min. The wave number of the peaks was obtained through the equipment software tool by using a 1 % reflectance threshold. The reflectance signal heights of each spectrum at 996, 1,014, 1,039, 1,075, and 1,145 cm−1 were recorded from each sample; the intensity ratios of the bands at 996 and 1,014 cm−1 (996/1,014) and at 1,039 and 1,014 cm−1 (1,039/1,014) were used as convenient indexes of short-range starch conformation (Lopez-Rubio et al. 2008; Shrestha et al. 2010; Liu et al. 2011). The intensity ratio of the bands at 1,145 and 1,075 cm−1 (1,145/1,075) was used as a convenient starch content index of the samples since these bands were not influenced by the short-range changes. The band heights were also studied for proteins (1,640 and 1,540 cm−1) and lipids (2,923, 2,853, and 1,740 cm−1) and were related to the starch main peak (996 cm−1), in order to establish normalized ratios in each spectrum among the principal constituents of the samples.
Determination of Amaranth Fractions Composition
The proximate composition of amaranth and amaranth fractions was determined in triplicate by official methods: moisture content (AOAC 2000, 925.09 method); starch (AACC 1995, 76–11 method); protein (AOAC 2005, 960.52 method); fat (AOAC 2005, 925.12 method); ash (AOAC 2005, 923.03 method).
Statistical Analysis
Significance of the effect of milling treatment and the effect of composition on amaranth seed fractions were evaluated by one-way ANOVA (significance level α = 0.05 %) with Tukey posttest using Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). In some cases, p value was calculated by using a t test in order to deeply analyze differences between samples.
Results and Discussion
The main components of control, starch- and lipid–protein-enriched fractions from abrasive milling, and starch from wet milling were determined, and the results are reported in Table 1. The samples obtained by ball milling (C-BM, SEF-BM1, and SEF-BM2) have the same starch content of that of the input sample (ball-milling treatment only changes the short-range starch conformation, but not its content). It can be seen that the abrasive-milling procedure successfully produced two well-enriched fractions, one in lipids and proteins and the other in starch. The enrichment level obtained in this work was higher in comparison with reported data of Tosi et al. (2000). SWM sample showed, as it was expected, higher starch purity in comparison to SEF sample obtained by abrasive milling. However, it is important to remark that from the industrial point of view, though in this procedure, two enriched fractions were produced together.
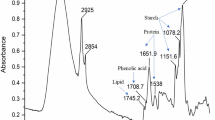
FT-IR spectroscopy was employed in order to study the main bands observed in the amaranth whole-flour control sample (Fig. 2) and in both enriched fractions obtained by abrasive milling (Fig. 3). Figure 2 shows a complex spectrum in which it is possible to identify three separated areas for analyzing starch, lipids, and proteins. The bands assignment based on the literature for similar flours is shown in Table 2. The main band at 996 cm−1 can be ascribed to starch contribution. This band showed two shoulders at 1,014 and 1,039 cm−1, being the latter more intense than the first one. Protein bands can be observed at 1,640 and 1,540 cm−1 and correspond to the amide I and II vibrations, which are the most common vibration to study proteins (Cremer and Kaletunç 2003; Barth 2007). Lipid contribution can be observed in the bands at 2,923, 2,853 and 1,740 cm−1, corresponding to C–H vibration. Similar spectra were obtained by Tapia-Blácido (2006) with edible films based on amaranth flour and starch.
The protein and lipid relative content after the different treatments was also analyzed by FT-IR spectroscopy. Figure 3 shows the spectra of control sample and those of the abrasive-milled amaranth flour. All the acquired spectra of the treated samples presented the same main bands observed in the control amaranth flour. It can be observed that SEF sample showed higher relative intensity of the starch bands (996 cm−1 and others) with respect to the control spectrum. Besides, LPEF showed higher relative intensities for protein and lipid bands and a distinct pattern of the carbohydrate region (1,200–700 cm−1), revealing that the main contribution to this band may be attributed to fiber of amaranth bran or starch of perisperm, which were removed by abrasive milling, supported by the lower starch content in this sample, as previously discussed.
Table 3 shows the main changes observed in the FT-IR spectra of the different fractions as a consequence of different milling treatments and aging. In order to avoid differences in bands intensity due to ATR data acquisition, normalization was performed by taking adequate intensity ratios. Then, the intensity ratios between the main lipid and protein bands with respect to starch main band (996 cm−1) for each fraction were compared among them, as is shown in Table 3. An increase from 0.76 to 1.07 in the 1,640/996 ratio and from 0.46 to 0.68 in 1,540/996 ratio were observed for lipid–protein-enriched fraction according to its higher protein proportion in relation with control. In the same way, the increase from 0.35 to 0.48 of 2,923/996 ratio reflected the major proportion of lipid in such enriched fraction.
Several FT-IR intensity ratios have been proposed for evaluating structural changes of starch-rich systems. The bands at 1,047 and 1,020 cm−1 have been assigned to ordered and amorphous structure of starch, respectively (Lopez-Rubio et al. 2008). The ratio between the bands at 1,039/1,014 and 996/1,014 cm−1 have been more frequently used to quantify the crystallinity degree in starches (Sevenou et al. 2002; Capron et al. 2007; Liu et al. 2011). The spectra for starch-enriched fractions (SEF and SEF-BM1) and starch extracted by wet milling showed differences in the crystalline/amorphous region, as shown in Fig. 4. The values of the 996/1,014 and 1,039/1,014 cm−1 intensity ratios for these samples are reported in Table 3. The ratio 1,039/1,014 cm−1 decreased from 0.896 (C) to 0.870–0.796 (SEF–SEF-BM2), indicating that the ordered structure in treated starch granules was partially destroyed, especially by ball-milling treatment and, in less extent, by abrasive milling. The ball-milling treatment caused a relative intensity decrease in the 1,039 cm−1 band, indicating a rise of amorphous structure in starch granules caused by this process. On the other hand, the 996/1,014 cm−1 ratio significantly decreased. The ball-milling treatment may induced an irreversible distortion of the ordered structure of the starch granule and generation of more amorphous phase which lead to the increase in the degree of disorder of treated starch granules (Xie et al. 2006; Liu et al. 2011). This phenomenon was further demonstrated by Liu et al. (2011) in maize starch with X-ray diffraction analysis, showing the reduction and even disappearance of the principal diffraction peaks along ball-milling treatment. The higher reduction of 1,039/1,014 ratio in the starch fraction obtained by wet milling can be explained by the annealing effect associated to the soaking temperature (50 °C) during wet-milling procedure (Calzetta Resio 2007).
Figure 5 shows the intensity ratios of selected peaks from FT-IR spectra of amaranth flour fractions obtained by abrasive milling (SEF and LPEF), control, and starch from wet milling as a function of their lipid, protein, and carbohydrate concentrations determined by means of standard analytical methods (Table 1). In Fig. 5, the fractions obtained from ball milling (C-BM, SEF-BM1, and SEF-BM2) and the aged lipid–protein-enriched sample were not included, since in ball milling, the thermomechanical treatment may change biomolecules conformation and the FT-IR pattern, and they cannot be compared to the abrasive-milled samples, as well as for the chemically modified samples by aging. While, as discussed before, in the starch-rich fractions, the 996/1,014 and 1,039/1,014 ratios are frequently related to structural aspects of starch, such as crystallinity loss and amorphicity, and they did not correlate with the starch content. It was found, however, that the ratio between the intensities at 1,145 and 1,075 (not shown in Table 3) correlated with the starch content of the samples. Good correlations were obtained for lipid, protein, and carbohydrate contents as can be observed in Fig. 5, with correlation coefficients higher than 0.984. It can be concluded that protein, lipid, and carbohydrate contents could be accurately obtained by FT-IR means for the following ranges of content: 5–47, 2–23, and 73–99 %, respectively.
Finally, the effect of planetary ball milling and of 6-month aging was studied on lipids and protein as shown in Fig. 6. The effect of planetary ball milling (C-BM sample) on lipid and protein bands in comparison with the control is also shown in Table 3. A reduction of 20 % was found for lipid band (2,923/996); besides, the amide I and amide II bands decreased 13.1 and 19.6 %, respectively. Besides, the ratio of 2,850/996 corresponding to lipid region also showed a similar decrease respect to control, as shown in Fig. 6. Prabhasankar and Rao (2001) have reported protein degradation (glutenin fraction) in wheat flour obtained by stone and plate milling. These authors also found a diminution of free lipid and unsaturated fatty acids contents as consequence of dry milling. They related the lower binding content of free lipids to other constituents to the heat generated during milling. Considering the high level of energy involved in planetary ball milling, the observed reductions of FT-IR bands can be attributed to thermomechanical degradation of grain components.
The aging effect can be also observed in terms of lipid intensity ratio (2,923/996) decrease in Table 3, which reached a 50 % reduction in comparison with the corresponding band of LPEF fraction. Besides, a lower reduction of 34.6 and 39.7 % was found for amide I and amide II protein bands, respectively. Lipid degradation during flour storage (aging) is caused by several factors, involving the presence of lipases (Doblado-Maldonado et al. 2012), increase of free radicals, and antioxidant degradation (Calucci et al. 2004). A decline in both soluble and storage protein contents is observed during accelerated aging in seeds of wheat (Calucci et al. 2004) and other plants (Basavarajappa et al. 1991), probably associated to protein bond breakage occurring in endosperm proteins during seed aging. Lipid oxidation and parallel protein modifications are promoted by shearing action as well as friction in the ball mill, and it continues during aging benefited by the minimum particle size of flour.
Conclusions
The characteristic FT-IR peaks of the main components in whole amaranth seeds and in milled fractions (starch, lipid, and protein) were identified. By calculating the ratios between height intensity of selected specific peaks and that at 996 cm−1, which corresponds to the C–O–C glycosidic starch bond and was the one with fairly constant intensity, several characteristics of the samples could be conveniently explained: increased protein content and lipid proportion of the LPEF; decrease of the crystallinity degree in the ball-milled samples due to starch amorphization during this process; and decrease of the C–H vibration band of the lipids in the aged samples. FT-IR analysis can be thus considered as a useful tool to determine the relative proportion of grain components within amaranth milling fractions as well as to investigate starch modifications provoked by abrasive and ball milling and lipid deterioration due to oxidative reactions. As a nondestructive, solvent-free, and sensitive technique, it results attractive to follow molecular and structural changes caused to the main food components during processing and storage.
The abrasive milling associated to planetary ball milling to obtain modified milling fractions or combined with wet milling to isolate starch fraction is presented as an interesting milling strategy of amaranth grain to overcome technological issues related to the processing of amaranth grain. This study provides further insights about the industrial use of amaranth, which possess interesting functional properties in starch production and for protein-enriched products, compared to other widely used grains.
References
AACC 76–11. (1995). Starch content method. Approved methods of the AACC (9th ed.). St Paul: American Association of Cereal Chemistry.
Achouri, A., Nail, V., & Boye, J. (2012). Sesame protein isolate: fractionation, secondary structure and functional properties. Food Research International, 46, 360–369.
AOAC 923.03. (2005). Ash of flour. Official methods of analysis, 18th ed. Association of Official Analytical Chemists: Gaithersburg, MD.
AOAC 925.09. (2000). Solids (total) and moisture in flour. Official methods of analysis, 17th edn. Association of Official Analytical Chemists: Gaithersburg, MD.
AOAC 925.12. (2005). Acid hydrolysis method. Official methods of analysis, 18th edn. Association of Official Analytical Chemists: Gaithersburg, MD.
AOAC 960.52. (2005). Microchemical determination of nitrogen. Official methods of analysis, 18th edn. Association of Official Analytical Chemists: Gaithersburg, MD.
Barth, A. (2007). Infrared spectroscopy of proteins. Biochimica et Biophysica Acta, 1767, 1073–1101.
Basavarajappa, B. S., Shetty, H. S., & Prakash, H. S. (1991). Membrane deterioration and other biochemical changes, associated with accelerated aging of maize seeds. Seed Science Technology, 19, 279–286.
Calucci, L., Capocchi, A., Galleschi, L., Ghiringhelli, S., Pinzino, C., Saviozzi, F., & Zandomeneghi, M. (2004). Antioxidants, free radicals, storage proteins, puroindolines, and proteolytic activities in bread wheat (Triticum aestivum) seeds during accelerated aging. Journal of Agricultural and Food Chemistry, 52, 4274–4281.
Calzetta Resio, N. (2007). Wet-milling of amaranth grain (A. cruentus). Study of the effects of processing conditions on yield and quality of its products and properties of the starchy fraction. PhD Thesis. Buenos Aires, Argentina: Department of Industry, University of Buenos Aires.
Calzetta Resio, N., Tolaba, M., & Suárez, C. (2006). Effects of steeping conditions on wet-milling attributes of amaranth. International Journal of Food Science and Technology, 41(2), 70–76.
Capron, I., Robert, P., Colonna, P., Brogly, M., & Planchot, V. (2007). Starch in rubbery and glassy states by FTIR spectroscopy. Carbohydrate Polymers, 68, 249–259.
Cremer, D., & Kaletunç, G. (2003). Fourier transform infrared microspectroscopy study of the chemical microstructure of corn and oat flour-based extrudates. Carbohydrate Polymers, 52, 53–65.
Doblado-Maldonado, A. F., Pike, O. A., Sweley, J. C., & Rose, D. J. (2012). Key issues and challenges in whole wheat flour milling and storage. Journal of Cereal Science, 56, 119–126.
Jiménez-Elizondo, N., Sobral, P. J. A., & Menegalli, F. C. (2009). Development of films based on blends of Amaranthus cruentus flour and polyvinyl alcohol. Carbohydrate Polymers, 75, 592–598.
Jiugao, Y., Ning, W., & Xiaofei, M. (2005). The effects of citric acid on the properties of thermoplastic Starch plasticized by glycerol. Starch-Starke, 57, 494–504.
Liu, T., Ma, Y., Yu, F., Shi, J., & Xue, S. (2011). The effect of ball milling treatment on structure and porosity of maize starch granule. Innovative Food Science and Emerging Technologies, 12(4), 586–593.
Lopez-Rubio, A., Flanagan, M., Shrestha, A., Gidley, M., & Gilbert, E. (2008). Molecular rearrangement of starch during in-vitro digestion: towards a better understanding of enzyme resistant starch formation. Biomacromolecules, 9, 1951–1958.
Martínez-Bustos, F., López-Soto, M., San Martín-Martínez, E., Zazueta-Morales, J., & Velez-Medina, J. (2007). Effects of high energy milling on some functional properties of jicama starch (Pachyrrhizus erosus L. Urban) and cassava starch (Manihot esculenta Crantz). Journal of Food Engineering, 78, 1212–1220.
Peiretti, G. (2009). Becoming real a new crop—25 years promoting the future of amaranth. In: Proceedings of the Amaranth Symposium, 22–23 October 2009. Buenos Aires, Argentina: La Plata. Book.
Prabhasankar, P., & Rao, P. H. (2001). Effect of different milling methods on chemical composition of whole wheat flour. European Journal of Food Research and Technology, 213, 465–469.
Roa-Acosta, D., Loubes, M., Baeza, R. & Tolaba, M. (2011). Following amaranth pearling by means of image analysis. In: Proceeding of Congreso Argentino de Ciencia y Tecnología de Alimentos, 19–21 October 2011, Buenos Aires, Argentina (CD-ROM).
Sevenou, O., Hill, S. E., Farhat, I. A., & Mitchell, J. R. (2002). Organization of the external region of starch granules as determined by infrared spectroscopy. International Journal of Biological Macromolecules, 31, 79–85.
Shrestha, K., Ng, C., Lopez-Rubio, A., Blazek, J., Gilbert, E., & Gidley, M. (2010). Enzyme resistance and structural organization in extruded high amylose maize starch. Carbohydrate Polymers, 80(3), 699–710.
Tapia-Blácido, D. (2006). Edible films based on amaranth flour and starch. PhD Thesis. Department of Food Engineering, Campinas-SP, Brazil.
Tosi, E., Re, E., Lucero, H., & Masciarelli, R. (2000). Amaranth (Amaranthus spp.) grain conditioning to obtain hyperproteic flour by differential milling. Food Science and Technology International, 5, 60–63.
Xie, X., Liu, Q., & Cui, S. W. (2006). Studies on the granular structure of resistant starches (type 4) from normal, high amylose and waxy corn starch citrates. Food Research International, 39, 332–341.
Zhang, Z., Zhao, S., & Xiong, S. (2010). Morphology and physicochemical properties of mechanically activated rice starch. Carbohydrate Polymers, 79, 341–348.
Acknowledgments
The authors acknowledge the financial support from PME-2006-01685, UBACYT (Project UBACyT 20020100100397 and 20020110200357), CONICET (PIP 100846), and ANPCYT (PICT 0928). PRS and MPB are members of CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roa, D.F., Santagapita, P.R., Buera, M.P. et al. Amaranth Milling Strategies and Fraction Characterization by FT-IR. Food Bioprocess Technol 7, 711–718 (2014). https://doi.org/10.1007/s11947-013-1050-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1050-7