Abstract
Mint (Mentha spicata L.) is a European aromatic plant, with its essential oil used in food, pharmaceutical and cosmetic industries. Supercritical fluid extraction (SFE) is important for natural products, because it is residue free and preserves thermolabile compounds and product characteristics. The aim of this work was to obtain mint essential oil by sub-/supercritical extraction, with and without modifier and in different operational conditions, by hydro-distillation and by Soxhlet with different solvents. The results indicated the SFE highest yield (2.38% w/w) was obtained at 50 °C and 300 bar, with the crossover of yield isotherms occurring between 140 and 170 bar. When using a cosolvent for SFE, the ethanol showed the highest yield, compared to ethyl acetate. The mint essential oil was rich in compounds with therapeutic activities and several substances of industrial interest, such as carvone, cineol, and pulegone, presenting also good antioxidant activity performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Lamiaceae family is formed by several representative types with considerable amount of essential oils (Aghel et al. 2004). The Mentha genus, with 25 to 30 species, is common in Europe, Asia, Australia, and South Africa (Dorman et al. 2003). The Mentha spicata L. (mint) is an aromatic plant, native from Central Europe and one of spearmint species most harvested in Brazil, because it is adequate for subtropical climate. Mint contains, among several others, the following properties: antifungal, antiviral, antimicrobial, insecticide, antioxidant, allergenic, diuretic, and stimulant. It is used to treat fever, bronchitis, colds, cramps, gastritis, headaches, indigestion, and nausea (Choudhury et al. 2006).
According to Dorman et al. (2003), foods enriched with natural antioxidants bring benefits to human health. Then, there is a large and growing interest for the use of these substances (Hras et al. 2000). Moreover, the application of synthetic antioxidants is restricted in many countries because of possible harm effects in human health. Natural antioxidants have been isolated from different parts of plants such as seeds, fruits, leaves, and roots. These compounds include flavonoids, phenolic acids, terpenes, tocopherols, phospholipids, organic acids, and also plant extracts (Gómez 2003; Mitra et al. 2009). Spices herbs such as mint are important natural antioxidants (Hras et al. 2000), which have significant antioxidant potential (Gómez 2003).
The extraction technique used to obtain high aggregate value compounds from natural raw materials defines the product quality. There are several well-established extraction procedures, called conventional methods, such as hydro-distillation (HD) and organic solvent extractions like Soxhlet (Sox) and maceration (Mac) techniques (Reverchon and De Marco 2006; Weinhold et al. 2008). The limitations of conventional methods are high energy costs, elevated solvent use, high temperatures, injurious for thermolabile substances, and solvent residue in the solute, reducing the product quality (Campos et al. 2008; Michielin et al. 2009).
The supercritical fluid technology is an alternative and innovative process which permits efficient recovery of natural aromatic compounds, besides volatile oils (Simões and Spitzer 2001; Pereira and Meireles 2009). Supercritical fluid extraction (SFE) presents advantages over other methods, such as the use of low temperatures, solvent recycling, reduced energy consumption, product quality due to the absence of solvent in solute phase, and operational flexibility (Ferreira et al. 1999; Michielin et al. 2005; Mezzomo et al. 2009). The solubility of low volatility substances in supercritical nonpolar gases decreases with increasing molecular weight and with increasing polarity and number of polar functional groups (Ferreira et al. 1999). Then, the solubility of these substances can be successfully increased by the addition of a cosolvent (Sovová et al. 1999).
Therefore, the aim of this work was to obtain mint (M. spicata L.) essential oil by different extraction techniques: sub-/supercritical fluid extraction with and without modifier, hydro-distillation and Soxhlet, evaluating the methods by extraction yield, chemical profile, and antioxidant activity of the extracts.
Materials and Methods
Sample Preparation
The raw material (M. spicata L.) was provided by Quimer Ervas e Especiarias (São Paulo, SP, Brazil) in dehydrated flakes. The moisture content was determined according to 950.46B method of AOAC (1990). The samples were grounded for 1 min in a domestic blender (LiqFaz, Wallita, São Paulo, SP, Brazil). The particles were classified by particle size in a vertical vibratory sieve shaker (Bertel Metalurgic Ind. Ltda., Caieiras, SP, Brazil). The fraction of mesh −48/+80 was selected for the extraction assays, and the evaluation of the particle size was done using scanning electronic microscopy (XL 30, Philips, Holand) in order to determine the medium diameter of particles.
Conventional Techniques
Soxhlet extraction (Sox) was performed according to Campos et al. (2008). Briefly, grounded mint leaves were packed inside a cartridge placed inside the 250-mL extractor device. The sample was submitted to Sox for 6 h at boiling temperature of each solvent used, in the proportion of 5 g of sample to 150 mL of solvent in a 250-mL Soxhlet flask. The extraction was performed at least in duplicate, with different solvents: n-hexane (Hx), dichloromethane (DCM), butanol (BtOH), ethyl acetate (EtAc), and ethanol (EtOH) (Nuclear, CAQ Ind. e Com. LTDA., Brazil), with polarities of 0, 3.4, 3.9, 4.3, and 5.2, respectively (Byers 2009), in order to evaluate the influence of their different polarities on extraction yield, extract composition, and antioxidant properties.
The HD method consists of placing 30 g of grounded mint leaves inside a 2-L flask of Clevenger type apparatus with 500 mL of distilled water for hydro-distillation and carried out at least in duplicate during a 6-h extraction process.
The resulting extracts, obtained by the different methods, were separated at reduced pressure by evaporating the solvents used in a rotary evaporator (Fisatom, 802, Brazil), with vacuum control and a thermostatic bath (Microquímica, MQBTZ 99-20, Brazil), obtaining the fraction of each solvent by each technique.
The global yield (X 0) for each low pressure extraction (Sox and HD) was obtained by the mean value of the ratio between extracted oil mass and mass of raw material used.
Sub- and Supercritical Fluid Extraction
The sub- and supercritical fluid extraction of M. spicata L. was performed in two dynamic extraction units previously described by Zetzel et al. (2003) and Danielski et al. (2007). A cosolvent (CS) pump (Constametric 3200, Thermo Separation Process, EUA) was connected to the extraction line in order to supply the modifier (organic solvent at high pressure) at pre-established flow rate to mix with CO2 flow before the extraction vessel. The extraction procedure, described by Michielin et al. (2005), consisted of placing a fixed mass of 15 g of mint particles inside the extractor cell to form the particles fixed bed, followed by the control of the process variables (temperature and pressure). The extraction was then performed, and the solute was collected in amber flasks after a 180-min extraction time and weighed in an analytical balance (OHAUS, Model AS200S, NJ, USA). The SFE assays developed to obtain the values of X 0 (ratio between extracted oil mass and mass of raw material) were performed in duplicates and divided in two groups: (a) pure CO2 assays, using carbon dioxide as solvent, where carried out at 30, 40, and 50 °C, at 100, 200, and 300 bar and at constant solvent flow rate of 5.0 ± 0.5 g/min; (b) the CS assays, where the organic solvents added to supercritical CO2 were ethanol and ethyl acetate in concentrations of 10%, 15%, and 20% to produce the solvent mixture. This group was performed at 40 and 50 °C, 150, 200, and 230 bar, 0.9 g/min of CO2, and 180 min of extraction. The cosolvent was separated from the solute as for conventional techniques. The process used CO2 99.9% pure delivered at a pressure of up to 60 bar (White Martins, Brazil), and for each operating condition, the values of solvent density were obtained according to Angus et al. (1976).
Extract Quality
DPPH Assay Method
The free radical scavenging activity of the M. spicata L. extracts was evaluated as described by Campos et al. (2008). Briefly, the mint extract was mixed with a 0.3 mM 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) ethanol solution, to give final concentrations of 5, 10, 25, 50, 125 and 250 µg of extract per milliliter of DPPH solution. After 30 min at room temperature, the absorbance values were measured at 517 nm in spectrometry (Genesys, 10 VIS, Rochester, NY, USA) and converted into percentage of antioxidant activity (% AA). This activity was also expressed as the inhibition concentration at 50% (IC50), i.e., the concentration of the test solution required to give a 50% decrease in the absorbance of the test solution compared to that of a blank solution.
Chemical Profile
The identification and relative quantification of the volatile compounds present in the M. spicata L. extracts were achieved by gas chromatography coupled to mass spectrometry analysis (GC-MS). The analysis was performed in a gas chromatograph (CP-3800, Varian, CA, USA) coupled with a mass detector (Saturn 2000, Varian) and a CP-Sil 8 CB Low Bleed/MS capillary column (30 m × 0.25 mm × 0.25 μm). The samples were dissolved in ethyl acetate and injected (1 µL) for analysis following the conditions: initial temperature of 60 °C and final temperature of 220 °C, with heating rate of 5 °C/min; detector temperature at 220 °C and hydrogen as carrier gas at 1 mL/min flow rate. The extract components were evaluated by recognition using the database for natural products Standard Reference Data Series of the National Institute of Standard and Technology (NIST Library, 2000), where the mass spectrometer results were compared, and with n-alcane standard injected (C10–C25). The extract samples evaluated were Sox with Hx, DCM, BtOH, EtAc, and EtOH; SFE with pure CO2 at 40 °C/100 bar, 50 °C/100 bar, 40 °C/200 bar, 50 °C/200 bar, 30 °C/300 bar, and 50 °C/300 bar; and SFE with CO2 plus 15% of EtOH at 40 °C/200 bar, plus 20% of EtOH at 40 °C/200 bar, 40 °C/150 bar, 50 °C/200 bar, and 50 °C/230 bar.
Statistical Analysis
The results were statistically evaluated by a one-way analysis of variance, applied using the Software Statistica for Windows 6.0 (Statsoft Inc., USA) in order to detect significant differences in X 0 and maximum antioxidant activity (MAA) values. The significant differences at level of 5% (p < 0.05) were analyzed by the Tukey test.
Results and Discussion
Raw Material Characteristics
The scanning electronic microscopy of mint sample used on extraction assays defined the M. spicata L. medium diameter of 0.362 mm. The moisture content of mint samples, determined according to “Sample Preparation”, was 10.7 ± 0.3% (w/w), and the SFE particle bed presented a density of 0.15 ± 0.02 g/cm3.
Global Yield (X 0)
Conventional Techniques
The results for extraction yield obtained by Sox extraction with organic solvents and HD are presented in Table 1, which compares the values for global yield obtained by SFE with and without cosolvent.
The HD extraction yield (0.23 ± 0.04%) was the lower values for all techniques and conditions evaluated in this study. Lucchesi et al. (2004) and Aghel et al. (2004) also obtained low HD extraction yield for mint: 0.09% and 0.6%, respectively. Besides the fact that water has affinity with polar compounds, the –OH group turns water into a bad solvent for organic compounds. Also, the high temperature applied (100 °C) and the long extraction time (240 min) may have destroyed volatile compounds, reducing the extraction yield. Although water presents higher polarity index than all other solvents used in Sox, the yield was lower, because the polarity is not the only factor affecting the extraction efficiency, and it is important to understand the different interactions between solute and solvent.
The extraction yield obtained by Sox with all solvents (Table 1) was higher than all other techniques and conditions evaluated, including SFE. The highest values were achieved with ethanol (23.33%) and butanol (12.76%), mainly because of their superior polarity, compared to the other solvents, adequate for the major compounds in mint extract. The high performance of Sox is also due to the superior amount of solvent used during extraction (approximately five times than other methods), the longer extraction time (two times higher) used in this technique, and the high temperature (solvent boiling point), which may result in a higher cost extraction process, compared to other methods.
Although Sox presented higher extraction yield than SFE, monoterpene compounds are sensible to chemical changes, and during solvent separation for conventional methods, some volatile compounds can be lost. The extracts obtained by these methods require a purification process, and thus, to choose the best extraction process, one must take into account the cost of the overall process (extraction + purification) (Quispe-Condori et al. 2005).
In addition, Soxhlet can be considered an analytical method to measure the theoretical maximum level of extraction yield and not an ideal process for industrial application.
As the Sox yield results were superior to those of SFE with pure CO2, we consider the use of an organic solvent as cosolvent on SFE from M. spicata L. Then, it is possible to increase the extraction yield, as well as broaden the spectrum of solid matrix compounds solubilized by solvent, since the CO2 extracts preferably nonpolar compounds.
Sub-/Supercritical Fluid Extraction with Pure CO2
The solubilization power of supercritical CO2 basically depends on its density, which increases with pressure at constant temperature and decreases with increasing temperature at constant pressure. The global yield results for SFE, presented in Table 1, confirm the above-mentioned behavior to increase with pressure at constant temperature.
The temperature effect on process yield is more complex. The isobaric increase of temperature diminishes the solvent density but also increases the solute vapor pressure. In the vicinity of the critical point, small changes in temperature produce large variations in solvent density. At higher pressures, the density variation with temperature is moderate, and the solute vapor pressure becomes the dominant factor. The competition between these two factors leads to the phenomenon of crossover isotherms (Michielin et al. 2005). The crossover phenomenon for M. spicata L. yield isotherms was detected between 140 and 170 bar, as presented in Fig. 1.
SFE with CO2 Plus Cosolvents
According to Sox yield results (Table 1), solvent polarity and extraction data from Lee et al. (2000), ethanol, and ethyl acetate were selected as cosolvents for SFE because of the higher performance compared to other solvents. The CS concentrations studied were 10%, 15%, and 20% (w/w).
The raise in the CS concentration increased the SFE yield (Table 1), indicating a decrease in solute–solid matrix interactions and the CS replacement in the active sites of the matrix, increasing the solute solubilization (Hollender et al. 1997; Lutermann et al. 1998).
The hydroxyl group from ethanol forms hydrogen bonds between EtOH molecules and the oil polar molecules, increasing the extraction of polar lipids while increasing the polarity of the supercritical mixture (Lee et al. 2000).
Ethyl acetate is a polar volatile ester with a C═O group and not able to form hydrogen bounds (Morrison and Boyd 1981). Thus, an increase in the EtAc concentration does not increase the extraction yield (maximum 0.9%).
As already mentioned, increasing pressure at constant temperature enhances the extraction efficiency. However, the SFE with 20% of ethanol at 40 °C increased the pressure from 200 to 230 bar and decreased the yield from 9.0% to 6.5% (Fig. 2). The same behavior was observed by Lucchesi et al. (2004) for SFE of Levisticum officinalis and Salvia officinalis, by Luengthanaphol et al. (2004) for SFE extraction of tamarind seeds, by Campos et al. (2008) for SFE of grape pomace, and by Kitzberger et al. (2007) for SFE of shiitake, using EtAc as cosolvent. This behavior can be explained by changes in solid matrix structure because of the high concentration of CS, ethanol, which slow diffusion process, and then reduced the extraction yield (Lucchesi et al. 2004).
Antioxidant Activity—DPPH Method
The results for the maximum antioxidant activity (MAA%) and IC50 (µg/mL) are presented in Table 2 for mint extracts obtained by Sox and SFE. IC50 values represent the extract concentration where 50% of antioxidant activity is observed.
The lowest MAA % values, among the low pressure methods, were obtained by Sox with Hx and by HD. Although water is a highly polar solvent, it was unable to extracts compounds with significant antioxidant activity. The extended use of high temperature in HD extraction (100 ºC for 240 min) may have degraded solute compounds, promoting reactions such as esters hydrolysis, aldehydes polymerization or other decompositions (Santos et al, 2004).
The antioxidant activity of Sox extracts was higher than all other methods, except for Sox/Hx. This good performance for MAA% is because of solvent recycling, high amount of solvent, high temperature, and extended extraction time for Sox extraction. These aspects increase solubility of polar compounds, like antioxidant substances.
Since ethyl acetate has higher polarity compared to butanol, its MAA values was also expected to be higher. A possible explanation for the opposite behavior detected is the higher boiling temperature of EtAc, compared to BtOH, which promoted higher degradation of compounds with antioxidant activity during extraction process. The antioxidant compounds are heat sensitive, requiring a very strict control of temperature to prevent its decomposition.
The results for IC50 for conventional techniques show values of 50 µg/mL, except for Hx and water. Although water is a polar solvent, the presence of –OH group, high temperature applied, and long time extraction turns water into a bad solvent, as already mentioned. Otherwise, Hx is a nonpolar solvent. Then, in addition to IC50 values of these solvents, it can be said that probably the antioxidants from M. spicata L. are preferentially polar.
The higher antioxidant activity of SFE extracts with CO2 was obtained at 40 °C (Table 2). The increase trend of antioxidant activity at lower temperatures suggests that the compounds of the extracts are heat sensitive (Hu et al. 2005).
The SFE with CO2 shows higher temperature influence than pressure on MAA% results. The higher MAA% value was at 100 bar and 40 °C (26.7%). The IC50 results (Table 2) for all conditions show values above 250 µg/mL of extract, i.e., to have 50% of inhibition of free radicals, the necessary amount of extract is superior of 250 µg/mL.
The AA results are shown in Fig. 3 by the DPPH method, represented by the AA curves for different mint extracts (Sox with Hx, EtAc, and EtOH, as well as by SFE with and without ethanol as CS).
The Sox extractions with ethanol, butanol, and ethyl acetate showed the highest antioxidant activity, with 95.2%, 94.0%, and 92.9%, respectively. The mint family is rich in phenolic compounds with antioxidant properties (Kanatt et al. 2007). The AA values for ethanol and butanol extracts were maximum at 50 µg/mL. For ethyl acetate and dichloromethane extracts, a gradual increase in AA was detected up to 92.9% and 86.3%, respectively, at 250 μg/mL.
Because of the various action mechanisms from antioxidant compounds and the different methods to evaluate the AA, Kanatt et al. (2007) advise that only one AA test suggests the antioxidant properties of the extract, while more tests can ensure the actual property.
The use of ethanol as CS increased the AA potential of the mint extract compared to extracts obtained by SFE without CS. This behavior is probably due to the enhancement in the extraction of polar compounds by the CS and because substances with high AA are polar ones (Hu et al. 2005). Based on the DPPH mechanism of molecule reduction, we suggest that the high AA of some polar extracts is due, at least in part, to the presence of substances with hydroxyl group (–OH), such as flavonoids (Silva et al. 2005).
IC50 values for the extracts obtained by SFE-CS (Table 2) were lower at the inferior conditions of temperatures and pressures. The cosolvent effect on product quality in not linear with CS concentration, because it depends on specific interactions between the solute, solvent, and CS (Sauceau et al. 2004). These interactions increase the extraction yield but not the MAA% at the concentration of 15% ethanol as CS, compared with results obtained at 10% and 20% ethanol as CS.
The effect of pressure and temperature on the MAA% of extracts obtained by 20% ethanol as CS indicated an increase in MAA% with temperature at 150 bar and a decrease with temperature at 200 and 230 bar. At 40 °C, higher pressures enhance the MAA%, while at 50 °C, the effect is opposite. This behavior is probably justified by the vulnerability of antioxidant compounds to pressure and temperature variations.
The chemical complexity of essential oils may cause different results of antioxidant activity, depending on the analytical method used. The DPPH method detects the antioxidant activity of hydrophilic and lipophilic species, providing a good comparison of the results of antioxidant activity of extracts (Sacchetti et al. 2005).
Chemical Profile
The chemical profile of the mint extracts were evaluated by GC-MS. The classification of the chemical constituents of essential oils is difficult, because they are formed by a mixture of several organic molecules with different properties, such as hydrocarbons, alcohols (bactericides, energizing, revitalizing, diuretics, and antiviral), esters (fungicides and antiinflammatory), aldehydes (antiinflammatory drugs, tranquilizers, sedatives, and antiviral), ketones (healing, mucolytic, dermatophilic, and lipophilic), and phenols (antibacterial, antifungal, immunostimulating, and invigorating) (ISO 2006).
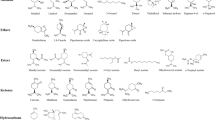
The compounds indentified by GC-MS are presented in Table 3. The data show the analytical results of different mint extracts obtained by Sox and SFE.
According to Table 3, the presence of the monoterpenes carvone, carveol, and cineol is noticeable. In particular, oxygenated monoterpenes, such as carvone, are recognized substances for food, cosmetic, and perfumery industries. Sesquiterpenes with oxygen, such as spathulenol, caryophyllene oxide, and cubenol (Table 3), normally present higher market value than its correspondent hydrocarbons identified on mint oil (β-caryophyllene, the β-elemene, and β-bourbonene—Table 3), due to its different uses in cosmetics, fragrances, or polymers substrates, among others.
The substances identified on mint extracts and listed in Table 3 present important properties. The cineol is a monoterpene of great interest for the perfume, food, and pharmaceutical industries. Its herbaceous odor assists in breathing problems, disinfects the airways, and stimulates bloodstream (Almeida et al. 2005). The carvone, which presents the menthol smell, has a sweet, minty, and refreshing flavor and has also antifungal, insecticidal, and antimicrobial properties (Carvalho and Fonseca 2006). The carveol contains a floral flavor formed by limonene hydroxylation and can be oxidized to carvone (Olsen et al. 2004). The pulegone, a terpenic unsaturated ketone, has astringent and deodorant properties. It chemically reacts with some aromatic molecules neutralizing its odor by turning them into non-aromatic molecules. The pulegone is used for indigestion and stomach pains (Abraroma 2006). The spathulenol has high antibacterial and moderate cytotoxic activities (Limberger et al. 2004). The palmitic acid, a fatty acid, is commonly used in the manufacture of shaving creams, due to its excellent detergency and foaming power. Palmitic acid is used in cosmetic creams and emulsion formulations (Aboissa 2006). The phytol, a component of the chlorophyll molecule, can be absorbed into the bloodstream and exert important functions in metabolic processes (Siqueira et al. 2003).
From Table 3, we can also observe that the compounds like diacetone, cineol, and pyranone were extracted only by Sox method. Otherwise, the components pulegone and carvone were obtained by both extraction techniques, Sox and SFE, although more pronounced relative concentrations were detected when Sox was applied. The 1-eicosene was extracted by both techniques, since the 3- and 9-eicosene were not extracted by SFE and Sox with Hx. The phytol and hexadecatrienoic acid were extracted in higher concentration by SFE.
The temperature and pressure effect on the extracts composition is also presented in Table 3. Carbon dioxide is a selective solvent, able to extract lipophilic components of low molecular weight and therapeutic properties (Ferreira et al. 1999; Michielin et al. 2005).
At 40 °C, the identified compounds were mostly presented in lower concentrations at 100 bar, if compared with results at higher pressures, because increasing solvent power enhances the solute dissolution. However, β-caryophyllene, caryophyllene oxide, and β-elemene were not detected at 200 bar. Palmitic acid presented high concentration at 300 bar, while cubenol, spathulenol, and phytols sesquiterpenes were extracted in larger quantities at 100 bar. The temperature effect at 200 bar shows a similar composition profile for the extracts at 40 and 50 °C.
The CO2 SFE is not always a selective method due to the simultaneous extraction of undesirable compounds, typically detected in essential oil extraction from herbal materials. SFE was conducted at conditions to produce high quality oil; cuticular waxes are coextracted because of its lipophilic characteristic and its location on the leaf surface (Ciurlia et al. 2009).
High concentrations of low volatility compounds (the aromatic substances) are extracted by supercritical fluids at high solvent density and temperature. However, the components’ stability to high temperatures and the operating costs at high pressures must be considered. The use of ethanol as CS in SFE improved the extraction of carveol, carvone, palmitic acid, ethyl pamitate, phytol, butyl palmitate, n-butyl benzenesulfonamide, and 6,9,12,15-docasatetraenoic acid. The higher amount of ethanol (20%) improved the SFE efficiency by increasing the compounds solubility in the solvent. Also, the use of CS decreased the extraction selectivity by increasing the number of extracted compounds, if compared with CO2 SFE. Some of these substances are dihydrocarveol, benzenepropanoic acid, humulene, α-cardinol, among others. Dihydrocarveol is a fragrance component used as flavoring ingredient (Abraroma 2006); humulene has antiinflammatory property (Michielin et al. 2009); α-cadinol is used in cosmetics and pharmaceutical industries (Higashi et al. 2005).
The effect of extracting condition on the concentration of some volatile compounds from mint extracts obtained by SFE with 20% of ethanol is represented in Fig. 4. The components selected were carveol, pulegone, dihydrocarbeol, phytol, 6,9,12,15-docosatetraenoic methyl ester, and butyl palmitate.
At 40 °C, the increase in pressure reduced the concentration of most compounds, probably because of the reduction in selectivity, decreasing the relative concentration of the selected substances. At 50 °C, increasing pressure also reduces the extraction of most compounds, except for phytol and butyl palmitate, which are best extracted at higher temperatures and pressures.
Some compounds can be exhausted during extraction, while others are extracted only after longer extraction time (high contact time between phases). The kinetics behavior of the mint oil composition was observed by oil samples collected at different stages of the SFE curve. The overall extraction curve for SFE of natural raw material present a typical behavior with a constant extraction rate (CER) period, followed by a falling extraction rate (FER) period, and finally, completed by a diffusional period (Dif) (Ferreira and Meireles 2002). Then, to evaluate the composition kinetics, three oil samples, representing CER, FER, and Dif, were submitted to chromatographic analysis. The samples were extracted at 200 bar and 40 °C, with CO2, and with 20% ethanol as CS. The time intervals for sample collection were 0–60, 60–120, and 120–240 min, which were determined based on curve behavior (upcoming results). The composition kinetics are presented in Table 4.
The results from Table 4 show that most compounds are detected in all samples, although higher concentrations were observed in the first and second intervals (0–60 and 60–120 min). For the SFE with CO2, only phytol was extracted in the second and third intervals. For the SFE with CS, the (+)epi-bicyclo sesquiphellandrene and calamenene were exhausted in the first stage while the β-bourbonene in the second one. Only the spathulenol was extracted from the second interval, and cineol is just extracted in the presence of CS.
Some compounds such as pulegone and carvone increased concentration in presence of CS, while others remained unaltered or decreased concentration when compared to the same conditions without CS. However, the composition kinetics showed that most components are extracted in the first two extraction periods, and probably their extraction is exhausted because of the disappearance in the third interval.
The extraction yield and the composition evaluation must be combined in order to adequately define the best extracting conditions for SFE of mint essential oil. Furthermore, it is important to consider other compounds not detected in this analysis, which may interfere with the extraction of desirable compounds and affect the scale-up determination of this process.
Conclusions
The results of mint essential oil extraction by different techniques and process conditions showed that the SFE allowed the extraction of mint essential oil rich in compounds with proven therapeutic activities. The crossover isotherms ranged between 140 and 170 bar for SFE with CO2. The use of CS also increased the extraction yield, compared with CO2 SFE. The best CS concentration in terms of process yield was 20% w/w, for ethanol and ethyl acetate. Ethanol as CS presented the best yield data compared with ethyl acetate for all concentrations, which places ethanol as attractive for industrial applications of SFE from mint essential oil. The different essential oils extracted presented good MAA% behavior, and the SFE is an adequate method to obtain antioxidants from M. spicata L. The composition analysis of mint extracts identified several compounds with industrial interest such as carvone, cineol, and pulegone.
References
Aboissa Óleos Vegetais. (2006). www.aboissa.com.br.
Abraroma. (2006). www.cassie.de.lyra.nom.br.
Aghel, N., Yamini, Y., Hadjiakhoondi, A., & Pourmortazavi, S. M. (2004). Supercritical carbon dioxide extraction of Mentha pulegium L. essential oil. Talanta, 62, 407–411.
Almeida, L. P., Ferri, P. H., Paula, J. R., & Santiago, M. F. (2005). Biotransformação do 1, 8-cineol por bactérias livres e imobilizadas. Revista Eletrônica de Farmácia, 2(1), 1–5.
Angus, S., Armstrong, B., & De Reuck, K. M. (1976). International thermodynamic tables of the fluid state: Carbon dioxide. Oxford: Pergamon.
AOAC—Association of Official Agricultural Chemists. (1990). Official methods of analysis (11th ed., p. 1141). Washington, DC: AOAC.
Byers, J. A. (2009). Phenomenex catalog. http://www.phenomenex.com/phen/Doc/z366.pdf.
Campos, L. M. A. S., Leimann, F. V., Pedrosa, R. C., & Ferreira, S. R. S. (2008). Free radical scavenging of grape pomace extracts from Cabernet sauvingnon (Vitis vinifera). Bioresource Technology, 99(17), 8413–8420.
Carvalho, C. C. C. R., & Fonseca, M. M. R. (2006). Carvone: Why and how should one bother to produce this terpene. Food Chemistry, 95, 413–422.
Ciurlia, L., Bleve, M., & Rescio, L. (2009). Supercritical carbon dioxide co-extraction of tomatoes (Lycopersicum esculentum L.) and hazelnuts (Corylus avellana L.): A new procedure in obtaining a source of natural lycopene. Journal of Supercritical Fluids, 49, 338–344.
Choudhury, R. P., Kumar, A., & Garg, A. N. (2006). Analysis of Indian mint (Mentha spicata) for essential, trace and toxic elements and its antioxidant behavior. Journal of Pharmaceutical and Biomedical Analysis, 3(7), 825–832.
Danielski, L., Michielin, E. M. Z., & Ferreira, S. R. S. (2007). Horsetail (Equisetum giganteum L.) oleoresin and supercritical CO2: Experimental solubility and empirical data correlation. Journal of Food Engineering, 78, 1054–1059.
Dorman, H. J. D., Kosar, M., Kahlos, K., Holm, Y., & Hiltunen, R. (2003). Antioxidant properties and composition of aqueous extracts from mentha species, hybrids, varieties, and cultivars. Journal of Agricultural and Food Chemistry, 51, 4563–4569.
Ferreira, S. R. S., & Meireles, M. A. A. (2002). Modeling the supercritical fluid extraction of black pepper (Piper nigrum L.) essential oil. Journal of Food Engineering, 54(4), 263–269.
Ferreira, S. R. S., Nikolov, Z., Doraiswamy, L. K., Meireles, M. A. A., & Petenate, A. J. (1999). Supercritical fluid extraction of black pepper (Piper nigrum L.) essential oil. Journal of Supercritical Fluids, 14(3), 235–245.
Gómez, M. E. D. B. (2003). Modulação da composição de ácidos graxos poliinsaturados ômega 3 de ovos e tecidos de galinhas poedeiras, através da dieta. I. Estabilidade oxidativa. São Paulo, 2003. Doctoring thesys on Food Science—Bromatology—Universidade de São Paulo, São Paulo.
Higashi, H., Iwai, Y., Miyazaki, K., Ogino, Y., Oki, M., & Arai, M. (2005). Measurement and correlation of solubilities for trifluoromethylbenzoic acid isomers in supercritical carbon dioxide. Journal of Supercritical Fluids, 33(1), 15–20.
Hollender, J., Shneine, J., Dott, W., Heinzel, M., Hagemann, H. W., & Gotz, G. K. E. (1997). Extraction of policiclic aromatic hydrocarbons from polluted soils with binary and ternary supercritical phases. Journal of Chromatography. A, 776, 233–243.
Hras, A. R., Hadolin, M., Knez, Z., & Bauman, D. (2000). Comparison of antioxidative and synergistic effects of rosemary extract with α-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chemistry, 71(2), 229–233.
Hu, Q., Hu, Y., & Xu, J. (2005). Free radical-scavenging activity of Aloe vera (Aloe barbadensis Miller) extracts by supercritical carbon dioxide extraction. Food Chemistry, 91, 85–90.
ISO TC 54. (2006). Business plan—essential oils. http://isotc.iso.org/livelink/livelink/971087/ISO_TC_054__Essential_oils_.pdf.
Kanatt, S. R., Chander, R., & Sharma, A. (2007). Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chemistry, 100(2), 451–458.
Kitzberger, C. S. G., Smânia, A., Jr., Pedrosa, R. C., & Ferreira, S. R. S. (2007). Antioxidant and antimicrobial activities of shiitake (Lentinula Edodes) extracts obtained by organic solvents and supercritical fluids. Journal of Food Engineering, 80, 631–638.
Lee, W. Y., Cho, Y. J., Oh, S. L., Park, J. H., Cha, W. S., Jung, J. Y., et al. (2000). Extraction of grapeseed oil by supercritical CO2 and ethanol modifier. Food Science and Biotechnology, 9(3), 174–178.
Limberger, R. P., Sobral, M., & Henriques, A. T. (2004). Óleos voláteis de espécies de Myrcia nativas do Rio Grande do Sul. Química Nova, 27(6), 916–919.
Lucchesi, M. E., Chemat, F., & Smadja, J. (2004). Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. Journal of Chromatography. A, 1043, 323–327.
Luengthanaphol, S., Mongkholkhajornsilp, D., Douglas, S., Douglas, P. L., Pengsopa, L., & Pongamphai, S. (2004). Extraction of antioxidants from sweet Thai tamarind seed coat—preliminary experiments. Journal of Food Engineering, 63, 247–252.
Lutermann, C., Willems, E., Dott, W., & Hollender, J. (1998). Effects os various binary and ternary supercritical phases on the extraction of polycyclic aromatic hydrocarbons from contaminated soils. Journal of Chromatography A, 816, 201–211.
Mezzomo, N., Martínez, J., & Ferreira, S. R. S. (2009). Supercritical fluid extraction of peach (Prunus persica) almond oil: Kinetics, mathematical modeling and scale-up. Journal of Supercritical Fluids, 51, 10–16.
Michielin, E. M. Z., Bresciani, L. F. V., Danielski, L., Yunes, R., & Ferreira, S. R. S. (2005). Composition profile of horsetail (Equisetum giganteum L.) oleoresin: Comparing SFE and organic solvents extraction. Journal of Supercritical Fluids, 33, 131–138.
Michielin, E. M. Z., Salvador, A. A., Riehl, C. A. S., Smânia, A., Jr., & Ferreira, S. R. S. (2009). Chemical composition and antibacterial activity of Cordia verbenacea extracts obtained by different methods. Bioresource Technology, 100(24), 6615–6623.
Mitra, P., Barman, P. C., & Chang, K. S. (2009). Coumarin extraction from cuscuta reflexa using supercritical fluid carbon dioxide and development of an artificial neural network model to predict the coumarin yield. Food and Bioprocess Technologies. doi:10.1007/s11947-008-0179-2.
Morrison, R. T., Boyd, R. N. (1981). Química Orgânica. 7 ed. Calouste Gulbenkian Foundation, São Paulo.
Olsen, M. H. N., Salomão, G. C., Fernandes, C., Drago, V., Horn, A., Jr., Filho, L. C., et al. (2004). Estudo da oxidação do limoneno utilizando catalisadores metaloporfirínicos com variação do sistema solvente/oxidante. Maringá, 26(1), 1–6.
Pereira, C. G., & Meireles, M. A. A. (2009). Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food Bioprocess Technology. doi:10.1007/s11947-009-0263-2.
Quispe-Condori, S., Sanchez, D., Foglio, M. A., Rosa, P. T. V., Zetzl, C., Brunner, G., et al. (2005). Global yield isotherms and kinetic of artemisinin extraction from Artemisia annua L leaves using supercritical carbon dioxide. Journal of Supercritical Fluids, 36, 40–48.
Reverchon, E., & De Marco, I. (2006). Supercritical fluid extraction and fractionation of natural matter. Journal of Supercritical Fluids, 38, 146–166.
Sacchetti, G., Maietti, S., Muzzoli, M., Scaglianti, M., Manfredini, S., Radice, M., et al. (2005). Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobial in foods. Food Chemistry, 91, 621–632.
Santos, A. S., Alves, S. M., Figueiredo, F. J. C., & Neto, O. G. R. (2004). Descrição de sistema e de métodos de extração de óleos essenciais e determinação de umidade de biomassa em laboratório. EMBRAPA, ISSN 1517–2244.
Sauceau, M., Letourneau, J. J., Freiss, B., Richon, D., & Fages, J. (2004). Solubility of eflucimible in supercritical carbon dioxide with and without a cosolvent. Journal of Supercritical Fluids, 31, 133–140.
Silva, C. G., Herdeiro, R. S., Mathias, C. J., Panek, A. D., Silveira, C. S., Rodrigues, V. P., et al. (2005). Evaluation of antioxidant activity of Brazilian plants. Pharmacological Research, 52, 229–233.
Simões, C. M. O., & Spitzer, V. (2001). Óleos voláteis. In: Farmacognosia: da planta ao medicamento, 3 edn. Ed. UFRGS/UFSC.
Siqueira, D. S., Pereira, A. S., Neto, F. R. A. A., Cabral, J. A., Ferreira, C. C., Simoneit, B. R. T., et al. (2003). Determinação de compostos de massa molecular alta em folhas de plantas da Amazônia. Química Nova, 26(5), 633–640.
Sovová, H., Rat, V., Khachaturyan, M., & Vlcek, D. (1999). Solubility of squalane and dinonyl phthalate in CO2 with entrainers. Journal of Supercritical Fluids, 14, 145–149.
Weinhold, T. S., Bresciani, L. F. V., Tridapalli, C. W., Yunes, R. A., Hense, H., & Ferreira, S. R. S. (2008). Polygala cyparissias oleoresin: Comparing CO2 and classical organic solvent extractions. Chemical Engineering and Processing, 47, 109–117.
Zetzel, C., Brunner, G., Meireles, M.A.A. (2003). Standardized low-cost batch SFE Units for University education and comparative research. In: Proceedings of the 6th International Symposium on Supercritical Fluids, vol. 1. Versailles, 577–581
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almeida, P.P., Mezzomo, N. & Ferreira, S.R.S. Extraction of Mentha spicata L. Volatile Compounds: Evaluation of Process Parameters and Extract Composition. Food Bioprocess Technol 5, 548–559 (2012). https://doi.org/10.1007/s11947-010-0356-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0356-y