Abstract
The methanolic extract of Cassia obtusifolia L. (Sicklepod) seed, an underutilized food legume from India, was analyzed for antioxidant and health relevant functionality. The total free phenolic content of the raw seeds was 13.33 ± 1.73 g catechin equivalent/100 g extract. The extract exhibited 1,292 mmol Fe[II] per milligram extract of ferric reducing/antioxidant power, 49.92% inhibition of ß-carotene degradation, 65.79% of scavenging activity against DPPH, and 50.78% of superoxide radicals. The in vitro starch digestion bioassay of the extract showed 79.80% of α-amylase and 81.04% of α-glucosidase enzyme inhibition characteristics. Sprouting + oil frying caused an apparent increase on the total free phenolic content with significant improvement on the antioxidant and free radical scavenging capacity of C. obtusifolia seeds, while soaking + cooking as well as open-pan roasting treatments show diminishing effects. Inhibition of α-amylase and α-glucosidase enzyme activity was 23.81% and 42.36%, respectively, following sprouting + oil-frying treatment. These enzyme inhibition values were similar to that of synthetic antidiabetic agent acarbose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cassia obtusifolia L. (Sicklepod) is an economically and medically important weed which belongs to Leguminosae family and Ceasalpinioideae subfamily. It is an annual herb widely distributed in the wastelands of India and West tropical regions. The sicklepod is a branched, annual or perennial herb, or undershrub which grows to a height of 2 m. It is extensively cultivated in Korea and China, and commonly consumed as roasted tea. In Bombay, Assam, and Goa regions of India, the seeds are used as a substitute for coffee. The tribal people living in the hilly region of Pune district, Maharashtra, India consume the roasted seeds and young pods as vegetables (Vadivel and Janardhanan 2002; Janardhanan et al. 2003).
The sicklepod seed has been reported to contain 18.5% to 22.9% crude protein, 5.3% to 7.4% crude lipid, 6.8% to 9.4% crude fiber, 5.1% to 5.8% ash, and 57% to 60% carbohydrate (Vadivel and Janardhanan 2002). The seed proteins exhibited relatively high levels of nonessential and essential amino acids, with the exception of threonine. The in vitro protein digestibility of this wild legume ranged from 74.66% to 81.44%. Favorable agronomic traits such as plant height, number of flowers per cluster, number of pods per cluster, pod length, seeds per pod, seed weight per pod, and seed recovery percentage are also reported (Vadivel and Janardhanan 2002).
The leaves are sauteed in castor oil and applied to ulcers (Vadivel and Janardhanan 2002). The sicklepod seeds are traditionally used in Korea, Japan, and China to treat eye inflammation, photophobia, and lacrimation (Zhu 1998). Leaves and pods are widely used as purgatives and laxatives. The sicklepod seed, called Juemingzi in Chinese, is widely used for the treatments of headache and dizziness, red and tearing eyes, and also as a cathartic and diuretic agent in traditional Chinese medicine (Guo et al. 1998; Lai et al. 2010). The seeds known as Ketsumeishi in Japan are used as a laxative, tonic, and diuretic (Kitanaka and Takido 1981).
Seeds of sicklepod have showed neuroprotective effects in Parkinson’s disease models (Ju et al. 2010). Such neuroprotective effects of the ethanolic extract of this seed are attributed to its anti-inflammatory effect (Kim et al. 2009a). It is also used as an antiseptic, diuretic, antidiarrhoic, antioxidant, and antimutagen (Sung et al. 2004). In India, the leaves of this species are used to treat tuberculosis and ringworms. The roots are also usually crushed, mixed with lime juice, and applied to ringworms. Ground seeds are mixed with sour buttermilk and used to ease the irritation of itchy eruptions (Vadivel and Janardhanan 2002).
In addition, it is reported that the sicklepod and its ingredients have estrogenic activities and inhibit histamine release from mast cells (Kitanaka et al. 1998; Wang et al. 2005). Sicklepod has also been shown to contain antimicrobial components (Kitanaka and Takido 1986). 1,2-Dihydroxyanthraquinone, isolated from the sicklepod seeds, inhibits the growth of Clostridium perfringens and Escherichia coli, while 1,2-, 1,4-, and 1,8-dihydroxyanthraquinones exhibited strong growth-promoting activity on Bifidobacterium bifidum (Sung et al. 2004). Kim et al. (2009b) assessed the positive effects of gluco-obtusifolin and its aglycone (obtusifolin), isolated from the sicklepod seeds, on learning and memory impairments. A HPLC fingerprint for the identification of sicklepod has been developed by Li et al. (2009) due to its immense medicinal use in the pharmaceutical industries. The seed extract is reported to exhibit neuroprotection in mouse hippocampal cultures (Drever et al. 2008). Emodin isolated from sicklepod seed showed larvicidal activity against three mosquito species (Yang et al. 2003).
Information regarding the health-relevant functionality of phenolic extract from sicklepod seed is scarce. Hence, the present study was designed to evaluate the total free phenolic content, antioxidant potential, and type II diabetes-related enzyme inhibition properties of methanolic extract of raw and traditionally processed sicklepod seeds.
Materials and Methods
Chemicals
(+)-Catechin hydrate, polyvinyl polypyrrolidone, butylated hydroxytoluene, 2,4,6-tris-(2-pyridyl)-s-triazine, 2,2-diphenyl-1-picryl-hydrazyl (DPPH), β-carotene, linoleic acid, Tween-40, riboflavin, methionine, nitro-blue tetrazolium, starch, acarbose, α-amylase, α-glucosidase, and p-nitrophenyl-α-d-glucopyranoside were procured from Sigma-Aldrich Chemicals, USA, and all other chemicals were received from Merck, Darmstadt, Germany.
Seed Samples
The seed material of C. obtusifolia was collected from different locations of Tamil Nadu, India (Pariyur, Bhavanisagar, Keriparai, Kalakkadu, and Pollachi) in April 2009. Seed samples from each location (about 500 g) were obtained from 8–15 plants belonging to the same species and mixed together to obtain a representative sample. After acquisition, the seed samples were frozen immediately and stored at −80 °C until further analysis. Each seed sample was then randomly divided into four batches with five replicates (each consisting of 25 g seeds) for experimentation using different processing methods. The first batch was stored without any treatment and considered as raw seeds, and the remaining three batches were processed as described below.
Processing Methods
The whole seeds of the second batch (25 g in each replicate) were soaked in distilled water in the ratio of 1:10 (w/v) for 8 h at 25 °C and then cooked with fresh distilled water (1:5 w/v ratio) at 85–90 °C for 30 min. The third batch of samples was added to the red soil suspension (1:5, w/v) and kept for 2 days in the dark under moist cloth. The sprouts were separated, washed with distilled water, and then fried in sunflower oil at 185–190 °C for 10 min. The fourth batch was roasted in an iron pot for 30 min at 120–130 °C. The seeds were then separated using a sieve and allowed to cool to room temperature.
Preparation of Methanolic Extract
All the raw and processed samples excluding the roasted seeds were frozen at −80 °C and freeze-dried in a lyophilizer (Virtis Freeze mobile 25 EL, New York) for 10 h. The samples were then ground into 1 mm particle size powder for further use. One gram of each sample was treated with petroleum ether (1:10 w/v) overnight on a magnetic stirrer and centrifuged at 3,000 rpm for 10 min. The supernatant was discarded and the defatted residue air-dried. The samples were sequentially extracted with 10 ml of 100%, 80%, 70%, and 50% methanol acidified with 1% conc. HCl in an ultrasonic bath for 10 min followed by extraction in a magnetic stirrer for 30 min. The contents were then centrifuged at 5,000 rpm for 10 min, and all the supernatants were pooled and made up to a known volume. The extract was treated with 5 g of polyvinyl polypyrrolidone at 0 °C for 30 min, and the supernatant was purified using a Solid Phase Catridge (Strata-x-33 um polymeric sorbent, L100-1105, 200 mg/6 ml sample, 8B-S100-FCH-S from Phenomenex, USA). Then, the phenolics were eluted with 10 ml of 50% and 100% methanol, and the solvent was evaporated using a rotary vacuum evaporator (Büchi Rotavapor–R, CH-9230, Switzerland) at 40 °C and then dried in a lyophilizer for 1 h. Finally, the residue was weighed, and total dry yield of the extract was calculated. The extract was then redissolved in water/methanol/formic acid (47.5%:47.5%:5%, v/v/v) solution in the ratio of 1 mg/ml and used for further analysis.
Analytical Methods
The total free phenolic content of methanolic extract of all the raw and processed samples in five replicates was estimated according to the method of Singleton et al. (1999). The ferric reducing/antioxidant power (FRAP) (Pulido et al. 2000), inhibition of β-carotene degradation (Miller 1971), radical scavenging activity against DPPH (Sanchez-Moreno et al. 1998), and superoxide (Zhishen et al. 1999) as well as α-amylase and α-glucosidase inhibition activities (Worthington 1993) of the methanolic extract were analyzed.
Statistical Analysis
All the data were analyzed and expressed as means ± standard deviation of five separate determinations (n = 5). One-way ANOVA with Dunnett’s posttest to determine the significant differences between the experimental batches and the correlation analysis were performed using GraphPad PRISM® version 5.00 for Windows, San Diego, CA, USA.
Results and Discussion
Total Free Phenolics
The total free phenolic content of methanolic extract from defatted raw sicklepod seed was 13.33 g catechin equivalent/100 g extract (Table 1). Such high yield of total free phenolics might be due to the repeated extraction of phenolic compounds using different concentrations of acidified methanol as a solvent. The seed coat color of sicklepod sample was brown. The relationship between seed coat color and the level of phenolics is still controversial. While Barampama and Simard (1993) found a positive correlation between the seed coat color and phenolic content, Guzman-Maldonado et al. (1996) did not find any link between the two. However, some reports are available showing a high correlation between cultivar lines and phenolic content (De Mejia et al. 2003). In addition to seed coat color, the quantity of phenolic compounds in a seed sample is influenced by soil, environmental conditions, genotype (cultivar/variety), agronomic practices (irrigation, fertilization, and pest management), maturity level at harvest, and post-harvest storage. For instance, low temperature during the onset and duration of seed fill was shown to increase the isoflavone content in soybean by several folds (Kim et al. 2006). Since sicklepod grows wildly in adverse environmental conditions such as drought and poor soil, a high phenolic content in the seed material might contribute to strong resistance to these conditions.
Presently, there is an increasing interest on the health benefits of phenolics, namely their antioxidant or free radical scavenging, antimicrobial, antimutagenic, therapeutic, and pharmaceutical properties. The seed coat of legume grains is reported to contain numerous types of phenolics, which are suggested to play an important protective role against oxidative damage in a consumer’s body (Troszynska et al. 2002). Hence, research efforts are under way to incorporate the wild-type legume grains like sicklepod in the formulation of supplementary therapeutic foods for the dietary management of various chronic diseases, including diabetes, obesity, and cardiovascular diseases.
Antioxidant Activity
The FRAP assay measures the antioxidant effect of a substance in terms of its reducing ability. FRAP reflects the total antioxidant power involving single-electron transfer reaction. The antioxidant potential of methanolic extract of sicklepod seed was estimated based on its ability to reduce TPTZ-Fe(III) complex to TPTZ-Fe(II) complex. The reducing power of methanolic extract from raw seed material of sicklepod was found to be 1,292 mmol Fe [II]/milligram extract dry matter (DM; Table 2). Inhibition of ß-carotene degradation (49.92%) demonstrated by the raw seed extract is similar to the positive control, BHT (Table 3). The results indicated that the presence of phenolic compounds in methanolic extract of sicklepod seed could moderately prevent the degradation of ß-carotene caused by radical reactions under in vitro conditions.
The DPPH radical scavenging activity of raw sicklepod seed extract was found to be 65.79% (Table 4). The interaction of phenolic compounds with DPPH varies with their structural conformation. Certain compounds can react very rapidly with DPPH radical and reduce a large number of DPPH molecules corresponding to the number of available hydroxyl groups. The free radical scavenging activity of the phenolic compound depends on different structural features such as O–H-bound dissociation energy, resonance delocalization of the antioxidant, and steric hindrance derived from bulky groups substituting hydrogen in the antioxidant compound. In this study, raw seed extract exhibited 50.78% of superoxide radical scavenging activity (Table 5).
The methanolic extract of raw sicklepod seed revealed a remarkable antioxidant property in terms of reducing power, inhibition of ß-carotene bleaching, DPPH and superoxide radical scavenging activities. These antioxidant characteristics confirmed the health-protective ability of sicklepod seed against various oxidative stress-related diseases including aging, cancer, atherosclerosis, Alzheimer disease, and diabetes. Hence, the incorporation of such nontraditional legume grains with appreciable amounts of phenolics in the regular diets of human beings could improve their antioxidant status with potential health benefits.
α-Amylase Inhibition Activity
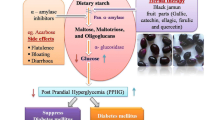
The consumption of foods rich in α-amylase and α-glucosidase inhibitors, the so-called hypoglycemic foods, has received a lot of attention and is being investigated extensively due to their diet-linked benefits in the management of type II diabetes. One way of controlling type II diabetes is to decrease the postprandial hyperglycemia which can be achieved by the inhibition of starch digestive enzymes. α-Amylase and α-glucosidase are two such enzymes whose inhibition could delay or prolong the overall carbohydrate digestion time causing a reduction in the rate of glucose absorption and consequently blunt the postprandial plasma glucose rise.
Acarbose, miglitol, and metformin are among the enzyme inhibitors that are commercially available for the clinical treatment of type II diabetes. However, these drugs are reported to cause various side effects such as abdominal distention, flatulence, and possibly diarrhea. Hence, at present, there is an increasing interest among the food scientists to find out an alternative natural source of α-amylase inhibitor with potential antioxidant activity without any side effects for the dietary management of type II diabetic patients.
In this connection, the methanolic extract from raw sicklepod seed showed 79.80% of α-amylase inhibition (Table 6). Nonetheless, low levels of α-amylase inhibition from natural fruits, vegetables, and legume grains have been reported to offer a good strategy in the control of postprandial hyperglycemia (McDougall et al. 2005; Kwon et al. 2006) since excessive inhibition of pancreatic α-amylase results in the abnormal bacterial fermentation of undigested carbohydrates in the colon. Thus, the α-amylase inhibition activity observed in raw sicklepod seed would not be suitable for use in the dietary management of type II diabetes.
α-Glucosidase Inhibition Activity
A high level of α-glucosidase inhibition (81.04%) was observed in the methanolic extract of sicklepod grain, which is similar to that of acarbose (Table 7). However, it should be noted that these results are based on in vitro biochemical tests and are indicative of antiglycemic effects in the prevention and management of type II diabetes and have limited implications on what happens under in vivo conditions.
Effect of Soaking + Cooking
A low reduction percentage in the total free phenolics was noticed during soaking + cooking treatment (13%; Table 1). Such nonsignificant loss during this treatment was attributed to the limited leaching out of total phenolics due to the presence of the hard seed coat. In contrast, 60% and 28% reduction in phenolics were observed during soaking + autoclaving in light brown- and dark brown-colored seeds of cowpea, respectively (Siddhuraju and Becker 2007); 82% loss in Vigna vexillata (Sowndhararajan et al. 2011) and 48% in Bauhinia vahlii seeds (Sowndhararajan et al. 2010).
Soaking + cooking significantly affected the antioxidant activity in terms of inhibition of ß-carotene degradation and DPPH radical scavenging activity as well as α-amylase and α-glucosidase inhibition properties of sicklepod seed (Tables 2, 3, 4, 5, 6, and 7). Similarly, Xu and Chang (2008) reported the loss of antioxidant activity during soaking (6–34%) as well as cooking (33–82%) of food legumes such as green pea, yellow pea, chickpea, and lentil. Additionally, cooking of legumes without prior soaking was reported to reduce the antioxidant activity of Lathyrus sativus (Starzynska-Janiszewska et al. 2008), Phaseolus vulgaris (Granito et al. 2008), and Chenopodium quinoa (Dini et al. 2010). Such decreased antioxidant and enzyme inhibition properties of sicklepod grain observed in the present study could be due to the degradation of phenolic compounds upon cooking at elevated temperature. Hence, soaking + cooking treatment would not be recommended for use in the preparation of C. obtusifolia seeds for consumption as a natural dietary source of antioxidants and type II diabetes-related enzyme inhibitors.
Effect of Sprouting + Oil Frying
An appreciable level of increase in the total free phenolics (28%) was observed during sprouting + oil frying of sicklepod grains (Table 1). Similarly, sprouting for 2 days followed by autoclaving was reported to increase the total free phenolics by 9%, 20%, 27%, and 50% in wheat, buckwheat, corn, and oats, respectively (Randhir et al. 2008). A substantial increase in the total free phenolics was also noticed in Vigna radiata (217%) after 7 days of germination (Fernandez-Orozco et al. 2008). Further, Zielinski (2003) reported that germination of Glycine max caused an increase in total free phenolics from 2.6 to 3.1 mg/g extract DM. A major portion of phenolic compounds is usually stored in seeds as soluble conjugates or insoluble forms. Hence, the significant level of increase exhibited by the sicklepod seed under sprouting + oil-frying treatment could be due to mobilization of stored phenolics as a result of the activation of enzymes like polyphenol oxidase during sprouting. The increase could also be due to the release of free phenolics from bound form through the breakdown of cellular constituents and cell walls during subsequent thermal process (oil frying).
Sprouting + oil frying was found to increase the antioxidant activity at significant level (Tables 2, 3, 4, and 5). The germination process has also been reported to increase the antioxidant activity of Mucuna pruriens seeds, lupin seeds, mung bean seeds, faba bean, peas, and common beans (Shetty et al. 2002; Randhir et al. 2004; 2009; Lopez-Amoros et al. 2006; Duenas et al. 2009). Similarly, sprouting + autoclaving was reported to significantly increase the antioxidant activity in wheat, buckwheat, corn, and oats (Randhir et al. 2008). Such significant increase of antioxidant properties of sicklepod seed was attributed to the elevation of phenolic levels during sprouting + oil-frying treatment. A significant level of positive correlation was noticed between the phenolic content and antioxidant properties of sicklepod seed (Table 8). Similarly, Karacabey et al. (2009) also noted a strong correlation between phenolic content and antioxidant activity of grape canes. The results of the present study emphasize that the chemical and functional properties of phenolic compounds are not affected by such treatment. Further, the Maillard reaction products that are formed during oil frying might also be responsible for such a rise in the antioxidant characteristics of sicklepod grains.
Nevertheless, significant losses of α-amylase and α-glucosidase inhibition activities were observed in the sicklepod grain after sprouting + oil-frying treatment (Tables 6 and 7). This is in agreement with earlier observation on M. pruriens seeds during germination (Randhir et al. 2009). However, the α-amylase and α-glucosidase inhibition levels recorded in sprouted + oil-fried seeds seem to be more desirable for the dietary management of type II diabetes without any adverse side effect. Such low level of α-amylase and moderate inhibition of α-glucosidase can regulate the blood sugar level of diabetic patients without any serious side effect. Thus, sprouting + oil frying could be recommended as a mild and favorable processing method in the exploitation of the sicklepod seed as a therapeutic food source.
Effect of Open-Pan Roasting
Open-pan roasting caused drastic losses of total free phenolics (33%) in the presently analyzed legume sample (Table 1). Similarly, mild reduction of the phenolics was recorded in moth bean (11%) (Siddhuraju 2006), dark brown seed-coated cowpea (16%) (Siddhuraju and Becker 2007), and B. vahlii (20%) (Sowndhararajan et al. 2010), while extreme losses of 48% and 64% were reported in light brown-colored cowpea and V. vexillata seeds, respectively (Siddhuraju and Becker 2007; Sowndhararajan et al. 2011). Degradation of phenolic compounds as a result of direct heat exposure could be a reason for such a strong reduction observed in sicklepod seed under open-pan roasting. This observation is in agreement with that of an earlier report on significant loss of polyphenolic compounds in apple pomace at high drying temperatures (50–80 °C) (Heras-Ramirez et al. 2011).
Severe losses of antioxidant activity and enzyme inhibition properties were caused by open-pan roasting (Tables 2, 3, 4, 5, 6, and 7). Similar reductions of antioxidant activity during roasting have been reported in black-eyed peas, kidney beans, and pinto beans (Boateng et al. 2008); amaranth, quinoa, wheat, and buckwheat (Alvarez-Jubete et al. 2010) and, almond nut (Bolling et al. 2010). As a result of the significant adverse effects of the open-pan roasting method on the total free phenolic content, diminishing effects were observed on functional properties of sicklepod seed. This could be due to the disintegration of phenolic compounds at the high temperature of roasting. There were no significant correlations between the enzyme inhibition properties and phenolic content even though a positive correlation was established between phenolic content and antioxidant properties of sicklepod seed (Table 8). Therefore, open-pan roasting would not be a suitable method to preserve the phenolic compounds and their antioxidant and health-relevant functionalities in sicklepod seed.
Conclusion
Methanolic extract of sicklepod seed was found to contain appreciable levels of total free phenolics with promising antioxidant potential and type II diabetes-related enzyme inhibition properties. A significant correlation between the phenolic content and antioxidant properties was identified, which was not observed in the case of enzyme inhibition characteristics. In regard to the effects of different indigenous processing methods, soaking + cooking exhibited mild losses in total free phenolics, antioxidant and functional properties. Open-pan roasting showed significant levels of reduction in total free phenolics and thereby drastically affected the antioxidant and starch digestive enzyme inhibition characteristics, and thus, it is considered to be the harsh preparation method. Alternatively, sprouting + oil frying was observed to increase the total free phenolic content as well as antioxidant properties extensively. Such viable processing technique could offer a good strategy to improve the phenolic content in sicklepod seed for enhanced antioxidant activity and functionality towards inhibition of starch digestive enzymes relevant to potential type II diabetes management. Therefore, such suitably processed underutilized legume grain could be envisaged as a dietary ingredient in the formulation of supplementary foods with therapeutic value. Further, identification of phenolic constituents using LC–MS and evaluation of in vivo antioxidant characteristics of methanolic extract from sicklepod seed are in progress.
References
Alvarez-Jubete, L., Wijngaard, H., Arendt, E. K., & Gallagher, E. (2010). Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chemistry, 119, 770–778.
Amarowicz, R., & Raab, B. (1997). Antioxidative activity of leguminous seed extracts evaluated by chemiluminescence methods. Zeitschrift für Naturforschung, 52, 709–712.
Anton, A. A., Ross, K. A., Beta, T., Fulcher, R. G., & Arntfield, S. D. (2008). Effect of pre-dehulling treatments on some nutritional and physical properties of navy and pinto beans (Phaseolus vulgaris L.). LWT Food Science and Technology, 41, 771–778.
Barampama, Z., & Simard, R. E. (1993). Nutrient composition, protein quality and antinutritional factors of some varieties of dry beans (Phaseolus vulgaris L) grown in Burundi. Food Chemistry, 47, 159–167.
Boateng, J., Verghese, M., Walker, L. T., & Ogutu, S. (2008). Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). LWT Food Science Technology, 41, 1541–1547.
Bolling, B. W., Blumberg, J. B., & Chen, C. Y. O. (2010). The influence of roasting, pasteurization, and storage on the polyphenol content and antioxidant capacity of California almond skins. Food Chemistry, 123, 1040–1047.
De Mejia, E. G., Guzman-Maldonado, G. H., Acosta-Gallegos, J. A., Reynoso-Camacho, R., & Ramirez-Rodriguez, A. (2003). Effect of cultivar and growing location on the trypsin inhibitors, tannins, and lectins of common beans (Phaseolus vulgaris L.) grown in the semi-arid high lands of Mexico. Journal of Agricultural and Food Chemistry, 51, 5962–5966.
Dini, I., Tenore, G. C., & Dini, A. (2010). Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT Food Science and Technology, 43, 447–451.
Drever, B. D., Anderson, W. G., Riedel, G., Kim, D. H., Ryu, J. H., Choi, D. Y., et al. (2008). The seed extract of Cassia obtusifolia offers neuroprotection to mouse hippocampal cultures. Journal of Pharmacological Sciences, 107, 380–392.
Duenas, M., Hernandez, T., Estrella, I., & Fernandez, D. (2009). Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chemistry, 117, 599–607.
Fernandez-Orozco, R., Frias, J., Zielinski, H., Piskula, M. K., Kozlowska, H., & Vidal-Valverde, C. (2008). Kinetic study of the antioxidant compounds and antioxidant capacity during germination of Vigna radiata cv. emerald, Glycine max cv. jutro and Glycine max cv. merit. Food Chemistry, 111, 622–630.
Granito, M., Paolini, M., & Perez, S. (2008). Polyphenols and antioxidant capacity of Phaseolus vulgaris stored under extreme conditions and processed. LWT Food Science and Technology, 41, 994–999.
Guo, H., Chang, Z., Yang, R., Guo, D., & Zheng, J. (1998). Anthraquinones from hairy root cultures of Cassia obtusifolia. Phytochemistry, 49, 1623–1625.
Guzman-Maldonado, G. H., Castellanos, J., & De Mejia, E. M. (1996). Relationship between theoretical and experimentally detected tannin content of common bean (Phaseolus vulgaris L.). Food Chemistry, 55, 333–335.
Heras-Ramirez, M.E., Quintero-Ramos, A., Camacho-Davila, A.A., Barnard, J., Talamas-Abbud, R., Torres-Munoz, J.V., et al. (2011). Effect of blanching and drying temperature on polyphenolic compound stability and antioxidant capacity of apple pomace. Food and Bioprocess Technology. doi:10.1007/s11947-011-0583-x.
Janardhanan, K., Vadivel, V., & Pugalenthi, M. (2003). Biodiversity in Indian under-exploited/tribal pulses. In P. K. Jaiwal & R. P. Singh (Eds.), Improvement strategies for leguminosae biotechnology (pp. 353–405). The Netherlands: Kluwer Academic Publishers.
Ju, M. S., Kim, H. G., Choi, J. G., Ryu, J. H., Hur, J., Kim, Y. J., et al. (2010). Cassiae semen, a seed of Cassia obtusifolia, has neuroprotective effects in Parkinson’s disease models. Food and Chemical Toxicology, 48, 2037–2044.
Karacabey, E., Mazza, G., Bayindirli, L., & Artik, N. (2009). Extraction of bioactive compounds from milled grape canes (Vitis vinifera) using a pressurized low-polarity water extractor. Food and Bioprocess Technology. doi:10.1007/s11947-009-0286-8.
Kim, J. A., Jung, W. S., Chun, S. C., Yu, C. Y., Ma, K. H., & Gwag, J. G. (2006). A correlation between the level of phenolic compounds and the antioxidant capacity in cooked-with-rice and vegetable soybean (Glycine max L.) varieties. European Journal of Food Research and Technology, 224, 259–270.
Kim, D. H., Hyun, S. K., Yoon, B. H., Seo, J. H., Lee, K. T., Cheong, J. H., et al. (2009). Gluco-obtusifolin and its aglycon, obtusifolin, attenuate scopolamine-induced memory impairment. Journal of Pharmacological Science, 111, 110–116.
Kim, D. H., Kim, S., Jung, W. Y., Park, S. J., Park, D. H., Kim, J. M., et al. (2009). The neuroprotective effects of the seeds of Cassia obtusifolia on transient cerebral global ischemia in mice. Food and Chemical Toxicology, 47, 1473–1479.
Kim, J. S., Hyun, T. K., & Kim, M. J. (2011). The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities. Food Chemistry, 124, 1647–1651.
Kitanaka, S., & Takido, C. (1981). Studies on the constituents of the seeds of Cassia obtusifolia: the structures of two new lactones, isotoralactone and cassialactone. Phytochemistry, 20, 1951–1953.
Kitanaka, S., & Takido, M. (1986). Studies on the constituents in the roots of Cassia obtusifolia L. and the antimicrobial activities of constituents of the roots and the seeds. Yakugaku Zasshi, 106, 302–306.
Kitanaka, S., Nakayama, T., Shibano, T., Ohkoshi, E., & Takido, M. (1998). Antiallergic agent from natural sources. Structures and inhibitory effect of histamine release of naphthopyrone glycosides from seeds of Cassia obtusifolia L. Chemical and Pharmacological Bulletin, 46, 1650–1652.
Kwon, Y. I., Vattem, D. V., & Shetty, K. (2006). Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pacific Journal of Clinical Nutrition, 15, 107–118.
Lai, Y. H., Ni, Y. N., & Kokot, S. (2010). Authentication of Cassia seeds on the basis of two-wavelength HPLC fingerprinting with the use of chemometrics. Chinese Chemical Letters, 21, 213–216.
Li, G., Xiao, Y., Zhang, C., Li, L., & Pang, Z. (2009). Comparison on the HPLC fingerprint of Cassia obtusifolia between the raw and roasted seeds. Zhongguo Zhong Yao Za Zhi, 34, 694–697.
Lopez-Amoros, M. L., Hernandez, T., & Estrella, I. (2006). Effect of germination on legume phenolic compounds and their antioxidant activity. Journal of Food Composition and Analysis, 19, 277–283.
McDougall, G. J., Shpiro, F., Doboson, P., Smith, P., Black, A., & Stewart, D. (2005). Different polyphenolic compounds of soft fruits inhibit α-amylase and α-glucosidase. Journal of Agricultural and Food Chemistry, 53, 2760–2766.
Miller, H. E. (1971). A simplified method for the evaluation of antioxidants. Journal of American Oil Chemical Society, 48, 91–92.
Nsimba, R. Y., Kikuzaki, H., & Konishi, Y. (2008). Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chemistry, 106, 760–766.
Oh, K. Y., Lee, J. H., Curtis-Long, M. J., Cho, J. K., Kim, J. Y., Lee, W. S., et al. (2010). Glycosidase inhibitory phenolic compounds from the seed of Psoralea corylifolia. Food Chemistry, 121, 940–945.
Pastor-Cavada, E., Juan, R., Pastor, J. E., Alaiz, M., & Vioque, J. (2009). Antioxidant activity of seed polyphenols in fifteen wild Lathyrus species from South Spain. LWT Food Science and Technology, 42, 705–709.
Pulido, R., Bravo, L., & Saura-Calixto, F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal of Agricultural and Food Chemistry, 48, 3396–3402.
Randhir, R., & Shetty, K. (2004). Microwave-induced stimulation of L-Dopa, phenolics and antioxidant activity in fava bean (Vicia faba) for Parkinson’s diet. Process Biochemistry, 39, 1775–1784.
Randhir, R., Lin, Y. T., & Shetty, K. (2004). Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochemistry, 39, 637–646.
Randhir, R., Kwon, Y. I., & Shetty, K. (2008). Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innovative Food Science and Emerging Technologies, 9, 355–364.
Randhir, R., Kwon, Y. I., & Shetty, K. (2009). Improved health-relevant functionality in dark germinated Mucuna pruriens sprouts by elicitation with peptide and phytochemical elicitors. Bioresource Technology, 100, 4507–4514.
Rocha-Guzman, N. E., Gonzalez-Laredo, R. F., Ibarra-Perez, F. J., Nava-Berumen, C. A., & Gallegos-Infante, J. A. (2007). Effect of pressure cooking on the antioxidant activity of extracts from three common bean (Phaseolus vulgaris L.) cultivars. Food Chemistry, 100, 31–35.
Sanchez-Moreno, C., Larrauri, J. A., & Saura-Calixto, F. A. (1998). A procedure to measure the antiradical efficiency of polyphenols. Journal of the Science of Food and Agriculture, 76, 270–276.
Shetty, P., Atallah, M. T., & Shetty, K. (2002). Effects of UV treatment on the proline-linked pentose phosphate pathway for phenolics and L-Dopa synthesis in dark germinated Vicia faba. Process Biochemistry, 37, 1285–1295.
Shobana, S., Sreerama, Y. N., & Malleshi, N. G. (2009). Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chemistry, 115, 1268–1273.
Shon, M. Y., Kim, T. H., & Sung, N. J. (2007). Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chemistry, 82, 593–597.
Shukla, S., Metha, A., John, J., Singh, S., Mehta, P., & Vyas, S. P. (2009). Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food and Chemical Toxicology, 47, 1848–1851.
Siddhuraju, P. (2006). The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chemistry, 99, 149–157.
Siddhuraju, P. (2007). Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated Tamarindus indica seed coat. LWT Food Science and Technology, 40, 982–990.
Siddhuraju, P., & Becker, K. (2007). The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chemistry, 101, 10–19.
Siddhuraju, P., & Manian, S. (2007). The antioxidant activity and free radical-scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds. Food Chemistry, 105, 950–958.
Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Sowndhararajan, K., Siddhuraju, P., & Manian, S. (2010). In vitro evaluation of the antioxidant activities in the differentially processed seeds from underutilized legume, Bauhinia vahlii Wight & Arn. Food Science and Biotechnology, 19, 503–509.
Sowndhararajan, K., Siddhuraju, P., & Manian, S. (2011). Antioxidant and free radical scavenging capacity of the underutilized legume, Vigna vexillata (L.) A. Rich. Journal of Food Composition and Analysis, 24, 160–165.
Starzynska-Janiszewska, A., Stodolak, B., & Jamroz, M. (2008). Antioxidant properties of extracts from fermented and cooked seeds of Polish cultivars of Lathyrus sativus. Food Chemistry, 109, 285–292.
Sung, B. K., Kim, M. K., Lee, W. H., Lee, D. H., & Lee, H. S. (2004). Growth responses of Cassia obtusifolia toward human intestinal bacteria. Fitoterapia, 75, 505–509.
Troszynska, A., Estrella, I., Lopez-Amores, M. L., & Hernandez, T. (2002). Antioxidant activity of pea (Pisum sativum L.) seed coat acetone extract. LWT Food Science and Technology, 35, 158–164.
Vadivel, V., & Janardhanan, K. (2002). Agrobotanical traits and chemical composition of Cassia obtusifolia L.: a lesser-known legume of the Western Ghats region of South India. Plant Foods for Human Nutrition, 57, 151–164.
Wang, C. Z., Wang, S. X., Zhang, Y., Chen, J. P., & Liang, X. M. (2005). In vitro estrogenic activities of Chinese medicinal plants traditionally used for the management of menopausal symptoms. Journal of Ethnopharmacology, 98, 295–300.
Worthington, V. (1993). Worthington enzyme manual (pp. 36–261). Freehold, NJ: Worthington Biochemical Corporation.
Xu, B., & Chang, S. K. C. (2008). Effect of soaking, boiling, and steaming on total phenolic content and antioxidant activities of cool season food legumes. Food Chemistry, 110, 1–13.
Yang, Y. C., Lim, M. Y., & Lee, H. S. (2003). Emodin isolated from Cassia obtusifolia (Leguminosae) seed shows larvicidal activity against three mosquito species. Journal of Agricultural and Food Chemistry, 51, 7629–7631.
Zhishen, J., Mengcheng, T., & Jianming, W. (1999). The determination of flavonoid contents on mulberry and their scavenging effects on superoxide radical. Food Chemistry, 64, 555–559.
Zhu, Y. P. (1998). Chinese materia medica: chemistry, pharmacology and applications (pp. 124–125). The Netherlands: Harwood Academic Publishers.
Zielinski, H. (2003). Contribution of low molecular weight antioxidants to the antioxidant screen of germinated soybean seeds. Plant Foods for Human Nutrition, 58, 1–20.
Acknowledgement
One of the authors (VV) is thankful to Alexander von Humboldt (AvH) Foundation, Bonn, Germany for the award of Post Doctoral Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vadivel, V., Kunyanga, C.N. & Biesalski, H.K. Antioxidant Potential and Type II Diabetes-Related Enzyme Inhibition of Cassia obtusifolia L.: Effect of Indigenous Processing Methods. Food Bioprocess Technol 5, 2687–2696 (2012). https://doi.org/10.1007/s11947-011-0620-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0620-9