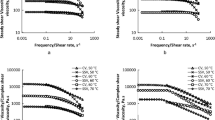Abstract
Different xanthan gum concentrations (0–0.8 %) were tested, and the rheological properties of ice cream mixes were characterized as linear viscoelastic solids. Ostwald de Waele was successfully used to fit the steady shear data of ice cream mixes exhibiting a pseudoplastic flow (R 2 > 0.982). The samples with xanthan gum were characterized as strong gel-like macromolecular dispersions with G′ much greater than G″ but without a cross-point in the whole range of frequency applied. Cox–Merz rule was not applicable to the ice cream mixes. Steady and dynamic rheology of the ice cream mixes changed with increasing xanthan gum concentration. Besides, the four-component Burger model consisted of the association in series of the Maxwell model and the Kelvin–Voigt model was used to characterize the viscoelasticity. It was also found that the final percentage recovery parameters; J SM, J ∞, J KV, and %R (compliance of Maxwell spring and dashpot, Kelvin–Voigt element and R, respectively) of the ice cream mixes were dramatically changed by the xanthan gum concentration, increasing the internal structure parameters G 0, G 1, η 0, and η 1 (elastic moduli of Maxwell and Kelvin–Voigt springs and corresponding dashpot viscosities, respectively).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polysaccharides are hydrocolloids that can interact strongly with water (Dogan et al. 2007). Xanthan gum, a hydrocolloid, is a heteropolysaccharide with a primary structure and an important industrial biopolymer consisting of repeated pentasaccharide units that are formed by two glucose units and one glucuronic acid unit (Garcia-Ochoa et al. 2000). Its annual worldwide production is 30,000 t corresponding to a market of $408 million, indicating that it is the most commercially produced industrial gum (Kalogiannis et al. 2003; Silva et al. 2009). Xanthan gum has been widely used as a thickening or stabilizing agent in the food industry due to its unique textural and rheological properties, namely high viscosity at low shear, shear thinning (pseudoplastic behavior), stability over a broad range of temperature and pH, and high resistance to shear degradation in aqueous solutions (Hsu and Lo 2003; Silva et al. 2009).
Texture and apparent viscosity are very important quality characteristics of ice cream (Arbuckle and Frandsen 1966; Dogan and Kayacier 2007). Many factors play an important role on the textural and apparent viscosity properties of ice cream. These are kind, quality and concentration of ingredients, processing and handling and composition of the mix; mainly fat and stabilizer. Final texture of the ice cream is also affected by various characteristics of the ice cream mix, including rheological properties of end product (Caillet et al. 2003; Chan 1984; Dogan and Kayacier 2007). For example, smooth texture and cooling sensation, which are the most commonly desired attributes of ice cream, can be achieved by optimization of rheological properties of the mix (Chang and Hartel 2002).
Ice cream mix, a complex colloidal system, includes some substances present in true solution, some of which are colloidally suspended and some of which are in dispersion (Goff 2002; Innocente et al. 2002). Among these substances are the stabilizers that have been used in the ice cream industry or in a combination with other constituents (Martinou Voulasiki and Zerfiridis 1990). Ice cream quality is affected by the stabilizers that are primarily used to increase the apparent viscosity of mix (Cottrell et al. 1980). When the viscosity increased, a resistance occurs against melting and smoothness of the body, leading a decrease in the rate of whipping (Arbuckle and Frandsen 1966). Additionally, increase in apparent viscosity leads to increase in resistance to melting of final product and enhanced smoothness. Therefore, desirable apparent viscosity of the mix can be obtained by controlling composition of the mix (Arbuckle and Frandsen 1966; Dogan and Kayacier 2007).
In this study, different concentrations of xanthan gum were added into ice cream mix formulations in order to examine its possible effects on the rheological properties of the mixes. It should be noted that, sensory quality, and consequently consumer preference are considerably influenced by the rheological properties of fluid foods. Of the rheological measurements, testing of viscosity is generally considered to be one of the most important tools for measuring of physical characteristics related to the quality of food products. Knowledge of the flow behavior is beneficial not only in quality control of the product but also for energy input calculations, process design, and equipment selection, particularly for heat exchangers and pumps (Ibanoglu and Ibanoglu 1998). Dynamic rheological testing is also a tool measuring viscoelastic characteristics of food systems. Characterization of texture and structure in foamed dairy emulsions has been completed using dynamic rheological measurements (Dogan and Kayacier 2007; Stanley et al. 1996; Wildmoser et al. 2004).
Studying the viscoelastic properties is a rheological characterization of a food system, which is important with respect to the engineering design of continuous process, development of new products and quality control during processing of emulsion type foods. The viscoelastic properties of complex food systems are studied by using oscillatory tests to determine storage (elastic) and loss (viscous) modulus and their complex viscosity (Dolz et al. 2006; Hernandez et al. 2004). However, another type of analysis is also required to determine the possible internal structure of a system and the structural changes depending on its composition (Dolz et al. 2008). In this respect, creep and recovery tests are among those that are the most commonly used methods. These are the methods in which the food system is deformed for a predetermined time period under a constant shear stress and the deformation is measured as a function of time. These are the creep and recovery phases in which the applied stress is removed and the deformation formation degree is measured within the similar time period (Dolz et al. 2008). In this test, mechanical simulation models, namely Maxwell model and Kelvin–Voigt models are used to reflect deformation of a system. Simplest approaches are used in these models. In the Maxwell model, either spring (representing purely elastic) or dashpot (representing purely viscous) and association of both elements are used in series. In the Kelvin–Voigt model, these elements are used in parallel (Dolz et al. 2008). In this respect, the four-component Burger model consisted of the association in series of the Maxwell model and the Kelvin–Voigt model is one of the most commonly used models (Yoo and Rao 1996; Dolz et al. 2008). However, a limited number of studies have appeared so far to investigate the creep and recovery properties of the ice cream mixes added with xanthan gum using mechanical approach.
In this study, we prepared ice cream mix samples added with different concentrations of xanthan gum. It was aimed to analyze the effect of different xanthan gum levels on the steady and dynamic shear properties of the ice cream mixes. It was also aimed to determine viscoelastic properties of ice cream mixes in terms of creep and recovery properties and simulate the effect of xanthan gum addition using a mechanistic approach.
Materials and Methods
Ingredients and Preparation of Ice cream Mixes
The ice cream mixes were prepared according to the traditional ice cream manufacturing method (Dogan and Kayacier 2007). In this respect, the following ingredients were used in the ice cream mix production (in g/100 g): 10 % sucrose, 7.0 % cream (including 70 % fat), 0.5 % emulsifier (saturated mono- and di-glycerides), and stabilizer system. The system consisted of 0.5 % salep and different concentrations (0, 0.4, or 0.8 %) of xanthan gum (Sigma, G1253, USA). After homogenization of mix in a single-state homogenizer (20.5 M Pa at 60 °C), the ingredients were pasteurized at 85 °C for 1 min with constant stirring. After the pasteurization, the ice cream mixes were rapidly cooled down to 4 °C. For ageing, the ice cream mixes were then stored at 4 °C for 24 h until the analysis.
Steady Shear Analysis
A controlled stress rheometer (RheoStress 1, HAAKE, Karlsruhe, Germany) equipped with a temperature control unit (Water bath K15, Thermo-Haake Karlsruhe, Germany) was used to conduct rheological analysis of the ice cream mixes. The mixes were sheared using a cone-plate configuration (cone diameter, 35 mm; angle, 4°; and gap size, 0.140 mm). Measurements were carried out in the shear rate range of 1–100 s−1 at 5 °C; 0.85-ml sample was placed between cone and plate, and the measurement was started immediately. During the shearing, a total of 25 data points were recorded at 10-s intervals. Each measurement was replicated with two repetitions. The apparent viscosity was determined as a function of shear rate. Obtained data were fitted to Ostwald de Waele model using RheoWin Data Manager (RheoWin Pro V. 4.0, HAAKE, Karlsruhe, Germany) and consistency coefficient and flow behavior index values were calculated according to the following model used to describe shear-induced behavior of the ice cream mix samples:
where η is apparent viscosity (in Pascal per second), K is consistency coefficient (in Pa sn), \( \mathop{\gamma }\limits^{ \cdot } \)is shear rate (in seconds), and n is flow behavior index (dimensionless).
The steady shear data were processed with respect to the effect of xanthan gum concentration on apparent viscosity of the ice cream mixes. The variation of apparent viscosity with concentration at the specified shear rate of 50 s−1 can be described by several models (Ibarz et al. 1987; Rao et al. 1984). These are generally power-law and exponential type models as following:
where η 50 is apparent viscosity at 50 s−1 (Pa s), C is concentration of xanthan gum (%), η 1 and η 2 are constant for concentration effect (Pa s), a 1 is constant (dimensionless) of Power law model and a 2 is constant ((%)−1).
Small Amplitude Oscillatory Shear Analysis
A dynamic oscillatory shear rheometer (RheoStress 1, HAAKE, Karlsruhe, Germany) was used to carry out the frequency sweep tests for all ice cream mixes. Mechanical spectra were plotted over a frequency (f) range of 0.1–10 Hz at 0.2 Pa (within the range of linear viscoelasticity) at 5 °C. In oscillatory shear tests, a sinusoidal oscillating stress or strain with an increasing frequency is applied to samples. The elastic or storage modulus G′ and the viscous or loss modulus G″ are calculated as a function of frequency (Steffe 1996):
Loss tangent, a ratio between viscous and elastic properties, is a dimensionless number, and it gives a clear indication of whether the material behavior is solid like or liquid like, was calculated according to the following equation (Gunasekaran and Ak 2000).
where δ is phase angle.
The overall response of the sample against to the sinusoidal strain may be characterized by using the equations of complex modulus G* and complex viscosity η*:
Plots of ω versus G′ and G″ dynamic rheological data were subjected to non-linear regression, and the magnitudes of intercepts (K′ and K″), slopes (n′ and n″), and R 2 were computed from raw data using the following equations (Rao and Cooley 1992; Yoo and Rao 1996):
Cox–Merz rule known to be valid for heterogeneous dispersions and commercial foods was used for the establishment of correlations between the values of oscillatory shear parameters (complex viscosity, η* and angular frequency, ω) and steady shear parameters (apparent viscosity, η and shear rate, \( \mathop{\gamma }\limits^{ \cdot } \)) (Gunasekaran and Ak 2000; Juszczak et al. 2004; Rao and Tattiyakul 1999). The relationship between the steady shear and dynamic shear data is valuable to correlate true material characteristics obtained from different tests (Gunasekaran and Ak 2000; Steffe 1996). The Cox–Merz rule is used to estimate steady shear viscosity from dynamic shear viscosity and vice versa (Steffe 1996):
Creep-Recovery Tests
A controlled stress rheometer (RheoStress 1, HAAKE, Karlsruhe, Germany) equipped with a temperature control unit (Waterbath K15, Thermo Haake Karlsruhe, Germany) and data acquisition software (RheoWin Pro V. 2.96, Haake, Karlsruhe, Germany) was used to determine creep and recovery behaviors of the ice cream mixes. The compliance measurements were carried out using a cone-plate configuration (cone diameter, 35 mm; angle, 4°; and gap size, 0.140 mm). Stress sweep tests were performed between 0.1 and 10 Pa to assure the tests being in the linear viscoelastic region (LVR). Creep tests were recorded at constant stress amplitude (0.2 Pa within the range of linear viscoelasticity) at 5 °C. The ice cream mix samples (each 0.85 ml) were placed between cone and plate and suddenly subjected to a constant shear within the LVR. Until deformation of the viscoelastic materials approaches a steady state in the time when the deformation rate remains constant, the stress is applied and then suddenly removed and analyzed for recoverable shear. In this respect, the stress was applied instantly and maintained for 150 s, and then released to allow sample recovery for 150 s in this study. Each measurement was replicated three times on three different samples.
Statistical Analysis
Data were subjected to statistical analysis using Minitab for Windows Release 14®. Standard errors were calculated using the Duncan’s multiple range test (MstatC 1986).
Results and Discussion
Steady Shear Properties
Figure 1 illustrates the shear stress (σ) versus shear rate (\( \mathop{\gamma }\limits^{ \cdot } \)) and apparent viscosity (η) versus shear rate (\( \mathop{\gamma }\limits^{ \cdot } \)) plots for the ice cream mixes prepared with different gum concentrations (0, 0.4, and 0.8 %) at 5 °C. The values of flow behavior index (n) ranging from 0.527 to 0.070 were observed for all the ice cream mixes indicating a non-Newtonian shear-thinning behavior (Table 1). This can be attributed to a complex involvement of partially broken-down micellar casein at the droplet surface in the ice cream mix (Dickinson and Stainsby 1982). These results were also in accordance with the information given by Goff and Davidson (1992) and Soukoulis et al. (2008) who reported that ice cream mixes exhibit non-Newtonian flow characteristics. Accordingly, as seen in Fig. 1, apparent viscosity decreased with the increase in shear rate, which was also related to the increased alignment of the constituent molecules (Rha 1975). Consistent results were reported in the literature. Kaya and Tekin (2001) reported the same flow behavior for a typical ice cream model mixes exhibiting a non-Newtonian flow in which apparent viscosity decreased with increasing of shear rate. On the other hand, n values decreased with gum concentration (Table 1). The Ostwald de Waele model was found to be adequate for describing the flow behavior of the ice cream mixes because determination coefficients (R 2) were higher than 0.984. Table 1 also indicates that increase in gum concentration caused a considerable non-linear increase in consistency coefficients (K), as could be expected. Higher K values indicated a higher viscous nature because of increase in apparent viscosity in ice cream mixes, and a consistent trend was also observed in K and n values depending on the gum concentration. In literature, similar trend was observed in the ice cream mix prepared with natural gums (Cottrell et al. 1980; Goff and Davidson 1994). It was also reported that polysaccharides such as xanthan gum showed a greater increase in non-Newtonian behavior with concentration than the anionic gums (Cottrell et al. 1980). In addition, Morris (1995) and Sworn (2000) reported that the xanthan gels freely flow and thus perform a strong shear thinning behavior. In our study, this was the case for the xanthan gum. This means that the gum may provide a higher shear thinning behavior, which may lead to system characteristics that could be exploited to stabilize particle suspensions at lower shear rate process conditions.
Effect of Gum Concentration on Apparent Viscosity (η 50)
The effect of xanthan gum concentration on η 50 values was also shown in Table 1. As can be seen, an increment in the η 50 values was observed as the gum concentration increased. It is well known that increasing solid content normally increases the consistency (Saricoban et al. 2010, 2008; Yapar et al. 2006). Additionally, the effect of gum addition to increase the apparent viscosity was well reported (Cottrell et al. 1980). Kaya and Tekin (2001) reported that the salep, a glucomannan-based hydrocolloid increased the consistency coefficient of ice cream. They found the consistency coefficient of ice cream mix containing 0.62 % salep to be 464.47 Pa sn while the consistency coefficient of ice cream mix containing 0.4 % salep was 143.19 Pa sn at 10 °C.
Power law and exponential type models were used to describe the variation of η 50 values with concentration. Linearized forms of Eqs. 2 and 3 were plotted and corresponding model parameters are presented in Table 2, which represents the values of the parameters of power law and exponential relationships. Depending on the coefficient of determination values obtained from two models, the exponential model (R 2 = 0.980) seems to describe the effect of gum concentration on η 50 values of the ice cream mixes clearly, but the power-law model did not absolutely (R 2 = 0.136).
Dynamic Shear Properties
Stress Sweep and Frequency Sweep Tests (Dynamic Viscoelastic Properties)
Stress sweep test was conducted in the range of 0.1–10 Pa at a fixed frequency (0.628 rad/s–0.1 Hz); therefore, 0.2 Pa (which is in the LVR) was selected as a constant stress to conduct frequency sweep. Dynamic oscillatory shear test can be used to determine viscoelastic properties of food materials. The elastic (or storage) modulus G′ expresses the magnitude of the stored energy in the material or recoverable per cycle of deformation. For completely hardened ice cream, all of which water is frozen, maximum storage modulus can be expected because of the maximum solids fraction (Wildmoser et al. 2004). However, the measure of magnitude of energy lost at viscous dissipation per cycle of deformation is called as viscous (loss) modulus G″. Therefore, all the energy is stored in a perfectly elastic solid, namely, G″ is 0, which means stress and strain will be in phase. On the contrary, all the energy in a liquid having no elastic properties is dissipated as heat, namely, G′ is 0, which means stress and strain will be out of phase by −90° (Sharoba et al. 2005).
Plots of frequency versus G′ and G″ dynamic rheological data were subjected to non-linear regression and the magnitudes of intercepts, slopes and R 2 are summarized in Table 3. In this respect, all the ice cream mixes studied showed similar flow characteristic; that is, shear-thinning behavior following power law model determined from G′ and G″ versus frequency plots. The possible reason could be attributed to the fact that the shear thinning region occurs as a result of a dramatic shear which induces the structural breakdown related to a mechanism of oil droplet deflocculation (Bower et al. 1999; Franco et al. 1998; Moros et al. 2002). Figure 2 demonstrates the changes in G′, G″, and η* values as a function of frequency for ice cream mixes prepared with 0, 0.4, and 0.8 % xanthan gum concentrations at 5 °C. As can be seen, the low-frequency dependency was particularly observed for 0.8 g/100 g xanthan addition in the ice cream mixes. In other words, gum addition into the ice cream mixes led increase of G′ and G″ with frequency to become small. Wildmoser et al. (2004) reported the similar behavior; namely, G′ was slightly dependent on the oscillation frequency referred to be a “plateau region” which is characterized by a decrease in the slope of both G′ and G″ (lower than 1) and possibly minimum in the G″. This is known to be an intermediate zone of the mechanical spectra between the “terminal” and the “transition” zones (Ferry 1980). In general, this was reported to be a characteristic behavior of protein-stabilized emulsion systems because an elastic network occurs due to the occurrence of an extensive bridging flocculation process (Franco et al. 1998, 1997; Pal 1995).
On the other hand, ln (G′,G″) of the ice cream mixes indicated the positive slopes in spite of their low frequency dependency. From a structure point of view, for true gels, ln (G′,G″) versus ln ω plots have 0 slope, while there are positive slopes and G′ is greater than G″ over a whole frequency ranges of ω studied for weak gels and highly concentrated dispersions (Ross-Murphy 1984). On the other hand, no cross-point was observed in the ice cream mixes added with xanthan gum within the whole range of frequency applied (Fig. 2). Therefore, the ice cream mixes added with xanthan gum were regarded to exhibit strong gel-like behavior because the slopes were positive (n′ = 0.904–0.150; n″ = 0.432–0.183) (Table 3) and almost parallel to each other (Fig. 2). The curves were qualitatively similar for the ice cream mixes added with xanthan gum. Although, the magnitude of K′ was lower than that of K″ in the ice cream mix without xanthan gum, an inverse result was the case in the ice cream mixes prepared with 0.4 and 0.8 % xanthan gum; namely, the magnitude of K′ was higher than that of K″ (Table 3). In addition, Fig. 2 shows that xanthan gum addition increased both G′ and G″ values, implying that gum addition contributed to the viscoelastic properties of the ice cream mixes, but their elastic properties rather than viscous properties. Accordingly, the magnitudes of G′ (K′ = 24.73–127.8) were always greater than that of G″ (K″ = 11.72–31.03) without exhibiting no cross-point of G′ and G″ along the whole frequency range studied (Fig. 2). It is clear from these results that the ice cream mixes were more predominantly elastic than viscous when added with xanthan gum. Wildmoser et al. (2004) indicated the similar results whereby the magnitude of G′ was observed to be greater than that of G″ without exhibiting no cross-point of G′ and G″ within a temperature range between −20 and 10 °C. They explained the rheological behavior of the ice-cream mixes by classifying this range into three temperature zones; the zone I (−20 to −10 °C), zone II (−10 to 0 °C), and zone III (0 to 10 °C). In the zone III, G′ and G″ values were observed to have a lower plateau level because all ice was melted in this temperature range, therefore leading only disperse air and fat phase to have an effect on the rheological characteristics.
Total resistance to flow of a material considered to be a viscous liquid refers to complex viscosity (η*) which is measured in Pa s (Dimitreli and Thomareis 2008). Figure 2 shows that the ice cream mixes added with xanthan gum displayed increased complex viscosity values depending on increase in the gum concentration and the decreased values over the entire frequency range (due to higher resistance), compared with those without xanthan gum (lower resistance).
Effect of increase in the gum concentration on the viscoelastic properties of the ice cream mixes is shown in Fig. 3a. The addition of xanthan gum to the ice cream mix resulted in an increase in G′, G″, and η* values, but the most pronounced effect was observed in the complex modulus (G*) values, the total resistance to deformation of a material considered to be elastic solid. Although these results can be expected to some extent, comparing these results with those in the literature is difficult because there have been very limited investigations studying the effect of xanthan gum addition on the viscoelastic properties of complex systems like formulated foods.
Another popular material function for the description of viscoelastic behavior is the loss tangent (G″/G′), which indicates whether elastic or viscous properties of the material. In the case of the ice cream mixes, it means that the larger the tan δ value, the more easily the ice cream mix flows. The loss tangent values showed an elastic behavior (tan δ < 1 or G′ > G″) for ice cream mixes over the increased frequency due to the existence of a phase lag between the input sinusoidal signal and the response one (Muñoz et al. 2007). Figure 3b indicates that the tan δ values decreased as the xanthan gum concentration increased. Decreasing tan δ values was the evidence of elastic characteristic of the ice cream mixes, implying that the elastic, in the other words, solid-like character increased as the gum concentration increased.
Applicability of Cox–Merz Rule
When frequency is equal to shear rate, dynamic shear viscosity is nearly equal to the steady shear viscosity (Cox and Merz 1958); in this case, the empirical Cox–Merz rule can be applied to predict dynamic oscillatory shear data from steady shear data and vice-versa by using steady shear data and the calculated shift factors (Rao and Cooley 1992). Accordingly, the Cox–Merz rule has been tested for many polymers, solution, and complex food systems (Da Silva and Rao 1992; Tiziani and Vodovotz 2005; Yasar et al. 2009; Yilmaz et al. 2010).
Figure 4 shows the applicability of the Cox–Merz rule (Eq. 11). The apparent viscosity, η and complex viscosity η* of the ice cream samples were plotted against shear rate, \( \mathop{\gamma }\limits^{ \cdot } \)and angular frequency (ω), respectively. As can be seen, the ice cream mixes did not obey Cox–Merz rule although there was a trend of the apparent viscosity, η to become parallel with the complex viscosity η* values. It was observed that the magnitudes of η* were higher than those of η, indicating that the Cox–Merz rule was not applicable to the ice cream mixes and, as mentioned before, they exhibited strong-gel properties although it was reported that departure from Cox–Merz superposition of η*(ω) versus η(\( \mathop{\gamma }\limits^{ \cdot } \)) is a characteristic feature of weak gel networks (Ndjouenkeu et al. 1995). It was also reported that biopolymer dispersions including either hyperentanglements or aggregates (Da Silva and Rao 1992) and complex food systems (Bistany and Kokini 1983) do not obey this rule.
In the ice cream mix samples without xanthan gum, there was a considerable deviation between the ln η* versus ln ω and ln η versus ln \( \mathop{\gamma }\limits^{ \cdot } \) data on the ice cream mix samples. However, the two lines appear to be more parallel to each other in the ice cream mixes added with different concentrations of xanthan gum. It can be concluded that the departures of ice cream mixes from the Cox–Merz rule was concentration-independent, but the deviation between steady and dynamic viscosity of the ice cream mixes decreased as the xanthan gum concentration increased.
It was difficult to directly compare our results with those in the literature. However, similar food environments like some food emulsions may be found suitable in this respect. For example, Manoi and Rizvi (2009) reported that cold gel-like emulsions produced from texturized whey protein concentrate obeyed only the modified version of Cox–Merz rule. It was also reported that O/W emulsions stabilized with egg yolk did not exhibit superposition of apparent viscosity and complex viscosity because they were weak gels with viscoelastic characteristic (Santipanichwong and Suphantharika 2009).
In structured polymer systems when aggregation takes place among polymer chains, the departures from the Cox–Merz rule have been reported to occur (Lapasin and Pricl 1999), which was attributed to decay of the structure resulted from strain deformation applied to the system (Chamberlain and Rao 1999). Applied strain is well known to be low in small amplitude oscillatory shear and sufficient enough in steady shear to break down structured inter- and intra-molecular associations of materials (Ahmed and Ramaswamy 2006; Gunasekaran and Ak 2000).
Creep and Recovery Properties of the Ice Cream Mixes
Creep Properties
Creep data are collected under constant stress (σ) over time (t) and can be defined in terms of a creep compliance (J) function, as represented by Eq. 12 in terms of shear deformation (γ):
Since the Burger model has been applied satisfactorily to emulsion systems, (Das and Ghosh 2006), the four-component Burger model (Burgers 1939) consisted of the association in series of the Maxwell model and the Kelvin–Voigt model (Fig. 5) was used in the creep analysis of the ice cream mixes in this study. The Burger model is one of the most commonly used models because it is relatively simple and acceptable results can be obtained using this model to analyze system deformation (Dolz et al. 2008). The system deformation per unit stress called J (compliance) is given by the following equation (Barnes 2000; Barry 1983; Steffe 1996):
where J(t) is the overall compliance at any time t in the creep phase, G 0 the instantaneous elastic modulus of the Maxwell unit, G 1 the elastic modulus of Kelvin–Voigt representing the contributions of the retarded elastic region to the total compliance, η 0 the dashpot of the Maxwell element representing the residual viscosity, and η 1 the dashpot related with Kelvin–Voigt representing the internal viscosity (Barry 1983). By calculating the values G 0, G 1, η 0, and η 1 (Fig. 5), it is possible to compare the internal structure of different systems. By this way, a mechanical model with behavior in response to deformation can be produced (Dolz et al. 2008).
The creep test results for the values of compliance J = γ/σ as a function of time are shown in Fig. 6 which shows the effect of xanthan gum concentration on the creep behavior of ice cream mixes in a time interval between 0 and 150 s.
Table 4 indicates the values of G 0, G 1, η 0, and η 1 with the standard errors and the determination coefficients. The fitting of J = f(t) in the interval 0 ≤ t ≤ 150 s based on four-component Burger model (Eq. 13) for the ice cream mixes as affected by different concentrations of xanthan gum yielded in the values of R 2 higher than 0.997 in all cases. Accordingly, Sherman (1966) used four-component mechanical model (instantaneous elastic compliance, J 0; a retarded elastic compliance, J 1, comprising an elastic modulus, E 1 and a viscosity, Π1; and a Newtonian compliance, t/ΠN, where t is time and ΠN is the Newtonian viscosity) to describe creep behaviour of melted ice cream. On the other hand, Sherman (1966) also reported that ice cream mix requires six elements since an additional elastic modulus (E 2) and a second viscosity (Π2) are associated with retarded elasticity, which were attributed to globules of 0.5 μ or less, which are separated by only a few Å after flocculation rather than the 50 Å or more separating larger globules. However, in our study, the R 2 values calculated at each xanthan gum concentration were determined to range between 0.997 and 0.999, which revealed that the four-component Burger model used seems to adequately describe the creep behavior of the ice cream mixes added with different concentrations of xanthan gum. This means that six-component mechanical model may not be necessarily needed to describe the creep behavior of the ice cream mix samples. In addition, the reason why the six-component mechanical model was proposed (Sherman 1966) to model creep behavior of the ice cream mixes might have been due to fat coagulation which occurs when ice cream mix is frozen and whipped. It was reported that the ice crystals disappear in order for the coagulation to be able to continue when frozen ice cream is thawed, leaving a melt with a weak structure, which is different from the fat network present in the original mix (Sherman 1966). Whereas in our study, the creep measurements were conducted on the ice cream mixes that were not frozen, which, in fact, implies the consistency between our mechanical modeling simulation and that of Sherman (1966).
Table 4 shows that the G 0, G 1, η 0, and η 1 values of the ice cream mixes dramatically increased with the xanthan gum concentration. This indicated that the deformation was minimum in the ice cream mixes including xanthan gum for the same stress value and the same time, thus suggesting that the gum concentration affected considerably the internal structures of the ice cream mixes. Figure 6 shows the variation of the shear creep and recovery compliance, J(t), with the xanthan gum concentration. As can be seen, the ice cream mixes with gum content exhibited lower J(t) values during creep and recovery, which expressed more elastic behavior, or recovery after the applied deformation or when the stress was removed from the system.
In the literature, very limited studies have appeared to be dealing with the effect of xanthan gum addition on the creep and recovery properties of ice cream. However, these results were comparable with those investigating the creep and recovery properties of some selected gums. In this respect, Dolz et al. (2008) reported that deformation was found to be greatest in locust bean gum (LBG) gel exhibiting the lowest G 0, G 1, and η 1 values. On the other hand, the values of the parameters of the gel including 0.1 % LBG and xanthan gum were reported to be higher than the rest of the gel samples studied, thus defining it to be the gel with the greatest opposition of deformation. To summarize, it can be concluded that the increase in the concentration of xanthan gum generally reinforces the structure, increasing the elastic moduli and viscosity of the elements present in Burger model.
Recovery Properties
Figure 5 also shows the three well differentiated regions in all recovery tests. J SM is the first recovery, practically instantaneous and the segment (AB) corresponds to the spring of the Maxwell element. J KV is the second recovery due to the Kelvin–Voigt element (curve posterior to B). This recovery is slower, has a decreasing exponential type, and tends towards an asymptote for t→∞. The third recovery is the residual deformation, J ∞ due to sliding of the Maxwell dashpot, indicating permanent deformation resulting from the irreversibility of the mentioned slide.
The following equation describes the compliance during the recovery test (Dolz et al. 2008):
where t is the time and B and C are parameters defining the recovery speed of the system. The Eq. 3 complies with certain clearly defined limiting conditions.
Regarding B and C parameters, they can be calculated from the derivative of the Eq. 14 with respect to time d(J(t))/dt, as following:
J SM, the deformation suffered by the Maxwell spring is initial shear compliance, which can be calculated by using the following equation:
where J MAX is the maximum deformation that corresponds to the compliance value for the longest time (t = 150 s) in the creep transient analysis.
Dolz et al. (2006) reported that J SM should be obtained indirectly using the Eq. 17 because the deformation is instantaneous and usually very small. Therefore, for t = 0, determination of J SM experimentally is difficult, on the other hand, the values for the rest of the compliances are more confident when the method used.
Full mechanical characterization of a system can be determined by calculating the contribution of each element in the Burger model with respect to J MAX to which the ice cream mixes are exposed. The following equation can be used to calculate the percentage deformation of each element presents in the Burger model:
where J element is the corresponding compliance parameters, J SM, J KV, or J ∞.
In addition, to calculate the final percentage recovery of the entire system, the following equation has been proposed (Dolz et al. 2008).
As to our recovery results for the ice cream mixes studied, the mixes reached the maximum deformation (J MAX) after 150 s of stress application. After removal of the stress applied at the time σ = 0, the remaining process was to measure the compliance values J = f(t) during the 150 s, which completes the recovery tests for each ice cream mix sample studied. Figure 6 also shows the experimental results for the recovery phase of the ice cream mix samples in a time interval between 150 and 300 s with the changing gum concentration. Deman and Beers (1987) reported that an increase in permanent deformation during recovery indicates the collapsed gel structure and irreversibly broken elastic bonds. Conversely, the ice cream mixes with increasing xanthan gum content had a liability to become firmer and harder, as can be judged by their lower J(t) values (Fig. 6).
Table 5 shows the J ∞, J KV, and B and C parameters as well as their determination coefficients calculated by fitting the J as a function of time based on Eq. 14. As can be seen, the J ∞ values dramatically decreased with xanthan gum concentration, confirming the above results. Regarding the speed of structural recovery that is absolute value of d(J(t))/d(t), speeds of recovery of the ice cream mixes were quite similar. This means that no clear effect of xanthan concentration was observed, as can be seen from the B and C values in the Table 5.
The contribution of the two components, the Maxwell and the global Kelvin–Voigt elements to the total deformation of the ice cream mixes is given in the Table 6. As can be seen, the contribution the Maxwell spring (J SM) to unit total deformation of the ice cream mixes is lower than that of the Kelvin–Voigt element (J KV). However, they were relatively small when compared with that of the Maxwell dashpot (J ∞). The contribution of J ∞ to deformation decreased with xanthan gum concentration, indicating that xanthan gum addition increased the resistance of ice cream mixes to the deformation. It can be concluded that the ice cream mix with 0.4 % xanthan gum was the system with higher percentage contribution of the Maxwell dashpot to the deformation, which was in accordance with the fact that this was the ice cream mix with the more liquid behavior. However, the ice cream mix without xanthan gum was the system with no percentage contribution of the Maxwell dashpot to the deformation, meaning that no recovery could be established for this mix. On the other hand, it should be pointed out here that, in the ice cream mixes, the total contribution of both Maxwell elements (spring J SM plus dashpot J ∞) to unit deformation was in the range of 2- to 4-fold more than that of the Kelvin–Voigt element (Table 6), indicating logically that the contribution of Kelvin–Voigt element to the deformation of the ice cream mix samples was smaller. The final percentage recovery (% R) values of the ice cream mixes are also presented in Table 6. The percentage recovery of the ice cream mixes increased as the xanthan gum concentration increased; however, no recovery was observed in the ice cream mix without xanthan gum, indicating the liquid behavior.
Gel Strength and Relaxation Exponent Parameters
The gel strength (S) and relaxation exponent (n) parameters of the ice cream mixes were calculated using the equation of Winter and Chambon (Nijenhuis 1997).
where S (Pa sn) is the gel strength parameter depending on the cross-linking density and the molecular chain flexibility, and n is related to the molecular structure and connectivity of the incipient gel (Nyström et al. 1996).
G(t), the relaxation function can be obtained from creep test data as the reciprocal of J(t) (Ferry 1980).
where m is the slope of the log–log plot of G(t). The relationship between G(t) and J(t) was confirmed by using the rheometer operating at a constant shear strain, and therefore providing direct G(t) measurements. In this study, it was confirmed that the m values of the ice cream mix without xanthan gum and that with 0.4 % xanthan gum were less than unity. However, it was higher than unity as the xanthan gum concentration increased up to 0.8 % (Table 7).
Table 7 also shows that the obtained gel strength S, which can be considered as a combination of hardness and ductility (Park 2005) and relaxation exponent n values. As can be seen, ice cream mix strength strongly increased with increasing xanthan gum content, suggesting that the gel structure was firmer and more stable depending on the increasing xanthan gum level. The other parameter is the exponent n that gives knowledge for the ice cream mix gel structure. It was reported that the cross-linker is in excess when the n value is lower than 0.5, but in the values higher than 0.5, the opposite is valid (Nijenhuis 1997). In this respect, the ice cream mix samples without xanthan gum exhibited the largest exponent, n, which is an indicator of a less orderly structure and lower cross-linking density of the ice cream mix. On the other hand, xanthan gum addition decreased n values of the ice cream mix samples, indicating the increased orderly structure and cross-linking density of the ice cream mixes.
Conclusions
In this study, ice cream mixes prepared with different concentrations of xanthan gum were studied with respect to steady and dynamic shear properties, indicating that all the ice cream mixes can be characterized as the linear viscoelastic solids exhibiting pseudoplastic flow. Also, the ice cream mix samples were determined to behave as strong gel-like macromolecular dispersions with G′ much greater than G″ within the whole range of frequency applied; however, exhibiting much pronounced independent behavior of both the storage and loss modulus with increasing frequency only in the xanthan gum added mix samples. The study of the establishment of correlations between the values of oscillatory shear parameters and steady shear parameters revealed the fact that the ice cream mixes did not obey the Cox–Merz rule.
Full mechanical characterization of the ice cream mix samples was also established to obtain internal structure of the samples using creep and recovery analysis, together with oscillatory measurements. Burger model was used to simulate the viscoelastic behavior of the ice cream as a function of xanthan gum concentration. Pronounced changes were determined in the mechanical properties of ice cream mixes when they were added with 0.4 and 0.8 % of xanthan gum, revealing that xanthan gum levels could remarkably change the internal structure and deformation properties of the ice cream mixes.
References
Ahmed, J., & Ramaswamy, H. S. (2006). Viscoelastic properties of sweet potato puree infant food. Journal of Food Engineering, 74, 376–382.
Arbuckle, W. S., & Frandsen, J. H. (1966). Ice cream. West Port: Avi Pub. Co.
Barnes, H. A. (2000). A handbook of elementary rheology. Aberystwyth: University of Wales, Institute of Non-Newtonian Fluid Mechanics.
Barry, B. (1983). Rheology of dermatological vehicles. Dermatological Formulations, Percutaneous Absorption (pp. 351–407). New York: Marcel Dekker.
Bistany, K., & Kokini, J. (1983). Dynamic viscoelastic properties of foods in texture control. Journal of Rheology, 27, 605.
Bower, C., Gallegos, C., Mackley, M., & Madiedo, J. (1999). The rheological and microstructural characterisation of the non-linear flow behaviour of concentrated oil-in-water emulsions. Rheologica Acta, 38, 145–159.
Burgers, J. (1939). Mechanical considerations-model systems-phenomenological theories of relaxation and of viscosity. First report on viscosity and plasticity. New York: Nordemann.
Caillet, A., Cogné, C., Andrieu, J., Laurent, P., & Rivoire, A. (2003). Characterization of ice cream structure by direct optical microscopy. Influence of freezing parameters. LWT-Food Science and Technology, 36, 743–749.
Chamberlain, E., & Rao, M. (1999). Rheological properties of acid converted waxy maize starches in water and 90 % DMSO/10 % water. Carbohydrate Polymers, 40, 251–260.
Chan, H. W. S. (1984). Biophysical methods in food research (vol. 5). Oxford: Wiley-Blackwell.
Chang, Y., & Hartel, R. (2002). Development of air cells in a batch ice cream freezer. Journal of Food Engineering, 55, 71–78.
Cottrell, J. I. L., Pass, G., & Phillips, G. O. (1980). The effect of stabilisers on the viscosity of an ice cream mix. Journal of the Science of Food and Agriculture, 31, 1066–1070.
Cox, W., & Merz, E. (1958). Correlation of dynamic and steady flow viscosities. Journal of Polymer Science, 28, 619–622.
Da Silva, J. A. L., & Rao, M. (1992). Viscoelastic properties of food hydrocolloid dispersions. In M. A. Rao & J. F. Steffe (Eds.), Viscoelastic properties of foods (pp. 285–315). London: Elsevier.
Das, S., & Ghosh, A. (2006). Study of creep, stress relaxation, and inverse relaxation in mulberry (Bombyx mori) and tasar (Antheraea mylitta) silk. Journal of Applied Polymer Science, 99, 3077–3084.
Deman, J., & Beers, A. (1987). Fat crystal networks: structure and rheological properties. Journal of Texture Studies, 18, 303–318.
Dickinson, E., & Stainsby, G. (1982). Colloids in food. London: Applied Science Publishers.
Dimitreli, G., & Thomareis, A. S. (2008). Effect of chemical composition on the linear viscoelastic properties of spreadable-type processed cheese. Journal of Food Engineering, 84, 368–374.
Dogan, M., & Kayacier, A. (2007). The effect of ageing at a low temperature on the rheological properties of Kahramanmaras-type ice cream mix. International Journal of Food Properties, 10, 19–24.
Dogan, M., Kayacier, A., & Ic, E. (2007). Rheological characteristics of some food hydrocolloids processed with gamma irradiation. Food Hydrocolloids, 21, 392–396.
Dolz, M., Hernández, M., & Delegido, J. (2006). Oscillatory measurements for salad dressings stabilized with modified starch, xanthan gum, and locust bean gum. Journal of Applied Polymer Science, 102, 897–903.
Dolz, M., Hernández, M., & Delegido, J. (2008). Creep and recovery experimental investigation of low oil content food emulsions. Food Hydrocolloids, 22, 421–427.
Ferry, J. D. (1980). Viscoelastic properties of polymers. New York: Wiley.
Franco, J., Raymundo, A., Sousa, I., & Gallegos, C. (1998). Influence of processing variables on the rheological and textural properties of lupin protein-stabilized emulsions. Journal of Agricultural and Food Chemistry, 46, 3109–3115.
Franco, J. M., Berjano, M., & Gallegos, C. (1997). Linear viscoelasticity of salad dressing emulsions. Journal of Agricultural and Food Chemistry, 45, 713–719.
Garcia-Ochoa, F., Santos, V., Casas, J., & Gomez, E. (2000). Xanthan gum: production, recovery, and properties. Biotechnology Advances, 18, 549–579.
Goff, H., & Davidson, V. (1992). Flow characteristics and holding time calculations of ice cream mixes in HTST holding tubes. Journal of Food Protection, 55, 34–37.
Goff, H., & Davidson, V. (1994). Controlling viscosity of ice cream mixes. Modern Dairy, 73, 12–14.
Goff, H. D. (2002). Formation and stabilisation of structure in ice–cream and related products. Current Opinion in Colloid & Interface Science, 7, 432–437.
Gunasekaran, S., & Ak, M. M. (2000). Dynamic oscillatory shear testing of foods—selected applications. Trends in Food Science and Technology, 11, 115–127.
Hernandez, M. J., Tarrega, A., Dolz, M., Costell, E., & Alfaro, M. C. (2004). In A. C. Diogo, N. B. Alvarenga, J. Canada, S. Ferro Palma, J. Dias (Eds.), Progress in rheology of biological and synthetic polymer systems. Instituto Palitecnica de Beja, G.E.R. Sevilla, 349–354.
Hsu, C. H., & Lo, Y. M. (2003). Characterization of xanthan gum biosynthesis in a centrifugal, packed-bed reactor using metabolic flux analysis. Process Biochemistry, 38, 1617–1625.
Ibanoglu, S., & Ibanoglu, E. (1998). Rheological characterization of some traditional Turkish soups. Journal of Food Engineering, 35, 251–256.
Ibarz, A., Vicente, M., & Graell, J. (1987). Rheological behaviour of apple juice and pear juice and their concentrates. Journal of Food Engineering, 6, 257–267.
Innocente, N., Comparin, D., & Corradini, C. (2002). Proteose-peptone whey fraction as emulsifier in ice–cream preparation. International Dairy Journal, 12, 69–74.
Juszczak, L., Witczak, M., Fortuna, T., & Bany, A. (2004). Rheological properties of commercial mustards. Journal of Food Engineering, 63, 209–217.
Kalogiannis, S., Iakovidou, G., Liakopoulou-Kyriakides, M., Kyriakidis, D. A., & Skaracis, G. N. (2003). Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochemistry, 39, 249–256.
Kaya, S., & Tekin, A. R. (2001). The effect of salep content on the rheological characteristics of a typical ice–cream mix. Journal of Food Engineering, 47, 59–62.
Lapasin, R., & Pricl, S. (1999). Rheology of industrial polysaccharides: theory and applications. New York: Aspen Publishers.
Manoi, K., & Rizvi, S. S. H. (2009). Emulsification mechanisms and characterizations of cold, gel-like emulsions produced from texturized whey protein concentrate. Food Hydrocolloids, 23, 1837–1847.
Martinou Voulasiki, I. S., & Zerfiridis, G. K. (1990). Effect of some stabilizers on textural and sensory characteristics of yogurt ice cream from sheep’s milk. Journal of Food Science, 55, 703–707.
Moros, J. E., Franco, J. M., & Gallegos, C. (2002). Rheology of spray dried egg yolk stabilized emulsions. International Journal of Food Science and Technology, 37, 297–307.
Morris, E. R. (1995). Polysaccharide rheology and in-mouth perception. In M. A. Stephen (Ed.), Food polysaccharides and their applications (pp. 515–546). New York: Marcel Dekker.
MstatC (1986). (4.00 ed.). East Lansing, M.I.: Michigan State University.
Muñoz, J., Rincón, F., Carmen Alfaro, M., Zapata, I., de la Fuente, J., Beltrán, O., & León de Pinto, G. (2007). Rheological properties and surface tension of Acacia tortuosa gum exudate aqueous dispersions. Carbohydrate Polymers, 70, 198–205.
Ndjouenkeu, R., Akingbala, J., Richardson, R., & Morris, E. (1995). Weak gel properties of khan flour from Belschmiedia sp.—a traditional food thickener from tropical West Africa. Food Hydrocolloids, 9, 165–172.
Nijenhuis, K. (1997). Thermoreversible networks. Berlin: Springer.
Nyström, B., Kjøniksen, A. L., & Lindman, B. (1996). Effects of temperature, surfactant, and salt on the rheological behavior in semidilute aqueous systems of a nonionic cellulose ether. Langmuir, 12, 3233–3240.
Pal, R. (1995). Oscillatory, creep and steady-state flow behaviour of xanthan-thickened oil-in-water emulsions. American Institute Chemical Engineers, 41, 783–795.
Park, J. W. (2005). Surimi and surimi seafood (2nd ed.). Boca Raton: CRC Press.
Rao, M., Cooley, H., & Vitali, A. (1984). Flow properties of concentrated juices at low temperatures. Food Technology, 38, 113–119.
Rao, M., & Cooley, H. (1992). Rheological behavior of tomato pastes in steady and dynamic shear. Journal of Texture Studies, 23, 415–425.
Rao, M., & Tattiyakul, J. (1999). Granule size and rheological behavior of heated tapioca starch dispersions. Carbohydrate Polymers, 38, 123–132.
Rha, C. (1975). Theory, determination and control of physical properties of food materials. Dordrecht: D. Reidel Pub. Co.
Ross-Murphy, S. (1984). Rheological methods. Biophysical methods in food research (pp. 138–199). London: Blackwell.
Santipanichwong, R., & Suphantharika, M. (2009). Influence of different [beta]-glucans on the physical and rheological properties of egg yolk stabilized oil-in-water emulsions. Food Hydrocolloids, 23, 1279–1287.
Saricoban, C., Yilmaz, M. T., Karakaya, M., & Tiske, S. S. (2010). The effect of different levels of sunflower head pith addition on the properties of model system emulsions prepared from fresh and frozen beef. Meat Science, 84, 186–195.
Sariçoban, C., Özalp, B., Yilmaz, M., Özen, G., Karakaya, M., & Akbulut, M. (2008). Characteristics of meat emulsion systems as influenced by different levels of lemon albedo. Meat Science, 80, 599–606.
Sharoba, A., Senge, B., El-Mansy, H., Bahlol, H. E., & Blochwitz, R. (2005). Chemical, sensory and rheological properties of some commercial German and Egyptian tomato ketchups. European Food Research and Technology, 220, 142–151.
Sherman, P. (1966). The texture of ice cream. 3: rheological properties of mix and melted ice cream. Journal of Food Science, 31, 707–716.
Silva, M. F., Fornari, R. C. G., Mazutti, M. A., de Oliveira, D., Padilha, F. F., Cichoski, A. J., Di Cansian, R. L., Luccio, M., & Treichel, H. (2009). Production and characterization of xantham gum by Xanthomonas campestris using cheese whey as sole carbon source. Journal of Food Engineering, 90, 119–123.
Soukoulis, C., Chandrinos, I., & Tzia, C. (2008). Study of the functionality of selected hydrocolloids and their blends with k-carrageenan on storage quality of vanilla ice cream. LWT- Food Science and Technology, 41, 1816–1827.
Stanley, D., Goff, H., & Smith, A. (1996). Texture–structure relationships in foamed dairy emulsions. Food Research International, 29, 1–13.
Steffe, J. F. (1996). Rheological methods in food process engineering. East Lansing: Freeman Press.
Sworn, G. (2000). Xanthan gum. In G. O. Phillips & P. A. Williams (Eds.), Handbook of hydrocolloids. New York: CRC Press. chap. 6.
Tiziani, S., & Vodovotz, Y. (2005). Rheological effects of soy protein addition to tomato juice. Food Hydrocolloids, 19, 45–52.
Wildmoser, H., Scheiwiller, J., & Windhab, E. J. (2004). Impact of disperse microstructure on rheology and quality aspects of ice cream. LWT-Food Science and Technology, 37, 881–891.
Yapar, A., Atay, S., Kayacier, A., & Yetim, H. (2006). Effects of different levels of salt and phosphate on some emulsion attributes of the common carp (Cyprinus carpio L., 1758). Food Hydrocolloids, 20, 825–830.
Yasar, K., Kahyaoglu, T., & Sahan, N. (2009). Dynamic rheological characterization of salep glucomannan/galactomannan-based milk beverages. Food Hydrocolloids, 23, 1305–1311.
Yilmaz, M. T., Karaman, S., Cankurt, H., Kayacier, A., & Sagdic, O. (2010). Steady and dynamic oscillatory shear rheological properties of ketchup-processed cheese mixtures: effect of temperature and concentration. Journal of Food Engineering, 103, 197–210.
Yoo, B., & Rao, M. (1996). Creep and dynamic rheological behavior of tomato concentrates: effect of concentration and finisher screen size. Journal of Texture Studies, 27, 451–459.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dogan, M., Kayacier, A., Toker, Ö.S. et al. Steady, Dynamic, Creep, and Recovery Analysis of Ice Cream Mixes Added with Different Concentrations of Xanthan Gum. Food Bioprocess Technol 6, 1420–1433 (2013). https://doi.org/10.1007/s11947-012-0872-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-0872-z