Abstract
The purification of lipoxygenase in soybeans (richest known source) was attempted by precipitation, using polyethylene glycol, followed by aqueous two-phase extraction (ATPE). The preliminary purification by precipitation was standardized which resulted in two-fold purification. The influence of process parameters such as phase forming salt, molecular weight of phase forming polymer, tie line length, and phase volume ratio on the partitioning behavior of lipoxygenase during ATPE was investigated. The aqueous two-phase system of polyethylene glycol 6000 and ammonium sulfate, with inherent pH of 5.2, was found to be most suitable. The tie line length of 41.25% and volume ratio of 1.7 have resulted in 125% activity recovery with the overall purification factor of 4.38 compared to crude enzyme extract.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aqueous extract of ground soybeans is a highly complex mixture of high molecular mass proteins, peptides, carbohydrates, oligosaccharides, and many low molecular mass compounds (Cole 1993; Agrawal et al. 2008). Soybean is the richest known natural source of lipoxygenases [LOX, EC 1.13.11.12], which belong to the family of oxidoreductases. These enzymes catalyze the oxygenation of polyunsaturated fatty acids such as linoleic and linolenic acids to form fatty acid hydroperoxides (Smith and Circle 1972; Gardner 1996). Soybean lipoxygenases contain a non-heme iron atom in the active site and have a manomeric structure with molecular mass of approximately 97 kDa. The protein is a single polypeptide of 839 amino acids comprising of two main domains (Fox 1998; Sudharshan and Appu Rao 1997).
The hydroperoxide derivatives formed by the action of LOX are used in the production of soap, dye, varnish, resin, and plastic products (Tukel et al. 2005). Other applications of LOX are in whitening of bread by oxidizing the carotenoids, increasing the volume of loaf and in the formation of volatile flavor compounds. Separation of lipoxygenase and other soybean proteins has been studied employing aqueous two-phase extraction and polyethylene glycol precipitation systems (Cole 1993); however, with no special attention to purification. Metal affinity-based aqueous two-phase extraction for the purification of soybean peroxidase was also reported (Silva and Franco 2000). LOX had been purified by conventional procedures including ammonium sulfate or acetone precipitation followed by chromatography, gel filtration, or combination of these methods (Walter 1969). However, the scale-up of these methods is difficult and involves multistep procedures (Menoncin et al. 2008; Oberoi et al. 2008). In the case of conventional methods such as centrifugation, electrophoresis, or column chromatography scale-up becomes uneconomical unless the product is of high value. Purification is often difficult because of the system complexity and the need to retain biological activity. About 50–90% of the production costs of biological products are determined by the purification steps involved (Diamond and Hsu 1992). Therefore, there is a need for alternative approaches to the problem. In this regard, aqueous two-phase extraction (ATPE) provides a powerful method for separating and purifying mixtures of proteins (Vaidya et al. 2006). ATPE has been widely used for the extraction and purification of many biological products due to their technical simplicity, ease of scale-up and high yields (Raghavarao et al. 1995). Thus, in the present work, the purification of LOX was carried out by extraction using PEG/sulfate system in combination with precipitation by PEG. The objectives of the present work are (a) to standardize the pre-purification step, i.e., precipitation with PEG to reduce the contaminant proteins and (b) to study the effect of process parameters such as pH, molecular weight of PEG, tie line length, and volume ratio on the partitioning behaviour of LOX in order to enhance the purity.
Material and Methods
Materials
Plant Material
The soybeans (Glycine Max) of variety JS 335 used in this study were obtained from National Seed Collection Centre, Mysore. The seeds were ground, defatted, and stored at 4 °C for the experiments.
Chemicals
Polyethylene glycol (MW 1,500, 4,000, 6,000, and 20,000) and BSA were procured from Sisco Research Laboratories, Mumbai, India. Potassium phosphate (K2HPO4 and KH2PO4), sodium phosphate (Na2HPO4), magnesium sulfate (MgSO4), ammonium sulfate ((NH4)2SO4), sodium sulfate (Na2SO4), sodium citrate, sodium acetate, borax were procured from Ranbaxy Chemicals, Mumbai, India. Linoleic acid was procured from Sigma Chemicals Co, St. Louis, MO, USA. All the chemicals used were of analytical grade.
Methods
Preparation of Crude Extract of Lipoxygenase
Enzyme was extracted as per the protocol detailed by Axelrod et al. (1981). Soybean seeds were finely ground in a burr mill (M/s Cadmach Machinary Co. Ltd, Ahmedabad, India), sieved (350 µm mesh), and then repeatedly extracted with hexane until the eluent became colorless. The defatted soybean meal was suspended in a 0.2-M sodium acetate buffer (pH 4.5) at a concentration of 10/100 g of total mass and extracted by slow mechanical stirring for 1 h with a magnetic stirrer (Remi-2MCH, Remi Motors, India). The suspension was filtered through cheesecloth and centrifuged at 10,000 rpm for 5 min (Eltek-TC 4100D, Elektrocrafts, Mumbai, India). The pH of the supernatant was adjusted to 6.8 using 2 N NaOH. All the steps were performed at 4 ± 1 °C and used for further experiments.
Precipitation
Precipitation was carried out by adding predetermined weighed quantity of PEG 20,000 to cold crude extract and kept for stirring for 10 min. The mixture was allowed to settle for 20 min for protein precipitation induced by PEG. It was then centrifuged to remove the precipitate of undesirable proteins, and clear supernatant containing LOX was used for ATPE experiments.
Aqueous Two-Phase Extraction
Predetermined weighed quantities of polymer and salts from the different phase diagrams reported in the literature (Zaslavsky 1995; Albertsson 1986) were added to the crude extract of LOX, making the total weight of the system 100% on w/w basis. During all the experiments, the crude enzyme extract was maintained at 30% (w/w) in the total phase system. The contents were mixed thoroughly for 1 h using magnetic stirrer and allowed to stand for phase separation. After clear separation, the top and bottom phases were collected and analyzed for protein concentration and enzyme activity as per the procedures reported in the following sections. All the experiments were performed in triplicates and the average values have been reported.
Tie Line Length
Tie line length (TLL) is a square root of the sum of the squares of the difference in PEG and salt concentrations between the top and bottom phases (Diamond and Hsu 1992). The TLL of the system was calculated from its reported phase diagram (Zaslavsky 1995) according to the following equation.
where C pt and C pb are the PEG concentrations (% w/w) in top and bottom phases, respectively, and C st and C sb are the salt concentrations (% w/w) in top and bottom phases, respectively.
Phase Volume Ratio
The phase volume ratio is defined as the ratio of volume of the top and bottom phases.
where V t and V b are the volumes of top and bottom phases, respectively.
Protein Concentration
Concentration of protein was estimated using Bradford method (Bradford 1976). The reagent was prepared by dissolving 100 mg of Coomassie Brilliant Blue G 250 in 50 ml of 95% ethanol and 100 ml of 85% phosphoric acid. After the dye was completely dissolved, the volume was made up to 1.0 l using double distilled water. Protein was estimated by adding 100 μl sample containing enzyme to 2.0 ml of Bradford reagent, and the volume was made up to 3.0 ml with potassium phosphate buffer (0.1 M, pH 7.0). Absorbance was measured at 595 nm (Spectronic UV-160A, Shimadzu, Japan). The protein content was inferred from the standard graph of bovine serum albumin (10–100 µg/ml).
Enzyme Activity
The activity of LOX was determined as per the procedure detailed by Axelrod et al. (1981). The assay mixture contains 2.9 ml of borate buffer, pH 9.0, containing 10 mM of linoleic acid substrate and 50 μl of crude enzyme. The reaction was carried out at 25°C in a quartz cuvette with 1.0 cm light path. Enzyme activity was measured as initial rate of product (hydroperoxide) formation by an increase in absorbance at 234 nm using UV spectrophotometer (Spectronic UV-160A, Shimadzu, Japan). One unit of LOX activity is defined as the amount of enzyme that will catalyze the oxidation of 1 µmol linoleic acid per minute.
Enzyme Activity Recovery
The enzyme activity recovery was calculated as per the following equation (Raghavarao et al. 1995). It was calculated based on bottom phase to which the enzyme preferentially partitioned.
where, A i and A b are the LOX activities (U/ml) in crude extract and bottom phase, respectively; V i and V b are the corresponding volumes.
Purification Factor
The purification factor of LOX is defined as the ratio of specific activity of LOX in bottom phase to that of crude extract and can be calculated according to the following equation:
where P i is the protein concentration in the crude extract.
Gel Electrophoresis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with 10% gel was performed using electrophoresis unit (Genei, Bangalore) and standard molecular weight markers. Electrophoresis was conducted at 50 V, 12.5 mA, for about 3 to 4 h. The gel was stained with 0.05% (w/v) Coomassie Brilliant Blue R-250 prepared in a staining solution containing methanol, distilled water, and acetic acid in the ratio of 5:4:1. The gel was destained using the solution without Coomassie Brilliant Blue.
Statistical Analysis
Significant differences between means were determined by t test (paired two samples for mean) using Microsoft Excel. Significance of differences was defined at p < 0.05.
Results and Discussion
The partitioning behavior of proteins in ATPE is very complex and depends mainly on size, charge, and hydrophobicity of the given biomolecule. Most of these properties depend on many factors such as (1) type of aqueous two phase system, (2) phase forming salt, (3) molecular weight of the phase forming polymer, (4) pH of the system, (5) tie line length, and (6) phase volume ratio.
Details of the selection of each of these parameters and their effect on partitioning of LOX have been discussed below.
Precipitation
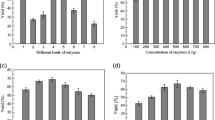
For the standardization of PEG concentration, precipitation was carried out at different concentrations of PEG (2–16%, w/w). It can be noticed from the Fig. 1 that LOX-specific activity in supernatant increased with an increase in the concentration of PEG up to 6% and later decreased with further increase in the concentration of PEG. Whereas, the protein concentration in the supernatant gradually decreased with an increase in PEG concentration. The decrease in LOX activity in the supernatant (above 6% of PEG) can be attributed to the precipitation of enzyme along with contaminating proteins. Precipitation using 6% PEG resulted in 2.0-fold purification of the enzyme. Generally PEG is used in the early purification steps, and similar results were reported elsewhere (Mahadevan and Hall 1992; Madhusudhan et al. 2008). The supernatant obtained was subjected to ATPE for further purification and concentration of the enzyme.
Selection of Type of Aqueous Two-Phase System
Using crude extract, both polymer–polymer and polymer–salt phase systems were employed in the initial extraction experiments. The results have indicated almost same purification in both systems. However, the polymer–salt systems are advantageous compared to polymer–polymer phase systems due to the low viscosity and shorter time for phase separation. Hence, polymer–salt system was selected for the studies.
Selection of Phase Forming Salt
In order to identify the most suitable salt for the purification of LOX, ATPE experiments were carried out employing phase system of PEG (MW 6,000) with different phase forming salts such as sodium sulfate, potassium phosphate, sodium citrate, magnesium sulfate, and ammonium sulfate. The results are shown in Table 1. It can be observed from the Table 1 that LOX partitioned selectively to the bottom phase in all the phase systems studied. Different salts studied with PEG 6,000 at the same composition have resulted in different pH (Table 1). The ammonium sulfate phase system resulted in higher purity (1.57-fold) as well as activity recovery (94%) when compared to other salts. Further, the LOX showed higher stability in case of ammonium sulfate compared to control (crude) as shown in Fig. 2. This confirms the biocompatible environment provided by aqueous two-phase system. Hence, PEG/ammonium sulfate system was selected for further studies.
Selection of Polymer Molecular Weight
Partition studies were carried out employing PEG/ammonium sulfate systems with different molecular weights of PEG (1,500, 4,000, 6,000, 20,000), and the results are presented in Table 2. Phase volume ratio, temperature, and pH of the system were kept constant at 1.0, 25 ± 2 °C, and 5.2, respectively. The specific activity of the enzyme in the bottom phase increased with an increase in the PEG molecular weight from 1,500 to 6,000 (p < 0.05). The free volume in the polymer (top) phase significantly decreases with an increase in the molecular weight of the polymer (PEG). As a result, the biomolecules selectively partitioned to the bottom phase due to volume exclusion effect. Further increase in molecular weight of PEG to 20,000 decreased the specific activity. It can be attributed to higher hydrophobicity of the polymer-rich phase. As a result, even the contaminating proteins partitions to the bottom phase, decreasing the specific activity as well as the purity (Babu et al. 2008; Nandini and Rastogi 2008).
Maximum specific activity (208 U/mg) was observed in the bottom phase of PEG 6,000/ammonium sulfate system with minimum activity in the top phase (Fig. 3). Hence, PEG 6000 was selected to study the effect of TLL. In general, recovery of the target protein is easier from the bottom (salt-rich) phase than from the top (polymer-rich) phase.
Effect of Tie Line Length
Experiments were carried out at four different TLL for PEG/ammonium sulfate system, and the results are shown in Table 3. An increase in the yield of LOX was observed with an increase in TLL, and a maximum yield of 97% was obtained at TLL 41.23% with an increase in purity from 0.86- to 1.89-fold (p < 0.05). Further increase in TLL (50.65%) resulted in a decrease in yield of LOX from 97% to 92%. This could be due to the increase in concentration of salt in the bottom phase at higher TLL, which effectively salts out the biomolecules as their solubility limits are reached. At this juncture, the protein precipitation was observed at the interphase. This indicates that the top phase has also reached the solubility limit. In general, salting out effect is observed in PEG/salt system with an increasing TLL, resulting in shift in the partitioning of proteins from salt-rich to polymer-rich phase (Rito-Palomares 2005). However, in the present study, as the PEG (top) phase also reaching the saturation limit with respect to solubility of LOX, precipitation was observed at the interphase. As a result, decrease in activity recovery as well as specific activities were observed at higher TLL. Hence, middle TLL (41.23%) was selected for further study.
Effect of Volume Ratio
In ATPE, the degree of purification and concentration of a given biomolecule can be varied by changing the volume ratio (v r) along the given TLL. In order to examine the effect of volume ratio, six different volume ratios were selected on a given TLL of 41.23%, and the results are shown in Table 4. An increase in purification fold (2.38), as well as activity recovery (125%), was observed with an increase in volume ratio from 0.5 to 1.7 (p < 0.05). With an increase in volume ratio, the volume of the bottom phase decreases. Due to the preferential partitioning of LOX to the salt-rich phase, the purity of the enzyme increases in the bottom phase with a decrease in its volume. At the same time, due to the reduction in volume of the bottom phase, the enzyme is getting concentrated resulting in high-activity recovery. However, with further increase in the volume ratio (1.7 to 4.75) activity recovery decreased (125% to 50%) while the purity remained almost the same. This could be attributed to the reduction in the total free volume in the bottom phase with a decrease in its volume resulting in precipitation of the enzyme at the interphase. At the same time, the purity of the enzyme did not get significantly affected as only the target enzyme is selectively (rather than contaminant proteins) partitioning to the bottom phase. Hence, at the phase volume ratio of 1.7, maximum specific activity (314.16 U/mg) was observed with 125% activity recovery in the bottom phase.
The higher activity recovery obtained (125%) in the present study can be attributed to the interaction of sulfate anions with various charged groups on a protein molecule, thereby enhancing solubility of the protein. Further, it is known that ammonium sulfate at low concentrations interacts with protein ionically/molecularly and prevents denaturation of the enzyme. Thus, the increased activity recovery could be attributed especially to the enhancement of solubility and removal of other contaminating proteins. Further, the high-enzyme activity recovery can be also due to the elimination of inhibitors from the extracting (bottom) phase during the purification process especially at standardized composition of the phase system (Mayerhoff et al. 2004).
Similar enhancement in enzymatic activity have been reported for liquid–liquid extractions of enzymes employing aqueous two-phase systems (Porto et al. 2008; Cavalcanti et al. 2006; Babu et al. 2008), reverse micelles (Cortez et al. 2001; Umesh Hebbar et al. 2008), and three-phase partitioning (Narayan et al. 2008)
Thus, precipitation using PEG followed by ATPE at standardized conditions resulted in the maximum recovery of 125% and purification factor of 4.38-fold. It may be noted that the purification of LOX from soybean has been attempted for the first time employing ATPE in combination with precipitation.
Recovery of Lipoxygenase
After the completion of ATPE, the bottom phase containing LOX was dialyzed against distilled water for the removal of salt. The obtained solution was analyzed for enzyme activity that showed 90% activity recovery, and the purity almost remained the same (4.4-fold).
SDS-PAGE
The purity of the LOX obtained from ATPE was confirmed using SDS-PAGE, and the profile is shown in the Fig. 4. From the SDS-PAGE (lane 4), it can be observed that the number of bands have been reduced after ATPE as compared to crude extract (lane 2), which indicates the purification of LOX.
Conclusions
Precipitation with nonionic polymer (PEG) followed by aqueous two-phase extraction could be effectively employed for the purification of lipoxygenase from soybean extract for the first time. PEG 6,000/ammonium sulfate system was found to be the most suitable aqueous two-phase system for the extraction and purification of LOX. The standardized conditions of tie line length 41.25%, phase volume ratio of 1.7 resulted in maximum activity recovery of 125%, and purity of 4.38-fold compared to crude extract.
References
Agrawal, Y. C., Seth, S., Ghosh, P. K., & Jayas, D. S. (2008). Effect of moisture content on the quality of soybean oil and meal extracted by isopropyl alcohol & hexane. Food Bioprocess Technology. doi:10.1007/s11947-008-0058-x.
Albertsson, P. A. (1986). Partition of cell particles and macromolecules (3rd ed., pp. 303–313). New York: Wiley.
Axelrod, B., Cheesebrough, T. M., & Laasko, S. (1981). Lipoxygenase from soybeans. Methods in Enzymology, 71, 441–451.
Babu, B. R., Rastogi, N. K., & Raghavarao, K. S. M. S. (2008). Liquid–liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chemical Engineering and Processing, 47, 83–89.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Cavalcanti, M. T. H., Porto, T. S., Neto, B. B., Lima-Filho, J. L., Porto, A. L. F., & Pessoa, A., Jr. (2006). Aqueous two-phase systems extraction of α-toxin from Clostridium perfringens type A. Journal of Chromatography B, 833, 35–140.
Cole, K. D. (1993). Separation of Lipoxygenase and the major soybean proteins using Aqueous two phase extraction and poly ethylene glycol precipitation systems. Journal of Agriculture and Food Chemistry, 41, 334–340.
Cortez, E. V., Felipe, M. G. A., Roberto, J. C., Pessoa, J. R. A., & Vitolo, M. (2001). Extraction by revesed micelles of the intracellular enzyme xylose reductase. Applied Biochemistry and Biotechnology, 91/93, 753–759.
Diamond, A. D., & Hsu, J. T. (1992). Aqueous two-phase systems for biomolecules separation. Advances in Biochemical Engineering Biotechnology, 47, 89–135.
Fox, B. G. (1998). Catalysis by non-heme iron. In M. Sinnott (Ed.), Comprehensive biological catalysis (Vol 3) (pp. 262–278). London: Academic.
Gardner, H. W. (1996). Lipoxygenase as a versatile biocatalyst. Journal of American Oil Chemical Society, 73, 1347–1357.
Madhusudhan, M. C., Raghavarao, K. S. M. S., & Nene, S. (2008). Integrated process for extraction and purification of alcohol dehydrogenase from Baker’s yeast involving precipitation and aqueous two-phase extraction. Biochemical Engineering Journal, 38, 414–420.
Mahadevan, H., & Hall, C. K. (1992). Theory of precipitation of protein mixtures by nonionic polymer. AIChE Journal, 38(4), 573–591.
Mayerhoff, D. V. Z. L., Roberto, C., & Franco, T. T. (2004). Purification of xylose reductase from Candida mogii in aqueous two-phase systems. Biochemical Engineering Journal, 18, 217–223.
Menoncin, S., Domingues, N. M., Freire, D. M. G., Toniazzo, G., Cansian, R. L., & Oliveira, J. V. (2008). Study of the extraction, concentration and partial characterization of lipases obtained from Penicillium verrucosum using solid-state fermentation of soybean bran. Food Bioprocess Technology.. doi:10.1007/s11947-008-0104-8.
Nandini, K. E., & Rastogi, N. K. (2008). Liquid-liquid extraction of lipase using Aqueous two- phase system. Food Bioprocess Technology. doi:10.1007/s 11947-008-0160-0.
Narayan, A. V., Madhusudhan, M. C., & Raghavarao, K. S. M. S. (2008). Extraction and purification of ipomoea peroxidase employing three-phase partitioning. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-008-8185-4.
Oberoi, H. S., Chavan, Y., Bansal, S., & Dhillon, G. S. (2008). Production of cellulases through solid-state fermentation using kinnow pulp as a major substrate. Food Bioprocess Technology. doi:10.1007/s11947-008-0092-8.
Porto, T. S., Medeiros e Silva, G. M., Porto, C. S., Cavalcanti, M. T. H., Neto, B. B., Lima-Filho, J. L., et al. (2008). Liquid–liquid extraction of proteases from fermented broth by PEG/citrate aqueous two-phase system. Chemical Engineering Process, 47, 716–721.
Raghavarao, K. S. M. S., Rastogi, N. K., Gowthaman, M. K., & Karanth, N. G. (1995). Aqueous two-phase extraction for downstream processing of enzymes/ proteins. Advances in Applied Microbiology, 41, 97–171.
Rito-Palomares, M. (2005). Practical application of aqueous two-phase partition to process development for the recovery of biological products. Journal of Chromatography B, 807, 3–11.
Silva, M. E., & Franco, T. T. (2000). Purification of soybean peroxidase (Glycine max) by metal affinity partitioning in aqueous two-phase systems. Journal of Chromatography B: Biomedical Sciences and Applications, 743(1–2), 287–294.
Smith, A. K., & Circle, S. J. (1972). Chemical composition of the seed. In A. K. Smith (Ed.), Soybeans: chemistry & technology, (Vol 1) (pp. 61–92). West Port: AVI.
Sudharshan, E., & Appu Rao, A. G. (1997). Rapid method to separate the domains of soybean lipoxygenase-1: identification of the inter domain interactions. FEBS Letters, 406, 184–188.
Tukel, S., Yildrim, D., Bilgin, R., & Yerebilgic, G. (2005). Purification of soybean lipoxygenase. FEBS Journal, 272(S1), 11–41.
Umesh Hebbar, H., Sumana, B., & Raghavarao, K. S. M. S. (2008). Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresource Technology, 99, 4896–4902.
Vaidya, B. K., Suthat, H. K., Kasture, S., & Nene, S. (2006). Purification of potato polyphenol oxidase (PPO) by partitioning in aqueous two-phase system. Biochemical Engineering Journal, 28, 161–166.
Walter, H. (1969). Modern separation methods of macromolecules and particles. New York: Wiley.
Zaslavsky, B. Y. (1995). Aqueous two phase partitioning. Physical chemistry and bioanalytical applications (pp. 641–667). New York: Marcel Dekker.
Acknowledgements
Authors thank Dr. V. Prakash, Director, CFTRI, Mysore, for his encouragement and keen interest in the area of downstream processing. MCL and MCM gratefully acknowledge CSIR, Government of India, for the Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakshmi, M.C., Madhusudhan, M.C. & Raghavarao, K.S.M.S. Extraction and Purification of Lipoxygenase from Soybean Using Aqueous Two-Phase System. Food Bioprocess Technol 5, 193–199 (2012). https://doi.org/10.1007/s11947-009-0278-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-009-0278-8