Abstract
Because many questions arise regarding the use of immobilization technology to consistently produce a high quality beer, this work focuses on the effects of using an immobilization matrix in the fermentation process. The aim of this study was to explore the feasibility and potential uses of immobilization on sensorial characteristics such as color, flavor, and headspace compounds of stout beer, when using batch fermentation. Batch production of beer was conducted as a standard ale process for stout beer production. For the immobilized yeasts fermentation, cells were microencapsulated in alginate, by using the Thiele modulus procedure for microcapsule design. Glucose concentration, cell multiplication, cell viability, specific gravity, pH, Brix, and ethanol were monitored throughout the fermentation process. Both, sensorial analysis (statistic triangle tests) and instrumental methods (gas chromatography to measure headspace compounds and visible spectrophotometer to quantify the color) were used to evaluate characteristics of the beer that was produced from immobilized and free yeast fermentations. Free and immobilized yeasts fermentation showed no significant difference (p > 0.05) for all variables of interest. The profile of headspace compounds was different, perhaps because of changes in yeast’s behavior and the presence of secondary metabolites. However, immobilization did not have a significant impact on the beer flavor, as detected by the sensorial triangle test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beer production is one of the pioneer applications of food fermentation. The oldest historical evidence of formal brewing dates to about 6000 BC in Babylonia (Nedovic and Willaert 2004). Traditionally, brewing has been a slow batch fermentation process, with freely suspended yeast, carried out in single fermentation vessels. This method has some economic disadvantages such as the high cost of vessels and the need to monitoring and control of each batch. During the past century, however, the immobilized system of beer production has been developed to produce continuous beer fermentation, as a more cost-effective method of making beer. This has attracted much attention during the last few decades. Various systems of immobilized fermentation have been developed and used (Pilkington et al. 1998; Smogrovicova et al. 1997): attachment to a surface (Cashin 1996; Van Iersel et al. 1998, 2000; Reddy et al. 2010), self aggregation (Karel et al. 1985), or entrapment within a porous support, e.g., gel microencapsulation, in which food-grade alginate is the main biopolymer used for that purpose (Nedovic et al. 2001; Smogrovicova et al. 2001).
Beer is a complex aqueous solution that contains carbon dioxide, ethanol, and more than 800 organic compounds (Hardwick 1995). Because one cannot estimate the quality of the product by only examining a single component (such as ethanol), in brewing, any introduction of technological innovation first requires an extensive investigation of technology’s potential influence on the sensorial qualities of the brewed product (Brányik et al. 2005). Despite the variety of technological development for continuous fermentation, the main problem is the negative impact on flavor (Nedovic and Willaert 2004; Willaert and Nedovic 2006). It is still not fully understood which factors affect the sensorial characteristics of the product developed by continuous fermentation. Nevertheless, it is clear that these types of fermentations change two macro parameters: an unstirred tank is modified in order to create a continuous process, and the free environment of the yeast is altered, trapping it into a solid three-dimensional microenvironment. Obviously, these modifications in process alter the quality of the final product: one or the other or both at the same time. These alterations could be exacerbated, if the design of the continuous process and immobilization are not appropriate, lacking aspects of engineering design such as mass transfer.
Most of the literature that details similar studies show results from experiments conducted in a continuous process (Nedovic and Willaert 2004; Willaert and Nedovic 2006), and it is unclear whether the source of the alteration in quality is the cell entrapment or that the process is continuous. Researches have not extensively studied the use of yeast immobilization in batch fermentation to obtain a product with specific characteristics. To our knowledge, no work has addressed the effect on quality when the product is obtained from both ways of processing: batch fermentation with free yeasts and batch fermentation with microencapsulated yeast.
The purpose of this work was to explore the singular effect of immobilization (alginate microcapsules) on characteristics of the ale beer that was fermented through a batch process. Stout beer, a kind of ale beer, was selected as the study model (it is a black top fermentation beer made from a mixture of pale malt and very highly colored malt). For this purpose, stout beer in unstirred tanks was produced under the traditional manufacturing method: one tank with microencapsulated yeasts and the other with free yeasts. In addition, an engineering procedure was considered for the microcapsule design that consisted of a simple approach based on mass transfer fundamentals.
Materials and Methods
Microcapsule Design
For the microcapsule design, the modified Thiele modulus (Φ) was used (Bailey and Ollis 1986). This dimensionless number has been used as design procedure to immobilize eukaryotic cells (Kim et al. 1998):
where Q is the rate of substrate consumption, R is the radius of microcapsule, D is the diffusion, and C is the substrate concentration.
However, if no diffusional restriction is present, one can estimate the glucose uptake (first fermentable sugar for yeast consume) as follows (Smogrovicova et al. 2001):
where r M is the maximum rate of substrate consumption, K is the saturation constant, and X is the biomass concentration.
As a general criterion, when the modified Thiele modulus is less than 0.3, diffusional restriction may not be considered (Bailey and Ollis 1986; Kim et al. 1998). In this case, one can use Eqs. 1 and 2 to write the next relation:
Equation 3 is dependent on the substrate concentration. When C < <K, one can figure the maximum biomass without diffusional restriction (X M) independent of the substrate concentration:
In our experience (Acevedo et al. 2009), the yeasts immobilized in alginate microcapsules can growth in wort up to approximately ten times the initial biomass. Consequently, to design a microcapsule without diffusional restriction in the fermentation process, the biomass load (X O) should be 1/10 of the maximum biomass (X M ≈10X O):
Parameters D and R were evaluated experimentally. Parameters r M and K were those reported by Smogrovicova et al. (2001) for immobilized beer fermentation.
Yeast Microencapsulation
Saccharomyces cerevisiae (Safale S-04) was purchased from Fermentis (Marcq-en-Baroeul, France) and sodium alginate (Food Grade) from Loba Chemie (Mumbai, India).
A sodium alginate solution was prepared at 4% w/v and sterilized for 20 min at 121 °C. Then, the sodium alginate was cooled at room temperature (20 °C approximately) and mixed with a yeast suspension at a 1:1 proportion to obtain microcapsules of 2% w/v alginate and a biomass of 25 (gDW/L). This biomass concentration was selected by using the Thiele modulus (see Microcapsule Design in “Results and Discussion” section). Approximately 25 (gDW) of biomass corresponds to 1011 cells.
The yeasts were microencapsulated in calcium alginate spheres by dropping the solution of sodium alginate (with yeast) into a sterile solution of CaCl2 (50 mM; Sigma-Aldrich, St. Louis, USA) and gently agitating it with a magnetic stirrer (Kierstan and Bucke 1977). The microcapsules were left 1 h in a calcium solution and washed them in sterile distilled water (Smogrovicova et al. 2001). Then, a sample of ten microcapsules was inspected with a microscope that was adapted with a digital camera (Nikon, Tokyo, Japan).
Glucose Diffusivity in Alginate Microcapsules
Microcapsules without cells were suspended in a glucose solution (10 g/L) and shaken (1,000 rpm) in a thermomixer machine (Eppendorf, Hamburg, Germany). After every minute, ten microcapsules were taken and disintegrated in 2 mL of a buffer phosphate (pH 7.0). Glucose concentration was measure by using a commercial GOD/PAP kit (Glucose Liquicolor; Human GmbH, Wiesbaden, Germany). The experimental data set was fitted to an integrated form of the Fick’s second law in its analogue form, as described by Oerther et al. (1999).
Beer Production
The batch production of beer was conducted by using the standard ale process to obtain stout beer (Bamforth 2004). Yeast (S. cerevisiae, Safale S-04) was purchased from Fermentis (Marcq-en-Baroeul, France). Cells were suspended in sterile water at 50 (gDW/L) and mixed during 30 min before wort inoculation or microencapsulation.
The grist composition to make the wort was 83% w/w Pale malt, 10% w/w Caramel malt, and 7% w/w chocolate malt. The mashing process consisted of infusing the compound at a constant temperature of 62 °C for an hour and a half. The wort was boiled for 1 h. The hops that were used (pellets type 90) were Cascade (7.5% w/w α-acids) and Kent Golding (5.0% w/w α-acids). The wort characteristics were as follows: pH 5.32, first wort gravity 19ºP, and original gravity 13ºP.
The primary fermentations with free and microencapsulated yeast were conducted in two independent batch bioreactors of 5 L, operating in similar conditions at room temperature (20 ± 5 °C). The yeast concentration pitched into the cold wort was 0.65 g of yeast per liter of wort. This concentration was the same in both bioreactors: free yeasts (standard beer) or microencapsulated yeasts. Before pitching the yeasts, the beer wort was aerated with sterile air (filtered at 0.22 µm) for two periods of 10 min each, with a space of 10 min between each aerating session to avoid over foaming. The bioreactors were operated without agitation and without oxygen supply during the main fermentation process. The primary fermentation was stopped when the specific gravity was constant (4 days in this case). The fermented liquid obtained, “called green-beer,” was matured by using previously carbonation (adding dextrose directly into the bottles). The beer was poured into 350-mL amber glass bottles and capped. After bottling the beer, it was maturated at room temperature for 10 days.
Cell Viability
Cell viability in the fermentation stage was determined by counts of yeast in the Neubauer Chamber, three times a day. To identify a viable from nonviable yeast, the cell suspension was previously stained with methylene blue solution (Sigma-Aldrich, St. Louis, USA) 0.1% w/v (Vairo 1961). To count the microencapsulated cells, the alginate microcapsules were dissolved in phosphate buffer (pH 5.2), using a proportion of ten microcapsules into 2 mL buffer.
Sensory Analysis
To evaluate sensorial differences between the obtained products, the statistic triangle test was applied on both green beer and beer (matured). The sensorial tests were carried out by at least 24 food scientists, advanced students trained in sensorial analysis, and regular beer consumers. Each group sampled the beer in trial tastings on the same day. Each panelist organoleptically evaluated 50 mL of beer, served in three nontransparent glasses, to identify the nonpair sample. The results were evaluated by using a statistical distribution specifically applied to the triangle test.
Glucose and Ethanol Assay
Glucose and ethanol were measured by using commercial spectrophotometer kits: GOD/PAP Glucose Liquicolor (Human GmbH, Wiesbaden, Germany) and Quanti-Chrom Ethanol Assay Kit DIET-500 (BioAssay Systems, Hayward, USA).
Headspace Analysis: Gas Chromatography/Mass Spectrum–Solid Phase Micro Extraction
Volatile organic compounds (VOCs) of the headspace were extracted by using (solid phase micro extraction, SPME) and analyzed with gas chromatography/mass spectrum (GC/MS; Acevedo et al. 2003, 2007). Beer sample (1 mL) was poured in headspace vials of 20 mL. Carboxen-PDMS fiber (Supelco, Bellefonte, USA) was exposed in the headspace for 30 min at 20 °C. Then, the fiber was injected in a GC/MS HP 6890 (with a HP MD5973 quadrupole mass spectrometer; Hewlet-Packard, Santa Clara, USA) under a split-less mode (2 min). Separation was performed in a HP-5MS column (Agilent Technologies, Santa Clara, USA). The fiber was exposed for desorption at the port of the chromatograph for 5 min at 250 °C. The identification of VOCs was performed on a mass spectrum, using the NIST-02 library.
Color Analysis
The beer color was analytically measured within the visible spectrum (400–700 nm), using a spectrophotometer (Model 8453 Hewlett Packard, Santa Clara, USA). Before the analysis, the beer was centrifuged (15,000 g × 10 min) and diluted to a 20% v/v, removing possible interference in the absorbance measurement and obtaining a linear signal. A blank of sterile distillate water was used in the analysis.
The whole color profiles obtained were statistically compared with a Hierarchical Cluster Analysis (Euclidean distance) by using Multiexperimental Viewer TM4 Software (Saeed et al. 2003). This statistical technique groups similar samples based on the behavior of the whole profile, using each wavelength (nm) as a variable (Brereton 2003). The color, then, can be distinguished and compared among samples.
Statistical Analysis
When necessary, a t Student test was used to compare between two means obtained from triplicate data.
Results and Discussion
Microcapsule Design
Microcapsules were designed by using Eq. 5 (see “Materials and Methods” section). This equation establishes the initial biomass to immobilize (X O) as a function of the microcapsule radius (R) and other design parameters (D, K, and r M). The average of microcapsules radius was estimated in 0.12 cm.
The diffusion coefficient (D) was obtained experimentally by fitting the experimental kinetic of glucose concentration to Fick’s second law (Oerther et al. 1999). Glucose diffusivity in the alginate microcapsule was estimated to be 2.4 × 10−2 (cm2/h). Others authors have published similar values (Tanaka et al. 1984; Hannoum and Stephanopoulos 1986). Parameters r M and K, used in the design, were those reported by Smogrovicova et al. (2001) for beer fermentation with immobilized yeast into alginate (r M = 0.35 g/gDWh and K = 19.4 g/L). Then, the design variable “X O ” was calculated in 25 gDW/L.
Figure 1a shows an alginate microcapsule with homogeneous and spherical shape. Figure 1b and c shows the yeast morphology before and after immobilization, respectively. No changes in yeast morphology and microcapsules topography were observed. Figure 2 shows the growth kinetics of immobilized yeast, it was verified that the growth lasted for more than 72 h, and the viability on the third day was more than 80%. These results confirm a correct microencapsulation design and procedure.
Fermentation
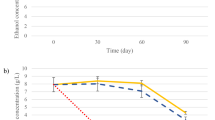
Figure 3 shows the kinetics of glucose uptake, specific gravity, and viable free yeast during fermentation. No significant differences (p > 0.05, t test) in glucose uptake and specific gravity were detected between the microencapsulated yeasts and the standard beer (using free yeasts). The velocity of the fermentation by immobilization process did not change, in comparison with nonimmobilized fermentation (standard beer fermentation). After the third day, when the fermentation stopped (constant specific gravity), the amount of free viable biomass in the bioreactor with microcapsules was higher than the one without microcapsules. Before the third day, the free viable biomass in both bioreactors was similar. Apparently, the yeasts released to the liquid controlled the fermentation velocity.
The panelists in the sensorial test found differences (p < 0.05) between both of the green beers (Table 1). This results could be an indication of a change in the behavior of the yeast when the cells are immobilized, likely producing compounds that modify the original color and flavor. It is well known that the metabolism of cells change when they are immobilized. The cells can recognize the extracellular matrix, in this specific case alginate and activate pathways, to regulate the production of metabolites (reviewed by Karel et al. 1985; Orive et al. 2002; and by Nedovic and Willaert 2004).
Though the flavor of the green beer produced by using immobilized technology is slightly different compared with that produced by standard fermentation, the process variables, such as sugar, alcohol, and biomass, behave the same in both processes. In this way, the advantages associated with this fermentation process are the known benefits of immobilized cell fermentation, such as the handling of the bioreactor.
Comparative Analysis Between Beers
Table 1 shows the parameters measured in the beer elaborated with microencapsulated yeasts and standard beer made by using free yeasts. No significant difference was detected (p > 0.05; t test) in pH, °Brix, specific gravity, and sugar and ethanol concentration. The sensorial triangle test showed no significant differences (p > 0.05), indicating a similar flavor. However, differences were found in the volatile release and in the color (Fig. 4). The beer made using microcapsules showed a slightly clearer color profile, indicated by the spectrum zone between blue and green (400–550 nm). This color might result from changes in yeast behavior, when it is entrapped in a three-dimensional structure such as alginate beads. This phenomenon has also been reported by other authors (Nedovic and Willaert 2004). Nevertheless, the consumers would not perceive the altered flavors (p > 0.05; Table 1).
Scientific studies describe that immobilized continuous beer production results in a product that has a poor flavor (Nedovic and Willaert 2004; Willaert and Nedovic 2006), but that an appropriated design of the fermentation parameters, such as a design of microcapsule, can enhance the sensorial profile (Brányik et al. 2005). No significant differences were found between microencapsulated and free yeast production of beer. From this result, an important observation is evident: if the microcapsule is designed correctly, the immobilization process will not be the main factor of the changes in flavor, in a continuous production of beer. Therefore, the continuous process should be recognized as the main factor that influences the flavor of the final product.
Color Profile
The visible spectrum (Fig. 4) shows color as a characteristic of the kind of fermentation, microencapsulated yeasts, or standard beer using free yeasts. For a more rigorous examination, a comparative study of other commercial ale beers, by using a cluster analysis, was developed (Brereton 2003; Fig. 5). This analysis shows that the color of beer that is brewed with microcapsules was different between pale ale, red ale, and amber ale. These three beers were consolidated into a homogeneous cluster. Beer prepared by using microcapsules was clustered together with commercial Stout, and the green beer elaborated with microcapsules was similar to the commercial Porter. The analysis also showed that the wort fermentation changes the color profile, depending on whether the yeast is microencapsulated or free.
Profile of Headspace Compounds
To verify changes in headspace flavors, the GC/MS-SPME was used (Balasubramanian and Panigrahi 2010). This technique allowed a rapid characterization and identification of VOCs in the headspace, indicating possible marker molecules. The advantage of this technique is that the sample is obtained directly from the headspace, not the liquid as in others techniques.
The profile of headspace compounds obtained by using this technique allowed for the separation and identification of 11 volatile compounds (Table 2), from which seven have been reported by others authors in standard beer fermentation, and five in immobilized fermentation (Bamforth 2004; Willaert and Nedovic 2006). It is interesting to note that in this study, six VOCs detected in the immobilized fermentation were not detected in the standard fermentation (Table 2): hexyl acetate, caprylic acid, caprylyl acetate, ethyl nonanoate, ethyl caprinate, and cariophyllene. This result shows that both fermentation procedures present important differences, even if the fermentation velocity did not change.
The presence of the alginate matrix creates a physical barrier, affecting diffusion into the microcapsules that could allow a kind of selectivity of molecules consumed by the yeasts. Other authors have described effect of diffusion into microcapsules, showing that small molecules can diffuse readily into microcapsules, but that other compounds only diffuse slowly into it (Orive et al. 2002). However, this hypothesis is unlikely because the amount of secondary metabolites produced in brewing (isoamyl acetate, ethyl caproate, hexyl acetate, ethyl caprylate; Lilly et al. 2000; Furukawa et al. 2003), was increased using immobilization technologies.
However, microencapsulated yeasts could be more active than free yeasts, producing and releasing volatile compounds. It is known that the growth and activity of cells increase when they are immobilized in an appropriated carrier (Nedovic and Willaert 2004). The production of the secondary metabolites, such as VOCs, could be increased up to the detection limit of the GC/MS. When the standard process is used (free yeasts), the concentration of VOCs is likely below the detection limit. We detected more VOCs in the immobilized fermentation. Because the panelists in the sensorial test accepted the beer, there is no evidence that suggests that the concentration of VOCs would lead to consumers rejecting the product. Based on this result, the microencapsulation could enhance flavor of the final product.
Conclusion
The use of an alginate matrix to immobilize yeasts inside a bioreactor does not significantly increase the velocity of the fermentation. In addition, the immobilization matrix does not alter the sensorial quality of the beer.
Microencapsulation of the yeast increases the production of secondary metabolism (as VOCs) compared to fermentation using free yeasts. This significantly affects the sensory characteristics of green beer, but it does not affect the sensorial quality of the beer or final product obtained from immobilized fermentation processes.
An appropriated design of the microcapsules enhances the production of the beer’s flavor compounds and color profile. This can be used to prepare a beer with specific characteristics, though the costs would be higher.
Brewers could use immobilization batch technologies to produce beer that would be accepted by consumers and with the practical advantages associated to immobilization, such as the handling of the bioreactor.
References
Acevedo, C., Sánchez, E., Young, M., & Simpson, R. (2003). Prediction correlation of vapor pressure for methyl jasmonate. Journal of Food Engineering, 59(4), 431–433.
Acevedo, C., Sánchez, E., Reyes, J., & Young, M. (2007). Volatile organic compounds produced by human skin cells. Biological Research, 40(3), 347–355.
Acevedo, C., Skurtys, O., Young, M., Enrione, J., Pedreschi, F., & Osorio, F. (2009). A non-destructive digital imaging method to predict immobilized yeast-biomass. LWT-Food Science and Technology, 42(8), 1444–1449.
Bailey, J., & Ollis, D. (1986). Biochemical engineering fundamentals. New York: McGraw-Hill.
Balasubramanian, S., & Panigrahi, S. (2010). Solid-phase microextraction (SPME) techniques for quality characterization of food products: A review. Food and Bioprocess Technology, doi:10.1007/s11947-009-0299-3, in press.
Bamforth, C. (2004). Beer health and nutrition. Oxford: Blackwell Science.
Brányik, T., Vicente, A., Dostalek, P., & Teixeira, J. (2005). Continuous beer fermentation using immobilized yeast cell bioreactor systems. Biotechnology Progress, 21(3), 653–663.
Brereton, R. (2003). Chemometrics: Data analysis for the laboratory and chemical plant. Chichester: John Wiley and Sons.
Cashin, M. (1996). Comparative studies of five supports for yeast immobilisation by adsorption/attachment. Journal of the Institute of Brewing, 102(1), 5–10.
Furukawa, K., Yamada, T., Mizoguchi, H., & Hara, S. (2003). Increased ethyl caproate production by inositol limitation in Saccharomyces cerevisiae. Journal of Bioscience and Bioengeneering, 95(5), 448–454.
Hannoum, B., & Stephanopoulos, G. (1986). Diffusion coefficients of glucose and ethanol in cell-free and cell-occupied calcium alginate membranes. Biotechnology and Bioengineering, 28(6), 829–835.
Hardwick, W. (1995). The properties of beer. In W. Hardwick (Ed.), Handbook of brewing (pp. 551–585). New York: Marcel Dekker.
Karel, S., Libicki, S., & Robertson, C. (1985). The immobilization of whole cells: Engineering principles. Chemical Engineering Science, 40(8), 1321–1354.
Kierstan, M., & Bucke, C. (1977). The immobilization of microbial cells, subcellualar organelles, and enzymes in calcium alginate gels. Biotechnology and Bioengineering, 19(3), 387–397.
Kim, S., Yu, S., Son, J., Hübner, H., & Buchholz, R. (1998). Calculations on O2 transfer in capsules with animal cell for the determination of maximum capsule size without O2 limitation. Biotechnology Letters, 20(6), 549–552.
Lilly, M., Lambrechts, M., & Pretorius, I. (2000). Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Applied and Environmental Microbiology, 66(2), 744–753.
Nedovic, V., & Willaert, R. (2004). Fundamentals of cell immobilisation biotechnology. Dordrecht: Kluwer Academic Publishers.
Nedovic, V., Obradovic, B., Leskosek-Cukalovic, I., Trifunovic, O., Pesic, R., & Bugarski, B. (2001). Electrostatic generation of alginate microbeads loaded with brewing yeast. Process Biochemistry, 37(1), 17–22.
Oerther, S., Le Gall, H., Payan, E., Lapicque, F., Presle, N., Hubert, P., et al. (1999). Hyaluronate-alginate gel as a novel biomaterial: Mechanical properties and formation mechanism. Biotechnology and Bioengineering, 63(2), 206–215.
Orive, G., Hernández, R., Gascón, A., Igarta, M., & Pedraz, J. (2002). Encapsulated cell technology: From research to market. Trends in Biotechnology, 20(9), 382–387.
Pilkington, H., Maragaritis, A., Mensour, N., & Russell, I. (1998). Fundamentals of immobilised yeast cell for continuous beer fermentation: A review. Journal of the Institute of Brewing, 104(1), 19–31.
Reddy, L., Reddy, L., Wee, Y., & Reddy, O. (2010). Production and characterization of wine with sugarcane piece immobilized yeast biocatalyst. Food and Bioprocess Technology, doi:10.1007/s11947-009-0321-9, in press.
Saeed, A., Sharov, V., White, J., Li, J., Liang, W., Bhagabati, N., et al. (2003). TM4: A free, open-source system for microarray data management and analysis. Biotechniques, 34(2), 374–378.
Smogrovicova, D., Domeny, Z., Germeiner, P., Malovikova, A., & Sturdik, E. (1997). Reactors for continuous primary beer fermentation using immobilised yeast. Biotechnology Techniques, 11(4), 261–264.
Smogrovicova, D., Domeney, Z., & Svitel, J. (2001). Modeling of saccharide utilization in primary beer fermentation with yeasts immobilized in calcium alginate. Applied Biochemistry and Biotechnology, 94(2), 147–158.
Tanaka, H., Matsumura, M., & Veliky, I. (1984). Diffusion characteristic of substrates in Ca-alginate gel beads. Biotechnology and Bioengineering, 26(1), 53–58.
Vairo, M. (1961). Methylene blue solutions for staining dead yeast cells. Stain Technology, 36, 329–330.
Van Iersel, M., Meersman, E., Arntz, M., Ronbouts, F., & Abee, T. (1998). Effect of environmental conditions on flocculation and immobilization of brewer’s yeast during production of alcohol-free beer. Journal of the Institute of Brewing, 104(3), 131–136.
Van Iersel, M., Brouwer-Post, E., Ronbouts, F., & Abee, T. (2000). Influence of yeast immobilization on fermentation and aldehyde reduction during the production of alcohol-free. Enzyme and Microbial Technology, 26(8), 602–607.
Willaert, R., & Nedovic, V. (2006). Primary beer fermentation by immobilised yeast: A review on flavour formation and control strategies. Journal of Chemical Technology and Biotechnology, 81(8), 1353–1367.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Almonacid, S.F., Nájera, A.L., Young, M.E. et al. A Comparative Study of Stout Beer Batch Fermentation Using Free and Microencapsulated Yeasts. Food Bioprocess Technol 5, 750–758 (2012). https://doi.org/10.1007/s11947-010-0379-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0379-4