Abstract
Radiant energy under vacuum (REV) as a rapid dehydration method was employed to dehydrate Lactobacillus salivarius 417 along with different concentrations of skim milk powder as protective medium. Two optimization methods: response surface methodology and random centroid analysis (RCO) were applied and compared to optimize the dehydration parameters for maximum viability—microwave power, absolute pressure in the drying chamber, and protective agent concentration. The study showed that both methods were suitable for the process optimization. Microwave power, concentration of skim milk powder, and absolute pressure had a significant effect on final viability while the speed of sample rotation in vertical axis had no effect (P < 0.05). The result also indicated that a microwave power of <250 W, 10–15% concentration of skim milk powder, and <1 mmHg absolute pressure was needed to achieve viability of equal or greater than 80% in L. salivarius. The RCO-optimized REV process yielded higher viability than the reference freeze drying method and was completed in less than an hour as compared to 64 h for freeze drying.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freeze drying is the most common method of drying and preserving microorganisms. In spite of being a long process (2–3 days), the low dehydration temperature and low oxidation potential make freeze drying a suitable process for dehydration of a wide range of microorganisms including fungi, yeasts, and bacteria (Abadias et al. 2001; Berny and Hennebert 1991; Morgan et al. 2006; To and Etzel 1997). On the other hand, the need for freezing equipment and frozen storage has increased the cost of the process. There is also a viability loss associated with freezing bacteria cells due to ice crystallization and concentration of solutes, bacterial membrane injury, and protein denaturation (Conrad et al. 2000; Thammavongs et al. 1996; Uzunova-Doneva and Donev 2000; Visick and Clark 1995).
Researchers have studied the effect of various compounds in improving the survival of bacteria during drying processes, among which one can name polysaccharides and disaccharides, proteins, and amino acids (Carvalho et al. 2004; Champagne et al. 1991; Leslie et al. 1995; Linders et al. 1997). Zayed and Roos (2004) reported 22.4% survival in Lactobacillus salivarius freeze-dried with skim milk powder as an excipient. De Giulio and colleagues (2005) reported trehalose as the most effective sugar among tested sugars in the survival of three bacterial strains, Lactobacillus delbrueckii bulgaricus, Lactobacillus acidophilus, and Streptococcus salivarius subsp. thermophilus, with a survival of 95%, 92%, and 87%, respectively.
Radiant energy under vacuum (REV), a form of vacuum microwave dehydration, is an efficient and rapid drying method which results in products with unique characteristics while retaining biological functions (Durance et al. 2007; Scaman and Durance 2005). By directing microwave energy in a generally unidirectional manner, in so-called traveling wave applicators, REV allows close regulation of sample temperature during dehydration. The retention of biological activity is enhanced by the low drying temperature and short drying time as compared to other drying processes. In other words, the drying process can occur with minimal damage to the final product compared to other conventional drying methods. Kim and colleagues (1997) studied the effect of temperature and water activity on the D values (decimal reduction time, the time required at a specified temperature to kill 90% of bacterial population) of bacterial cultures in vacuum microwave dried yogurt. They found that the survival of the cultures was much higher for vacuum microwave dried yogurt as compared to spray-dried or even freeze-dried samples. Our preliminary studies showed that viability of REV-dehydrated microorganisms depends on the type and concentration of protective agents, time and temperature of drying process, as well as final moisture content of dry material (Ahmad et al. 2007). This suggests that the REV process can be optimized to improve the drying condition to achieve a higher survival of dried microorganisms.
The aim of the present study was to optimize drying conditions for Lactobacillus salivarius 417 using REV dehydration with inclusion of skim milk powder as an excipient. In addition, we compared two statistical approaches for optimization of the process: response surface methodology (Koh et al. 2010; Ming et al. 2009; Montgomery 1991; Neter et al. 1996) and random centroid optimization (Nakai 1981, 1990; Neter et al. 1996).
Materials and Methods
Preparation of Bacterial Cultures
Pure culture of probiotic bacteria L. salivarius 417 (isolated from healthy chicken intestine, identified using API50CHL strip) was kindly provided by Neovatech, Abbotsford, BC, Canada. The bacterium was cultured at 37 °C for 24 h under anaerobic conditions in De Man Rogosa and Sharpe (MRS) broth (Fisher Scientific USA). Anaerobic conditions were achieved using an anaerobic jar containing BD BBL™ Gas Pak™ Plus (N2 + H2; Becton Dickinson and Company, USA). Stock culture was made by mixing the bacteria which was cultured overnight in glycerol and immediately freezing it at −80 °C. For subsequent experiments, bacteria were grown from these stock cultures under the same conditions as described above (Yaghmaee and Durance 2005). Experiments were conducted to monitor the growth stage (Fig. 1) of L. salivarius 417. Cells from stationary phase were used for subsequent drying experiments.
Sample Preparation Before Drying
All samples were sub-cultured three times from the frozen state before drying. Cells were subsequently harvested by centrifugation at 4,500×g for 10 min at 4 °C, then washed by re-suspension in sterile peptone water (0.1% (w/v); Bacto™ Peptone, Becton Dickinson and Company, Sparks, MD, USA) and centrifuged again under the same conditions (Yaghmaee and Durance 2005). The harvested pellet was mixed directly either with skim milk powder (on wet weight basis, Fluka Analytical, Sigma–Aldrich Chemie GmbH, Riedstr. Steinheim, Switzerland) or skim milk powder solution in sterilized distilled water (w/v).
Enumeration Before Drying
A representative sample was taken before drying and diluted serially in 0.1% peptone water solution and appropriate dilutions were plated in duplicate on 3M petrifilms (3M Petrifilms™, 3M Microbiology Products, St Paul, MN, USA) and incubated anaerobically at 37 °C for 48 h. Colony-forming units were counted after 48 h. Plates with 25–250 colonies were considered for calculation.
Enumeration After Drying
Samples were weighed after drying and rehydrated with MRS Broth to the original weight and serially diluted in 0.1% peptone water solution and appropriate dilutions were plated in duplicate on 3M petrifilms. Plates were incubated anaerobically at 37 °C for 48 h before enumeration. Percent survival and percent log survival was calculated using Eqs. 1 and 2, respectively.
Where
- N 1 :
-
Final population of microorganism (CFU/g solid)
- N 0 :
-
Initial population of microorganism (CFU/g solid)
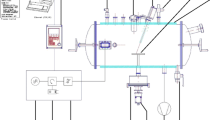
REV Drying
All samples were dehydrated in REV equipment (bioREV, EnWave Corp. Vancouver, BC) at various microwave powers, revolution per minute (rpm) about the vial vertical axis, and absolute pressure levels. Dehydration times varied from 3 to 52 min.
Freeze Drying
Samples dried in freeze drier were first quick-frozen at −80 °C for 4 h. Frozen samples were dehydrated in freeze drier at 15 °C shelf temperature, condenser temperature of −35 °C, and absolute pressure of 0.01 mm Hg for 60 h.
Moisture and Water Activity Measurement
Moisture of samples was measured by titration method (AOAC 977.10, 2002) using AQUA COUNTER (Coulometric Karl Fischer Titrator, Hiranuma Sangyo Co. Ltd., Japan). Water activity was measured by a water activity meter (Aqua lab, Model series 3, Decagon Devices, Inc., Washington, USA).
Experimental Design 1 on Response Surface Methodology
Survival of L. salivarius 417 after REV dehydration was studied using a face-centered central composite design with three variables and a response surface methodology (RSM; Neter et al. 1996). After preliminary trials, microwave power, absolute pressure, and protectant concentration were chosen as independent factors (variables). In these experiments, rpm was kept constant as zero. The design contained three coded factor levels: +1 denotes the high level, 0 mid level, and −1 the lower level for each factor. The actual level of each factor was calculated using Eq. 3 (Neter et al. 1996).
The factors and their respective coded and uncoded levels are presented in Table 1. The effect of the three independent variables on survival (Y) was modeled with a polynomial response surface. In the present design, the second-order response function is explained by the Eq. 4.
Where
- x 1 :
-
Microwave power (Watts)
- x 2 :
-
Absolute pressure (mmHg)
- x 3 :
-
Protectant (%)
x 1, x 2, and x 3 represent the coded values of the factors and β 0, β 1,……. β 23 are constant coefficients.
The statistical significance of the second-order model was checked by an F test (ANOVA) with data shown in Table 3. Regression models were used to construct the response surface and contour plots using Minitab software (version 13.3; 2003).
Experimental Design 2 on Random Centroid Optimization
In the second method, random centroid optimization (RCO) was used to optimize four process variables: power, absolute pressure, protectant concentration, and the spinning speed to achieve a high viability (Nakai 1990). The maximum and minimum values used for microwave power (100–800 watts), absolute pressure (0–40 mmHg), protectant (5% to 25%), and spinning speed (0–200 rpm) were entered into RCO program (RCO 2007) for first cycle random design search. After a set of nine experiments, centroid search was used to narrow down the most suitable combination of the four factors, which gave higher percent viability compared to the rest. According to the results of the first cycle, the maximum and minimum ranges for the four factors were narrowed down in the second cycle (Nakai et al. 1998).
Results
Based upon the preliminary work, skim milk powder was preferred over other protecting agents (lactose, raftilose synergy1, etc.) and used in the present study to better retain the viability of bacteria during the drying process (Ahmad et al. 2007). The objective of the present study was to find the best combination of power, absolute pressure, and protectant level to maximize the survival of bacteria after REV dehydration. In the case of RCO, a fourth process variable, rpm, was examined as well. All samples were dried to water activity of 0.3–0.4.
Response Surface Methodology
A set of experiments was carried out according to Tables 1 and 2. All three independent factors had a significant effect on the survival of the bacteria after dehydration. The coefficient of determination (R 2) of the polynomial model was 0.983, indicating that 98.3% of the variability in the response (percent viability) could be explained by the second polynomial equation. The lack of fit was non-significant (P = 0.162). ANOVA results showed that this model is a good fit for the optimization of the drying conditions for L. salivarius 417 (Table 3).
The significance of each coefficient of the model was determined by Student’s t test and p value (Table 4). The smaller p value and larger absolute t value determine the significance of the corresponding coefficient (Adinarayana et al. 2003). Thus, power, absolute pressure, and protectant were highly significant with p values of Px 1 < 0.0001, Px 2 < 0.0001, and Px 3 < 0.001, respectively (Table 4). The quadratic main effects of power were less significant (Px 21 < 0.01) than its respective first-order effects (Px 1 < 0.0000), while the quadratic main effects of protectant concentration (Px 23 < 0.000) were more significant than its respective first-order effects (Px 3 < 0.001). Among the interaction effects, the effects of power absolute pressure (Px 1 x 2 < 0.022) and power protectant (Px 1 x 3 < 0.025) were significant, while that of absolute pressure protectant was not significant (Px 2 x 3 > 0.166).
The surface plots of RSM as a function of two factors at a time, holding the third factor at fixed level (middle value), are helpful to understand the main and interaction effects of these factors (Cheynier et al. 1983). Surface plots for the survival of L. salivarius 417 are given in Figs. 2, 3, and 4.
Figure 2 represents the effect of varying levels of power and absolute pressure on the survival of the L. salivarius 417 while the concentration of skim milk powder is fixed at central concentration. It is evident from Fig. 2 that not only was the survival of L. salivarius 417 steadily increased (<96% log) when the power and absolute pressure levels were decreased but also their interaction had a significant effect on the survival. For example, at higher power level, the percent survival increased very slowly when absolute pressure decreased and vice versa. As we can see, maximum survival of the bacteria at lower level power and low absolute pressure confirms the data obtained in our preliminary trials (Ahmad et al. 2007). This could be related to increase in the temperature of the product at higher power levels. In other words, when bacteria are exposed to higher temperatures during drying, survival will be low. The same trend was observed with absolute pressure level—the lower the absolute pressure, the lower boiling point or sublimation of water. Therefore, the dehydration process occurs at lower temperature and results in higher survival. These findings are in agreement with our study (Ahmad et al. 2007) and study reported by Kim and colleagues (1997) which indicated higher dehydration temperature result in lower survival.
The effects of varying levels of power and the concentrations of protectant are shown in Fig. 3. As is evident from Fig. 2, lowering the power from 300 to 150 W resulted in an increase in the survival of L. salivarius 417 to the maximum level at 93% log survival when 10% skim milk powder was used as protectant. The effect of the absolute pressure level and protectant concentration on the survival of L. salivarius 417 is shown in Fig. 4 while keeping power level fixed at 200 W. Both of these factors have their own significant effects on the survival, but their interaction effects are non-significant.
Random Centroid Optimization
The set variables and percent survival for random results for RCO are given in Table 5. After carrying out 13 experiments during the first cycle of RCO, the maximum and minimum ranges of factors were narrowed. The maximum viability of the first cycle random search was 25% and for the centroid search was 35.4%. During the second cycle centroid search, the percent viability decreased as compared to the random search. So, the experiments were stopped at that point and mapping was done to find out the optimum combination of the factors for maximum viability according to the method of Nakai (1990). Maps for the optimization of the drying factors are shown in Fig. 5a, b, c, and d. The arrow at the bottom of each map shows the estimated optimum location for each factor. The maximum viability obtained in the second cycle was 89.1% by the combination of power (252 watts), absolute pressure (0 mmHg), protectant (14%), and spinning speed (1,129 rpm) and for the centroid search, it was 38.6%. The presence of trend lines on each map is an indicator of the likely optimum condition (Nakai et al. 1998). In this study, trend lines with clear directions were observed on the maps of power, absolute pressure, and percent protectant (Fig. 5 a, b, and c), while for rpm map, the trend lines had undefined directions (Fig. 5d). These findings suggest that survival of L. salivarius 417 is strongly affected by microwave power, absolute pressure, and protectant levels, and the spinning speed had less effect on the survival.
At the end of the experiment, the optimum concentration of skim milk powder was used to compare the freeze-dried (FD) viability with REV. The result showed that the REV-dried samples have a better or equal viability compared to FD samples with the same formulation. For L. salivarius with 10% skim milk powder, the percent viability of FD and REV were 54.9% and 49.2%, respectively, while for 14% skim milk powder, FD samples showed 36.4% viability, while viability for REV samples was 89%. As the process was optimized for REV process, an optimum viability in FD process is not expected.
In this study, RCO was found to be a much easier approach to optimizing the process variables. It has a very comprehensive step by step menu. The range of factors can be adjusted manually, which allows the experimenter to incorporate other insights or even intuition, which makes it more flexible. Number of experiments is reduced as compared to other designs, and one can stop experiments at a certain point when the response appears optimal or acceptable. During mapping, if a certain factor does not have defined trend lines, it can easily be eliminated and one can continue with optimization of the other factors.
Response surface methodology (RSM) is a more widely used statistical method than RCO. It shows the interaction of all variables and improves statistical interpretation. Factors can be varied simultaneously with a fixed number of experiments. In the present work, RCO found an optimum combination of drying conditions which yielded >89% survival versus 49% for RSM. With RSM, the experimenter has the option of repeating the optimization in a more focused range of variables to find a better optimum. However, this would require more experimental trials than RCO. Nonetheless, if the goal is to find the optimum result as quickly as possible, in our view, RCO is a more efficient approach.
Conclusion
In this study, two different statistical approaches were taken to find the optimum condition for REV dehydration of L. salivarius along with skim milk powder. Both approaches showed that a combination of low power, low absolute pressure, and concentration of 10–14% skim milk powder results in the highest percent viable cells in dehydrated products. In other words, the results of RCO are in agreement with the results of RSM, but RCO found conditions for better survival than RSM. Drying at even lower pressure could be a future step for the REV process. However, as performed here, REV resulted in equivalent or better survival when compared to freeze drying and was completed in less than 1% of the time.
References
Abadias, M., Benabarre, A., Teixido, N., Usall, J., & Vi, I. (2001). Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. International Journal of Food Microbiology, 65, 173–182.
Adinarayana, K., Ellaiah, P., Srinivasulu, B., Bhavani, R., & Adinarayana, G. (2003). Response surface methodological approach to optimize the nutritional parameters for nomycin production by Streptomyces marinensis under solid-state fermentation. Process Biochemistry, 38, 1565–1572.
Ahmad, S., Yaghmaee, P., Durance, T. D. (2007). Survival of probiotic bacteria and food yeast dehydrated with microwave energy under vacuum. 41st Annual Microwave Symposium Proceedings, 30–34.
Association of Official Analytical Chemists. (2002). Official methods of analysis of AOAC International (17th edition). Gaithersburg: AOAC International.
Berny, J. F., & Hennebert, G. L. (1991). Viability and stability of yeast cells and filamentous fungus spores during freeze-drying: effects of protectants and cooling rates. Mycologia, 83, 805–815.
Carvalho, A. S., Silva, J., Ho, P., Teixerira, P., Malcata, F. X., & Gibbs, P. (2004). Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnology Progress, 20, 248–254.
Champagne, C. P., Garnder, N., Brochu, E., & Beaulieu, Y. (1991). The freeze drying of lactic acid bacteria. Canadian Institute of Food Science and Technology Journal, 24, 118–128.
Cheynier, V., Feinberg, M., Chararas, C., & Ducauze, C. (1983). Application of response surface methodology to evaluation of bioconversion experimental conditions. Applied and Environmental Microbiology, 45, 634–639.
Conrad, P. B., Miller, D. P., Cielenski, P. R., & De Pablo, J. J. (2000). Stabilization and preservation of Lactobacillus acidophilus in saccharide matrices. Cryobiology, 41, 17–24.
De Giulio, B., Orlando, P., Barba, G., Coppola, R., De Rosa, M., Sada, A., et al. (2005). Use of alginate and cryo-protective sugars to improve the viability of lactic acid bacteria after freezing and freeze-drying. World Journal of Microbiology & Biotechnology, 21, 739–746.
Durance, T. D., Yaghmaee, P., Ahmad, S., Zhang, G. (2007). Method for dehydrating biological material. PCT/CA2007/000134.2007.
Kim, S. S., Shin, S. S., Chang, K. S., Kim, S. Y., Noh, B. S., & Bhomik, S. R. (1997). Survival of lactic acid bacteria during microwave vacuum drying of plain yoghurt. Lebensmittel-Wissenschaft und Technologie, 30, 573–577.
Koh, S. P., Tan, C. P., Lai, O. M., Arifin, N., Yusoff, M. S. A., & Long, K. (2010). Enzymatic synthesis of medium- and long-chain triacylglycerols (MLCT): optimization of process parameters using response surface methodology. Food and Bioprocess Technology, 3, 288–299.
Leslie, S. B., Israeli, E., Llighthart, B., Crowe, J. H., & Crowe, L. M. (1995). Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Applied and Environmental Microbiology, 61, 3592–3597.
Linders, L. J. M., de Jong, G. I. W., Meerdink, G., & van’t Riet, K. (1997). Carbohydrates and the dehydration inactivation of Lactobacillus plantarum: the role of moisture distribution and water activity. Journal of Food Engineering, 31, 237–250.
Ming, L. C., Rahim, R. A., Wan, H. Y., & Ariff, A. B. (2009). Formulation of protective agents for improvement of Lactobacillus salivarius I 24 survival rate subjected to freeze drying for production of live cells in powderized form. Food and Bioprocess Technology, 2(4), 431–436.
Montgomery, D. C. (1991). Design and analysis of experiments (3rd ed.). New York: John Wiley & Sons.
Morgan, C. A., Herman, N., White, P. A., & Vessey, G. (2006). Preservation of microorganisms by drying; a review. Journal of Microbiological Methods, 66, 183–193.
Nakai, S. (1981). Comparison of optimization techniques for application to food product and process development. Journal of Food Science, 47(144–152), 157.
Nakai, S. (1990). Computer-aided optimization with potential application in biorheology. Journal of Japanese Biorheology Society, 4, 143–152.
Nakai, S., Dou, J., Lo, V., & Scaman, C. H. (1998). Optimization of site-directed mutagenesis. 1. New random-centroid optimization program for Windows useful in research and development. Journal of Agricultural and Food Chemistry, 46, 1642–1654.
Neter, J., Kutner, M. H., Nachtsheim, C. J., & Wasserman, W. (1996). Applied linear statistical models. Chicago: McGraw-Hill.
Random Centroid Optimization (RCO). (2007) Instructions of the RCO program. In: S. Nakai (Ed.). Canada: Faculty of Food and Land systems, University of British Columbia.
Scaman, C. H., & Durance, T. D. (2005). Combined microwave vacuum drying. In Emerging technologies for food processing (1st ed.). London: Elsevier.
Thammavongs, B., Corroler, D., Panoff, J. M., Auffray, Y., & Boutibonnes, P. (1996). Physiological response of Enterococcus faecalis JH2-2 to cold shock: growth at low temperatures and freezing/thawing challenge. Letters in Applied Microbiology, 23, 398–402.
To, B. C. S., & Etzel, M. (1997). Survival of Brevibacterium linens (ATCC 9174) after spray drying, freeze drying, or freezing. Journal of Food Science, 62, 167–170.
Uzunova-Doneva, T., & Donev, T. (2000). Influence of the freezing rate on the survival of strains Saccharomyces cerevisiae after cryogenic preservation. Journal of Culture Collection, 3, 78–83.
Visick, J. E., & Clark, S. (1995). Repair, refold, recycle: how bacteria can deal with spontaneous and environmental damage to proteins. Molecular Microbiology, 16, 835–845.
Yaghmaee, P., & Durance, T. D. (2005). Destruction and injury of Escherichia coli during microwave heating under vacuum. Journal of Applied Microbiology, 98, 498–506.
Zayed, G., & Roos, Y. H. (2004). Influence of trehalose and moisture content on survival of Lactobacillus salivarius subjected to freeze-drying and storage. Process Biochemistry, 39, 1081–1086.
Acknowledgement
The authors thank the Natural Sciences and Engineering Research Council of Canada (NSERC) student fellowship and are grateful for the NSERC research grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, S., Yaghmaee, P. & Durance, T. Optimization of Dehydration of Lactobacillus salivarius Using Radiant Energy Vacuum. Food Bioprocess Technol 5, 1019–1027 (2012). https://doi.org/10.1007/s11947-010-0437-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0437-y