Abstract
The effects of gelatinization and gel storage conditions on the formation of canna resistant starch (RS) were investigated. Starch slurries (10%, dwb) were autoclaved at 121 °C for 30, 60, and 120 min. The gels obtained were subsequently stored at different temperatures (4 °C, 30 °C, and 100 °C) and times (0, 1, 3, 5, and 7 days). Analyses of the RS content in gelatinized starch samples in comparison with that in granular starch showed that the RS fraction in granular starch was very high (97.3% w/w); however, nearly all of the RS was thermally unstable, as indicated by a great reduction in RS content (to 1.9% w/w) after cooking at 100 °C for 20 min. The RS contents in gelatinized starch samples were 12.0–15.9% w/w, which were reduced to 7.9–10.8% w/w after cooking. Storage of gels resulted in a significant increase in the amount of the thermally stable RS fraction, e.g., a thermally stable RS content of 16.8% w/w was found in the gel sample gelatinized for 120 min and stored at 4 °C for 3 days. This indicated that the ordered structures of the RS portion were tightened under the storage conditions. The gelatinization temperature of canna starch was 72.2 °C, whereas the RS products exhibited two melting temperature ranges, 51.1–76.3 °C and 163.1–165.1 °C, indicating that the newly formed crystals were very strong.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary starch has different physiological effects in humans depending on its rate and extent of digestion. Starch has been classified into rapidly digestible (RDS), slowly digestible (SDS), and resistant starch (RS) according to the rate of glucose release and its absorption in the gastrointestinal tract (Englyst et al. 1992). RDS leads to a rapid increase of blood glucose and insulin levels, whereas SDS has moderate glycemic and insulinemic responses. RS reduces starch availability for digestion and produces short-chain fatty acids in the large bowel through fermentation; this is beneficial for colon health and may protect against colorectal cancer (Sajilata et al. 2006). Consequently, starch ingredients with high levels of SDS and RS can improve the nutritional function of foods (Gelencsér et al. 2008, 2010).
RS has been categorized into four types. Among these, RS type 3 (retrograded starch) seems to be of particular interest because of its physical and nutritional functionality and thermal stability. RS type 3 is basically produced by disruption of the starch granules by gelatinization followed by reassociation of the starch molecules dispersed (especially the amyloses), which is usually referred to as retrogradation, into the more ordered structure (crystalline form). Extent and quality of RS formed depend on several factors including intrinsic properties of the starch (amylose/amylopectin ratio, molecular size of amylose, other minor components, molecular structure of amylopectin), gelatinization conditions (starch concentration, gelatinization temperature, and time), and storage conditions (temperature and time).
So far, research strategies to enhance RS type 3 formation have involved: using a high-amylose starch as a starting material, complete dispersion of starch granules, increasing starch concentration, reducing the molecular size and branch chain of starch molecules, and optimizing the storage conditions. To achieve complete dispersion of starch granules, extending gelatinization time, increasing gelatinization temperature with applied pressure, and repeating boiling and cooling cycles have been investigated (Sajilata et al. 2006). Studies have also been conducted on partial hydrolysis of gelatinized starch to promote RS formation—by weak acids such as citric acid or lactic acid (Onyango et al. 2006; Wang et al. 2007) or by enzymes such as α-amylase or pullulanase (Lehmann et al. 2002). Time and temperature of gel storage as well as repeated heating/cooling cycles have been shown to affect RS type 3 formation. Cold storage seems to promote an increase in RS content. In commercial practice, RS type 3 products are manufactured from high amylose (about 70%) maize starch by repeated cycles of retrogradation (Haralampu 2000).
Canna starch, a kind of starch extracted from rhizomes of the edible canna plant (Canna edulis Ker.), is mostly used for preparing transparent starch noodles, a traditional food of Southeast Asia. Canna starch is characterized by very large granules, high amylose content, clear paste, high viscosity, low breakdown, high retrogradation, and high resistance to hydrolysis by α-amylase (Puncha-arnon et al. 2007; Thitipraphunkul et al. 2003a). High retrogradation of canna starch is supposed to be due to a combined effect of the following factors: considerably high amylose content (about 30%; Puncha-arnon et al. 2007); small size of amylose molecules (≈1,600 dp), with low value of average number of branch chains; and high value of average chain length of amylopectin (Thitipraphunkul et al. 2003b). Therefore, canna starch should be a good candidate for use as a starting material for RS production. This study aimed to investigate the effects of gelatinization and gel storage temperature and time on the formation of RS in canna starch. The RDS, SDS, and RS in cooked and uncooked native granular starch and gelatinized starch powders were analyzed, and the impact of the formation conditions on each starch fraction was discussed in relation to the properties of canna starch.
Materials and Methods
Materials
Eight-month-old rhizomes of edible canna plant were obtained from the Rayong Field Crops Research Center, Rayong, Thailand, and the starch was isolated according to a procedure described by Puncha-arnon et al. (2007). Alpha-amylase (type VI-B from porcine pancreas, A-3173, 26 U/mg), amyloglucosidase (EC 3.2.1.3, from Aspergillus niger, 300 U/ml) and a glucose oxidase–peroxidase assay kit (cat. no. GAGO-20) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Novelose 260 (RS type 2) and Novelose 330 (RS type 3) were provided by National Starch and Chemical Co. (Bridgewater Township, NJ, USA).
Preparation of Powdered Gelatinized Starches and Powdered Gels After Storage
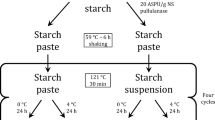
Two sets of canna starch slurries (10% w/v; 50 g of starch in 450 ml of distilled water) were pre-gelatinized at 70 °C for 15 min with moderate stirring. The pre-gelatinized starches were then gelatinized by autoclaving at 121 °C for 30, 60, or 120 min. The first set of gelatinized starch samples was directly oven dried at 50 °C for 20 h without storage. In the following sections, the gelatinized starch powders obtained are denoted as G30min, G60min, and G120min for samples autoclaved for 30, 60, and 120 min, respectively. The second set of gelatinized samples was stored at 4 °C, 30 °C, and 100 °C for 1, 3, 5, and 7 days and then dried at 50 °C for 20 h. All dried samples were ground in a mortar and pestle and passed through a 140-mesh sieve. The products obtained were named as GtimeStemperature/time. For example, G30minS4°/1day meant a powder prepared by autoclaving for 30 min and storing at 4 °C for 1 day.
Determination of RDS, SDS, and RS
The RDS, SDS, and RS were determined according to the method of Englyst et al. (1992) with modifications. Porcine pancreatic α-amylase (5 g) was dispersed in 50 ml of sodium acetate buffer (0.1 M, 4 mM CaCl2, pH 5.2), and centrifuged at 5,000 rpm for 10 min. The supernatant (4.5 ml) was transferred to a beaker, and 0.5 ml of amyloglucosidase (140 U/ml) was added to the solution. This enzyme solution was freshly prepared for each digestion. An uncooked starch sample (100 mg, db) and 5 ml of the enzyme solution were added to an Erlenmeyer flask, mixed for 1 min, and then incubated in a shaking water bath (37 °C, 130 strokes/minute). After 20 and 120 min of incubation, 0.2 ml of aliquot was added to 4 ml of absolute ethanol, mixed well, and centrifuged at 5,000 rpm for 10 min. The liberated glucose in the supernatant was determined using a GAGO-20 glucose oxidase/peroxidase assay kit. Starch fractions which were digested within 20 min and between 20 and 120 min were classified as RDS and SDS, respectively; those which were undigested within 120 min were classified as RS.
For the determination of RS in cooked samples, sample powders (100 mg, db) and water (2 ml) were added to an Erlenmeyer flask and mixed for 1 min. The sample was heated in a boiling water bath for 20 min and then placed into a water bath at 37 °C to equilibrate for 10 min. The enzyme solution was then added, and the procedure continued according to the steps mentioned above.
Differential Scanning Colorimetry
Thermal properties of starches were determined by a differential scanning calorimeter (DSC; Pyris 1, Perkin Elmer, Norwalk, CT, USA). Starch (10 mg) was weighed in a DSC pan, and water (20 mg) was added. The pan was sealed and allowed to stand for 24 h at 4 °C. The scanning temperature range and the heating rate were 30–160 °C and 5 °C/min, respectively. An empty pan was used as a reference. The transition temperatures reported are the onset temperature (T o), peak temperature (T p), and conclusion temperature (T c). The enthalpy of gelatinization (∆H) was estimated by integrating the area between the thermogram and a baseline under the peak, and was expressed in terms of joules per gram dry starch.
Statistical Analysis
For the effect of gelatinization time, one-way analysis of variance (ANOVA) was performed using Duncan’s multiple-range test to compare treatment means at P < 0.05. The effects of storage temperature and time on the levels of SDS and RS of G30min and G120min were evaluated using two-way ANOVA (three temperatures × four times). If there were any interactions between the two factors, the simple main effect was then analyzed. Post hoc analysis was carried out using Duncan’s test (P < 0.05). The software used for all analyses was SPSS (version 16.0). If not specified, all tests were carried out with two replications.
Results and Discussion
Effect of Gelatinization (Autoclaving) Time on RDS, SDS, and RS Contents
In order to assess the thermal stability of the products obtained from different treatments, all samples including the native starch were cooked at 100 °C for 20 min, and the cooked samples were analyzed in parallel with the uncooked counterparts. The amounts of RDS, SDS, and RS in native canna starch and gelatinized starches prepared by autoclaving for 30, 60, and 120 min are presented in Fig. 1.
RDS, SDS, and RS Contents in Uncooked Samples
RS contents of Novelose 260 and Novelose 330 were 46.7% and 34.8%, respectively (Fig. 1a), whereas that of raw canna starch was very high at 97.3%. The digestion of raw starch granules is a complex process which involves the accessibility (diffusion and adsorption) and hydrolytic activity of the enzyme toward the substrate. The accessibility of α-amylase is determined principally by the physical appearance (i.e., smoothness of granule surface, peripheral pores and channels inside the granule, and specific surface area) of starch granules, whereas the hydrolytic activity depends mainly on the supramolecular arrangement of starch molecules in granules (Watcharatewinkul et al. 2010). The high resistance of canna starch granules to enzyme hydrolysis has been reported by Hung and Morita (2005), Srichuwong et al. (2005), and Puncha-arnon et al. (2007), and was suggested to be attributed to granular features and structure—including large granular size (10–100 μm), quite smooth surface, and B-type crystalline pattern—as well as to the mode of degradation, which was identified as “exocorrosion” mode (outwardly hydrolyzed) (Puncha-arnon et al. 2007).
When canna starch was subjected to heat gelatinization for 30, 60, and 120 min, most of the RS was converted to SDS and RDS. The RS remaining in the gelatinized products (in a dried powder form) was only 12.0–15.9%, whereas the SDS and RDS increased from 2.0% to 4.0–7.1% and from 0.7% to 80.0–82.6%, respectively. The destruction of granular starch into granule fragments and the dispersal of starch molecules by autoclaving facilitated the accessibility of amylase to the substrate, thereby substantially reducing the RS content. Extending the autoclaving time from 30 to 60 min resulted in a decrease in RS content, from ≅16% to ≅12%. However, the contents in 60-min and 120-min autoclaved samples were comparable (12–13%). The RS fraction in G30min and G120min might be the starch fragments that remained after autoclaving, and/or be a newly formed crystal from reassociation of amylose into a tightly packed structure, stabilized by hydrogen bonding during drying at 50 °C for 20 h.
RDS, SDS, and RS Contents in Cooked Samples
Most starch in food products has usually undergone some kind of cooking such as boiling, steaming, extrusion, etc., which involves heating in the presence of moisture. Therefore, the data of RDS, SDS, and RS contents in cooked samples might be more useful in practice than those in raw samples. As a consequence, this study also reported the analyses of RDS, SDS, and RS contents in starch samples after cooking at 100 °C for 20 min.
As shown in Fig. 1b, the amounts of RS in Novelose 260 and Novelose 330 were not significantly reduced by cooking (from 46.7% and 34.8% to 35.0% and 29.5%, respectively). On the other hand, when raw canna starch, which contained a very high RS content (97.3%), was cooked, its RS was greatly reduced to 1.9%, whereas RS contents in the G30min, G60min, and G120min were reduced to some extent (from 15.9%, 12.0%, and 13.4% to 10.8%, 7.9%, and 10.8%, respectively). The results obtained suggested that nearly all of the RS in raw canna starch was thermally unstable, whereas RS in the gelatinized starches as well as in the commercial products (Novelose 260 and Novelose 330) was comparatively stable when undergoing the cooking process. As reported by Sajilata et al. (2006), the melting temperatures of Novelose 260 and Novelose 330 were 114.4 °C and 121.5 °C, respectively. This result was in accord with the starch paste appearance (figure not shown). After cooking at 100 °C for 20 min, the Novelose 260 in a water sample was still nonviscous and displayed a turbid white color. Their granules precipitated when the flask was left for a while. On the other hand, raw canna starch when gelatinized resulted in a clearer and more homogeneous solution compared to the G30min, G60min, and G120min. Some flake-like pieces which were found in the starch gel solutions of the G30min, G60min, and G120min might be remaining starch fragments and/or retrograded starch formed during drying the gelatinized starches at 50 °C for 20 h. These fractions were responsible for the higher resistance of the G30min, G60min, and G120min to enzyme hydrolysis.
Effect of Gel Storage Conditions on RS and SDS Formation
The starches gelatinized for 30 and 120 min were stored at 4 °C, 30 °C, and 100 °C for 1, 3, 5, and 7 days. After drying at 50 °C for 20 h, they were ground to obtain samples in a powder form. The RS and SDS in each dried gel sample before and after cooking were then analyzed; the data of mean and standard deviation values of the starch fractions are summarized in Table 1.
SDS and RS Formation in G30min
A two-way ANOVA was conducted to examine the effects of storage temperature and storage time as well as the interaction between the effects of storage temperature and storage time on RS and SDS formation. The P values from the F test as shown in Table 2 revealed that there were significant interactions between the effect of storage temperature and storage time on SDS and RS in both cooked and uncooked samples. The results of significant interactions were followed up by running tests for simple main effects of storage time—that is, the mean differences in RS (or SDS) of gels stored for 1, 3, 5, and 7 days at each storage temperature. Different letters in the same column of Table 1 were used to indicate significant differences (p ≤ 0.05) of RS (or SDS) at the same storage temperature.
Without Cooking
The initial content of RS in uncooked G30min was 15.9%, whereas the RS contents in stored gels varied from 13.1% to 18.3%. Storage time had no significant effect on RS formation when gels were stored at 4 °C and 30 °C, but at 100 °C, a significant effect was found. At 100 °C, the RS content in gels stored for 1 and 3 days was highest (18.3%), but it tended to decrease when the gels were stored longer and became 13.1% after storage for 7 days. For SDS, the initial content in uncooked G30min was 4.1% and changed to 2.5–11.7% after storage. Statistical analysis revealed that storage time affected SDS formation at all storage temperatures, i.e., longer storage tended to result in higher SDS (at 4 °C, gels stored for 3, 5, and 7 days had higher SDS contents than those stored for 1 day; at 30 °C, gels stored for 5 and 7 days had higher SDS contents than those stored for 3 days; and at 100 °C, gels stored for 7 days had higher SDS contents than those stored for 1, 3, and 5 days). It was noticed that a few cases which displayed an insignificant change in SDS (G30minS100°/1day and G30minS100°/3days) concurrently displayed a high content of RS. This indicated the potential of transformation between the SDS and RS.
After Cooking
The RS contents in all stored G30min gels decreased drastically when cooked. Among the cooked samples, only a few cases of stored gels (G30minS4°/7days, G30minS100°/3days, and G30minS100°/5days) displayed an increase in RS content (12.8–14.0%) compared to that of unstored samples (10.8%). Storage time had a significant effect on RS formation at all temperatures (P ≤ 0.05). At 4 °C, the RS content in gel stored for 7 days (14.0%) was higher than those in gels stored for 1, 3, and 5 days (10.0–10.4%). Conversely, the RS content in gel stored for 7 days was lower than those in gels stored for 1, 3, and 5 days when gels were stored at 30 °C and 100 °C. For SDS, the contents in gels stored at all conditions also decreased significantly when cooked. The initial content in cooked G30min was 4.8% and changed to 0.8–5.8% after storage. All stored gels had a comparable or a lesser amount of SDS than the G30minS0day. Statistical analysis revealed the effect of storage time on SDS formation at all storage temperatures; however, tendencies on the effect were inconclusive.
SDS and RS Formation in G120min
Without Cooking
Storage of G120min under various conditions resulted in a clear increase in both RS and SDS compared to G30min (Table 1). Except for G120minS30°/1day, G120minS30°/3days, G120minS100°/5days and G120minS100°/7days, the RS in G120min increased from an initial content of 13.4% to 15.8–19.5%. Similarly, except for G120minS4°/1day, G120minS30°/3days, and G120minS100°/1day, the SDS increased from 4.0% to 6.1–11.0%. This finding indicated that the gel characteristics (degree of granule dispersion) before storage had a great effect on inducing the formation of starch crystals; that is, the starch molecules in well-dispersed granules (G120min) can reassociate better than those in the less-dispersed sample (G30min).
According to the P values in Table 2, there were interactions between the effect of storage temperature and storage time on both SDS and RS formations in uncooked samples. Therefore, the simple main effect of storage time at each storage temperature was further analyzed. At storage temperature of 4 °C, gel stored for 5 days contained lower amount of RS than gels stored for 3 and 7 days. At 30 °C, gels stored for 5 and 7 days had higher RS than gels stored for 1 and 3 days; whereas at 100 °C, the RS content decreased as the storage time increased, and gel stored for 5 days contained the lowest amount of RS. Storage time affected SDS formation at all storage temperatures. Longer storage tended to result in higher SDS when gels were stored at 4 °C (gels stored for 3, 5, and 7 days had higher SDS contents than that for 1 day) and at 100 °C (7 days > 3 and 5 days > 1 day); whereas at 30 °C, storage time had less influence on SDS formation. The results obtained indicated a complicated interaction between the effects of storage temperature and storage time on RS and SDS formation.
After Cooking
The effect of gel storage was much more clearly observed in G120min. Although the cooked samples had a lower amount of RS than the uncooked ones, the differences between them were not as high as those of G30min. In addition, the SDS contents of cooked stored G120min gels in some cases were higher than that of the uncooked samples. These results indicated that the RS and SDS in stored G120min had higher thermal stability than those in the stored G30min. Heat stability of RS and SDS in stored gel depended on storage conditions. Unstable RS can be converted to SDS, and also unstable SDS can be converted to RDS, by heat processing.
The P values, as shown in Table 2, indicated that there was significant interaction between the effect of storage temperature and storage time on RS formation. Although there were some fluctuations in RS contents in cooked samples of G120min, there was a recognizable trend of the RS change with time at each storage temperature. At a storage temperature of 4 °C, the RS content of G120min stored for 3 days (16.8%) was significantly higher than that stored for 1 day (12.7%). An increase in storage time from 3 days to 7 days had no significant effect on the RS content. When the gel was stored at 30 °C, the RS content consistently increased with storage time and reached a maximum of 16.6% after 7 days of storage; whereas at 100 °C, the RS showed little change, fluctuating around 14–15%. Unlike the uncooked samples, there was no interaction between the effect of storage temperature and storage time on SDS formation (P = 0.069). Both storage temperature (P = 0.041) and storage time (P = 0.000) had significant effects on SDS contents. Storage of G120min at 30 °C resulted in the highest SDS. Gels stored for 1 and 5 days contained higher amounts of SDS compared to those stored for 3 and 7 days.
When SDS was taken into account, the sum of RS and SDS at 4 °C was highest after 5 days of storage (Fig. 2). Except for the first day, the sum of RS and SDS increased with an increase in storage time at 100 °C. Onyango et al. (2006) investigated the effects of incubation temperature (−20 °C to 100 °C) and time (6–48 h) on RS formation in autoclaved (1 h at 121 °C) cassava starch in water, and reported that at 100 °C, the RS increased significantly (to about 9–10%) at the later period of storage, whereas at 4 °C, the RS was considerably high after 6 h but remained constant after that (about 5–6%). Eerlingen et al. (1993) showed that the RS of gelatinized wheat starch stored at 100 °C quickly increased to a maximum (about 10%) after an induction time of about 150 min, which was higher than the maximums obtained at storage temperatures of 0 °C and 68 °C (about 4–6%). The effect of the relationship between incubation temperature and time on RS was interpreted in terms of recrystallization in an amorphous matrix (starch gel is a partially crystalline polymer system). Polymer recrystallization is a three-stage process that involves nucleation (formation of critical nuclei), propagation (crystal growth from the nuclei formed), and maturation (continued crystal growth and perfection). For a partially crystalline system, crystallization can occur only at a temperature between T g and T m, i.e., in a rubbery state (Levine and Slade 1988; Marsh and Blanshard 1988). Starch gel containing more than 27% water has a T g of about −5 °C (Slade 1984), and the T m of the amylose crystals is about 150 °C (Ring et al. 1987); therefore, crystallization of amylose can occur only between these temperature limits. Eerlingen et al. (1993) found that upon incubation of wheat starch gel at 0 °C, the nucleation rate was high, and the propagation rate was low; the opposite was the case upon incubation at 100 °C. However, that trend could not be clearly observed in our study. The deviation from theoretical recrystallization of canna starch gel might be due to its very high viscosity which is a typical characteristic of canna starch.
Thermal Properties
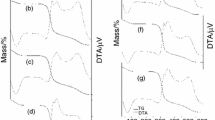
DSC thermograms of native canna starch as well as G120min stored at different conditions are shown in Fig. 3. Native canna starch exhibited an obvious sharp peak around a temperature range of 70–76 °C (T o = 69.7 °C, T p = 72.2 °C, T c = 75.6 °C) with ΔH of 17.0 J/g, and a very tiny peak at 158 °C. After gelatinization for 120 min (G120min), the sharp peak had disappeared, and a broad peak around 50–78 °C was found instead. Two other tiny peaks could be observed at 158 °C and 178 °C as well. The broad peak at low temperature with ΔH of 4.0–7.2 J/g was still observed in gels stored at all conditions. However, the endotherms at high temperature were varied depending on the storage condition. In some storage conditions, very small peaks in the region of 100–130 °C were found. The most obvious effect of storage was reflected by the endotherms found in a temperature range of 150–180 °C. The endotherms displayed a bit more visiblity when the gel was stored at 100 °C, compared to that at 4 °C.
The broad endothermic peak around 50–78 °C has been ascribed to retrogradation of the amylopectin component (Silverio et al. 2000; Fredriksson et al. 1998; Yuan et al. 1993; Xie et al. 2004). Amylopectin molecules associate by crystallization of the outer short branched chain (DP 14–20) to form a network (Ring et al. 1987). Due to the limited dimensions of the chains, the melting temperature of retrograded amylopectin is rather low. As a consequence, the stability of these crystallites is lower than that of those formed from amylose (Eerlingen and Delcour 1995), and there is less impact on RS and SDS in most heat-processed food products. Very small peaks around 100–130 °C may correspond to the transition of the amylose–lipid complex, as reported by Eliasson (1994) and Zaidul et al. (2008). The more prominent endothermic peaks that indicate RS crystal formation are the peaks around 150–180 °C. Similar melt transitions have been reported for RS type 3 from other starchy materials (Sievert and Wursch 1993; Shamai et al. 2003; Onyango et al. 2006) and have been ascribed to the dissociation of recrystallized amylose. The enthalpies associated with endotherms for heating enzyme-resistant retrograded starches in water represent a quantitative measure of ordered structure loss. It has been suggested that melting of double helices, and not disruption of crystalline packing, is the primary determinant of endotherm enthalpy (Cooke and Gidley 1992; Gidley et al. 1995). As seen in Fig. 3, the endothermic peaks and the baseline of peaks around 150–180 °C were not clear, so exact calculation of transition enthalpies is quite difficult. However, the transition enthalpies of these RS fractions were estimated to be 0.5–3.7 J/g. These were in a similar range (0.2–1.6 J/g) as that reported for RS type 3 from cassava starch (Onyango et al. 2006).
Conclusion
Canna starch in the granular form contained a very high amount of RS (97.3%) due to its lower susceptibility to enzyme hydrolysis. However, the RS content was reduced greatly (to 1.9%) when cooked, indicating that nearly all of the RS in raw canna starch was thermally unstable. Gelatinization time (autoclaving for 30, 60, and 120 min) did not have much effect on the thermally stable RS in canna starch (8–10%). However, storage of gels with a higher degree of disintegration (autoclaving for 120 min) resulted in a clear increase in both RS and SDS compared to those with a lesser degree of disintegration (autoclaving for 30 min). This finding indicated that the gel characteristics before storage had a significant effect on inducing the formation of the starch crystals. In this study, no recognizable trend in the effects of storage time and temperature on SDS and RS formation was observed. In most cases, there were significant interactions between the effects of storage temperature and storage time on RS and SDS formation. The amount of RS in cooked granular starch could be increased from 1.9% to 16.8% by simple gelatinization and storing. This is evidence that canna starch has high potential for use as a starting material for RS production. Using the enzymes (such as amylase and pullulanase) to hydrolyze starch before storage might enhance RS formation.
References
Cooke, D., & Gidley, M. J. (1992). Loss of crystalline and molecular order during starch gelatinisation: origin of the enthalpic transition. Carbohydrate Research, 227, 103–112.
Eerlingen, R. C., & Delcour, J. A. (1995). Formation, analysis, structure and properties of type III enzyme resistant starch. Journal of Cereal Science, 22, 129–138.
Eerlingen, R. C., Crombez, M., & Delcour, J. A. (1993). Enzyme-resistant starch: I. Quantitative and qualitative influence of incubation time and temperature of autoclaved starch on resistant starch formation. Cereal Chemistry, 70, 339–344.
Eliasson, A.-C. (1994). Interactions between starch and lipids studied by DSC. Thermochimica Acta, 246, 343–356.
Englyst, H. N., Kingman, S. M., & Cummings, J. H. (1992). Classification and measurement of nutritionally important starch fractions. European Journal of Clinical Nutrition, 46, S33–S50.
Fredriksson, H., Silverio, J., Andersson, R., Eliasson, A.-C., & Aman, P. (1998). The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydrate Polymers, 35, 119–134.
Gelencsér, T., Gál, V., Hódsági, M., & Salgó, A. (2008). Evaluation of quality and digestibility characteristics of resistant starch-enriched pasta. Food and Bioprocess Technology, 1, 171–179.
Gelencsér, T., Gál, V., & Salgó, A. (2010). Effects of applied process on the in vitro digestibility and resistant starch content of pasta products. Food and Bioprocess Technology, 3, 491–497.
Gidley, M. J., Cooke, D., Darke, A. H., Hoffmann, R. A., Russell, A. L., & Greenwell, P. (1995). Molecular order and structure in enzyme-resistant retrograded starch. Carbohydrate Polymers, 28, 23–31.
Haralampu, S. G. (2000). Resistant starch—a review of the physical properties and biological impact of RS3. Carbohydrate Polymers, 41, 285–292.
Hung, P., & Morita, N. (2005). Physicochemical properties and enzymatic digestibility of starch from edible canna (Canna edulis) grown in Vietnam. Carbohydrate Polymers, 61, 313–321.
Lehmann, U., Jacobasch, G., & Schmiedl, D. (2002). Characterization of resistant starch type III from banana (Musa acuminata). Journal of Agricultural and Food Chemistry, 50, 5236–5240.
Levine, H., & Slade, L. (1988). Water as a plasticizer: physico-chemical aspects of low-moisture polymeric systems. In F. Franks (Ed.), Water science reviews (Vol. 3, pp. 79–185). Cambridge: Cambridge University Press.
Marsh, R. D. L., & Blanshard, J. M. V. (1988). The application of polymer crystal growth theory to the kinetics of formation of the β-amylose polymorph in a 50% wheat-starch gel. Carbohydrate Polymers, 9, 301–317.
Onyango, C., Bley, T., Jacob, A., Henle, T., & Rohm, H. (2006). Influence of incubation temperature and time on resistant starch type III formation from autoclaved and acid-hydrolysed cassava starch. Carbohydrate Polymers, 66, 494–499.
Puncha-arnon, S., Puttanlek, C., Rungsardthong, V., Pathipanawat, W., & Uttapap, D. (2007). Changes in physicochemical properties and morphology of canna starches during rhizomal development. Carbohydrate Polymers, 70, 206–217.
Ring, S. G., Colonna, P., l’Anson, K. J., Kalichevsky, M. T., Miles, M. J., Morris, V. J., et al. (1987). The gelation and crystallization of amylopectin. Carbohydrate Research, 162, 277–293.
Sajilata, G., Singhal, R. S., & Kulkarni, P. R. (2006). Resistant starch—a review. Comprehensive Reviews in Food Science and Food Safety, 5, 1–17.
Shamai, K., Bianco-Peled, H., & Shimoni, E. (2003). Polymorphism of resistant starch type III. Carbohydrate Polymers, 54(3), 363–369.
Sievert, D., & Wursch, P. (1993). Thermal behavior of potato amylose and enzyme-resistant starch from maize. Cereal Chemistry, 70(3), 333–338.
Silverio, J., Fredriksson, H., Andersson, R., Eliasson, A.-C., & Aman, P. (2000). The effect of temperature cycling on the amylopectin retrogradation of starches with different amylopectin unit-chain length distribution. Carbohydrate Polymers, 42, 175–184.
Slade, L. (1984). Staling of starch-based products. Cereal Foods World, 29, 508.
Srichuwong, S., Sunarti, T. C., Mishima, T., Isono, N., & Hisamatsu, M. (2005). Starches from different botanical sources—I: contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohydrate Polymers, 60(4), 529–538.
Thitipraphunkul, K., Uttapap, D., Piyachomkwan, K. A., & Takeda, Y. (2003a). A comparative study of edible canna (Canna edulis) starch from different cultivars—part I: chemical composition and physicochemical properties. Carbohydrate Polymers, 53, 317–324.
Thitipraphunkul, K., Uttapap, D., Piyachomkwan, K. A., & Takeda, Y. (2003b). A comparative study of edible canna (Canna edulis) starch from different cultivars—part II: molecular structure of amylose and amylopectin. Carbohydrate Polymers, 54, 489–498.
Wang, J., Jin, Z., & Yuan, X. (2007). Preparation of resistant starch from starch–guar gum extrudates and their properties. Food Chemistry, 101, 20–25.
Watcharatewinkul, Y., Uttapap, D., Puttanlek, C., & Rungsardthong, V. (2010). Enzyme digestibility and acid/shear stability of heat-moisture treated canna starch. Starch/Starke, 62, 205–216.
Xie, F., Dowell, F. E., & Sun, X. S. (2004). Using visible and near-infrared reflectance spectroscopy and differential scanning calorimetry to study starch, protein, and temperature effects on bread staling. Cereal Chemistry, 81(2), 249–254.
Yuan, R. C., Thompson, D. B., & Boyer, C. D. (1993). Fine structure of amylopectin in relation to gelatinization and retrogradation behavior of maize starches from three wx-containing genotypes in two inbred lines. Cereal Chemistry, 70(1), 81–89.
Zaidul, I. S. M., Absar, N., Kim, S.-J., Suzuki, T., Karim, A. A., Yamauchi, H., et al. (2008). DSC study of mixtures of wheat flour and potato, sweet potato, cassava, and yam starches. Journal of Food Engineering, 86, 68–73.
Acknowledgments
We gratefully acknowledge grants from the Thailand Research Fund under TRF-MAG Project MRG-WII515S078 and the Commission on Higher Education, Thailand under KMUTT National University Research Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wandee, Y., Puttanlek, C., Rungsardthong, V. et al. Effects of Gelatinization and Gel Storage Conditions on the Formation of Canna Resistant Starch. Food Bioprocess Technol 5, 2665–2673 (2012). https://doi.org/10.1007/s11947-011-0629-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0629-0