Abstract
The effect of pectin surface density (ρ s) on the engineering properties of high methoxyl (HM) pectin-based edible films was determined in order to explore the role of ρ s on structure and functional properties. Films at different ρ s values (2.5, 3.2, 3.8, 4.5, 5.1, 5.8 mg cm−2) were analyzed by means of microscopy, thermal, mechanical, and barrier (water vapor permeability WVP, oxygen permeability \( {\text{kP}}_{{{\text{O}}_2 }} \), carbon dioxide permeability \( {\text{kP}}_{{{\text{CO}}_2 }} \)) properties. Microscopy, thermal, and mechanical results showed that by increasing ρ s from 2.5 to 5.8 mg cm−2, the film structure does not change. HM pectin-based film has a tensile strength of 20 ± 7 MPa and an elastic modulus (E) equal to 2,400 ± 200 MPa. However, it is quite brittle as the elongation to break (e) is close to 1%. Although the film structure was unaffected by ρ s, WVP increased with the rise in ρ s while \( {\text{kP}}_{{{\text{O}}_2 }} \) and \( {\text{kP}}_{{{\text{CO}}_2 }} \) decreased. On the whole, HM pectin-based film showed barrier properties comparable to biodegradable commercial film and low selectivity.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent interest in biopolymer films and coatings from polysaccharide, protein, and lipid materials has increased due to their ability to extend food shelf life. Indeed, almost every food suffers from at least one mass transfer problem, whether it is moisture migration, oxygen inclusion, aroma loss or gain, or oil migration. Thus, edible films or coatings can be placed either on the surface of the food or between components of multi-component food products to reduce water vapor, oxygen, lipid, and flavor migration or to stabilize water activity gradients and hence preserve their different textural properties.
A number of polysaccharides, including alginate, k-carrageenan, chitosan, cellulose derivates, plant gum, starch, and pectin, have been used as base materials for preparing edible films (Coffin and Fishman 1993; Sriamornsak and Kennedy 2006; Villalobos et al. 2005; Di Pierro et al. 2007; Giosafatto et al. 2007). In general, they form moderately resistant films, and their barrier properties against oxygen and organic vapor, such as volatile aromatic compounds, are good under low relative humidity (RH) conditions (<50%)(Sothornvit and Pitak 2007; Garcia et al. 2000; Miller and Krochta 1997). In contrast, owing to the inherently hydrophilic nature of polysaccharides and to the considerable amount of hydrophilic plasticizers incorporated into the films (Chillo et al. 2008; Kester and Fennema 1986; Guilbert 1986; Nisperos-Carriedo 1994), their water vapor barriers are poor, but can be enhanced by including wax or other lipid materials in their formulation (Gennadios et al. 1994; Garcia et al. 2000; Maftoonazad et al. 2007). Furthermore, the functionality of the film is affected by several factors such as polymer structure, solvent and other factors related to film dissolution, permeability, and diffusion properties (Chen 1994; Banerjee and Chen 1995; Krochta and De Mulder-Johnston 1997; Park et al. 2001; Gennadios 2002).
Pectin is an anionic polysaccharide mostly derived from citrus fruits. Its backbone is composed of α-(1,4) d-galactopyranosyluronic acid units with some (1,2)-linked l-rhamnopyranosyl units (Voragen et al. 1995). It is generally classified by the extent of methylesterification as high methoxyl pectin if half or more of the carboxyl groups are esterified or as low methoxyl pectin if less than half of the carboxyl groups are esterified (Iijima et al. 2000). As reported by Coffin and Fishman (1993), a number of studies have been carried out on pectin films, dating mostly from the 1930s to the 1950s, focusing on derivatized pectins and the use of polyvalent cations such as calcium. In more recent years, although the pectin (mainly low methoxyl pectin) has been studied for its ability to form gel and film (Lootens et al. 2003; Löfgren and Hermansson 2007), almost all researches has been focused on the influence of process parameters on the gel properties (Walkinshaw and Arnott 1981; Clark et al. 1994; Clark and Farrer 1996) and on the properties of pectin-based blends obtained with other polysaccharides or protein (Coffin and Fishman 1993; Fishman and Coffin 1998; Fishman et al. 2000, 2004; Di Pierro et al. 2005).
Pectin and pectin/starch films all exhibited high initial modulus values, but had low elongations to break (1–3%) and were fairly brittle. However, the addition of a plasticizer resulted in a very definite loss of brittleness of the film, making them much more flexible. Moreover, pectin can partially replace industrial film fabricated from poly(vinyl alcohol): They are miscible in all proportions, and together, they form films which also have excellent mechanical properties (Fishman and Coffin 1998).
In order to gain a better understanding of relationships between film-forming constituents and film properties, Maftoonazad et al. (2007) studied the effect of pectin, sorbitol and beeswax concentration on film barrier (WVP), and mechanical and optical properties. From the results, they concluded that film components had a marked influence on film properties. In particular, pectin and sorbitol concentrations had a significant effect on WVP and mechanical properties. The beeswax concentration influenced WVP, mechanical properties, and opacity significantly.
In a previous work (Giancone et al. 2008) investigating the rheological properties of HM pectin-based films made with different ρ s, it was found that a ρ s increase does not affect network formation. However, no information was collected on film functional properties. In order to understand how this result affects the functional properties of HM pectin films, the film engineering properties at different ρ s values were studied. In particular, the objectives of the work were to study the influence of ρ s values on film microstructure, solubility, thermal, and mechanical and barrier properties (WVTR, \( {\text{kP}}_{{{\text{O}}_2 }} \), \( {\text{kP}}_{{{\text{CO}}_2 }} \)).
Materials and Methods
Materials
Pectin from citrus fruits (galacturonic acid content 93.5%; methoxyl content 9.4%; dry matter 55.3%; pK a = 3.0–4.5) was purchased from Sigma Chemical (St. Louis, MO, USA). All other chemicals were of analytical grade.
Film-Making Procedure
To prepare pectin film, pectin was dissolved in deionized water at a level of 16 g dm−3 at pH 2. The pectin solution concentration was optimized in a previous work (Giancone et al. 2009). Prior to film casting, the solution was de-aerated under vacuum to prevent pinhole formation and then poured on to leveled 56.7-cm2 polystyrene Petri dishes. Appropriate volumes of solution were used to vary the pectin surface density (ρ s) between 2.5 and 5.8 mg cm−2 (2.5, 3.2, 3.8, 4.5, 5.1, 5.8 mg cm−2). The pectin surface density was calculated as:
Where C is the pectin solution concentration (g dm−3), V is the volume of the solution poured into the Petri dish (dm−3), and A is the surface area of the Petri dish (cm2).
All film-forming solutions were allowed to dry at 37°C and 50% RH overnight under air circulation. The dried films were peeled from the Petri dishes and stored at 20°C in a desiccator at 50% RH for at least 48 h.
Film Thickness Measurement
Film thickness was measured using a micrometer model HO62 with a sensitivity of ±2 µm (Metrocontrol Srl, Casoria, NA, Italy). Film strips were placed between the jaws of the micrometer, and the gap reduced until the instrument fell in contact with the film. Mean thickness (µm) of films was determined by averaging ten measurements at different locations.
Scanning Electron Microscopy Analysis
Microstructural characteristics of film samples at three different pectin surface densities (2.5, 3.8, 5.8 mg cm−2) were examined using an LEO EVO 40 scanning electron microscope (Zeiss, Oberkochen, Germany). All film samples were dried in a desiccator containing lithium chloride (a w = 0.113 ± 0.003) and then manually fragmented. Dried strip fragments of films were mounted on specimen stubs with the cross-section oriented up and coated with a thin layer of gold by a DC sputter coater (AGAR B7340, Agar Scientific, Stansted, UK). Digital images of film cross-section were collected at a tilt angle of 0° to the electron beam using an acceleration voltage of 20 kV.
Film Solubility
Film solubility was tested with a procedure similar to that described by Stuchell and Krochta (1994). Small pieces of films (20–25 mg) were dried at 70°C and 6.67 kPa in a vacuum oven for 24 h and then weighed to the nearest 0.0001 g to determine the initial dry weight of the film. Each film piece was incubated at 25°C for 24 h in a screw-top tube (150 × 15 mm) with 10 mL of 0.1 M acetate (pH 4.0), phosphate (pH 6.0), or Tris–HCl (pH 8.0) buffer solution. At the end of the incubation, the samples were poured onto Whatman no. 1 qualitative filter paper. The non-dissolved material, removed from the filter by using 10 mL of distilled water, was dried at 70°C and 50 Torr in a vacuum oven for 24 h and then weighed. The percentage of total soluble matter (TSM) was calculated as follows:
where dm is the dry matter, and subscripts i and f correspond to the initial and final dry matter. Tests were carried out in triplicate and averages are reported.
Thermogravimetric Analysis
Thermogravimetric analysis was performed with a thermobalance TGA 7 (Perkin-Elmer Norwalk, CT, USA). Edible films were cut using a sharp razor blade and placed in platinum sample pans. Weight loss as a function of temperature was monitored at the heating rate of 10°C/min, from room temperature to 500°C, in an inert atmosphere (20 mL/min N2).
Mechanical Analysis
Mechanical analysis was carried out at room temperature using an Instron Universal Testing Machine (mod. 4467 High Wycombe, UK) equipped with a 1,000 N load cell. Ten film specimens (10 mm × 80 mm strips) were cut using a sharp razor blade to prevent nicks and tears, conditioned at 20°C and 50% RH, and then mounted between the grips of the tester. The films were submitted to a uniaxial tensile test at 30 mm/min. Each test was carried out over a period of about 3 min to minimize exposure of the samples to the ambient environment. The test was considered valid when the film break occurred in the midpoint. By allowing for toe compensation, due to the take-up of slack, alignment, or seating of the specimen, to assess the corrected zero point on the strain or extension axis, tensile strength (TS) and percent elongation at break (e%), as well as elastic modulus (E), were calculated according to ASTM (2001).
Permeability Analysis
Water vapor permeability (WVP) of films was evaluated by a gravimetric test according to ASTM (1993) by means of a Fisher/Payne permeability cup (Carlo Erba, Italy). Three grams of silica gel was introduced into each cup. The film samples with a diameter of about 6 cm were placed on top of the cups and sealed by means of a top ring kept in place by three tight clamps. The film area exposed to vapor transmission was 10 cm2. The cups containing silica gel were weighed and then placed in a desiccator containing a saturated KCl solution, which provided a constant water activity of 0.8434 at 25°C. The desiccator was stored in a Heareus thermostated incubator (Binder KBF240, Turin, Italy) at 25.0 (±0.1°C). Cups were weighed at scheduled times, and the amount of water vapor transmission rate through the film was estimated by the linear portion of the diagram obtained by plotting the weight increment of the cup as a function of time. It was assumed that the steady state was reached once the regression analysis made by using the last four data points resulted in R 2 ≥ 0.998. The WVP was calculated from the equation
where dm/dt is the slope of the cup weight versus time curve once steady state was reached, X is the film thickness, A is the film exposed area, and Δp is the water vapor pressure across the film. Assuming that the vapor pressure inside the cup, due to the presence of silica gel, may be equal to zero, Δp becomes equal to the vapor pressure inside the desiccator and is calculated by multiplying water activity by the water tension (P 0) at 25°C (P 0 = 3.167 kPa).
Permeability of films to oxygen (\( {\text{kP}}_{{{\text{O}}_2 }} \)) and carbon dioxide (\( {\text{kP}}_{{{\text{CO}}_2 }} \)) were examined at 30°C by using a modified manometric standard method according to Di Pierro et al. (2005). The tests were performed at 51.4%RH and a ∆P of 100 kPa for each gas. Ten independent tests per film were performed.
Data Analysis
To assess the effect of pectin surface density (ρ s) on the functional properties of pectin-based films, six ρ s levels (2.5, 3.2, 3.8, 4.5, 5.1, 5.8 mg cm−2) were tested, each level being replicated three times. The reliance of ρ s on film functional properties was assessed by ANOVA analysis by using SPSS 13.0 for window (SPSS, Milan, Italy). Duncan’s test was carried out to find the source of the significant differences within the samples examined. Significance of differences was defined at p ≤ 0.05.
Results and Discussion
Thickness and Microscopy Analysis
In measuring film thickness (h F) against pectin surface density (ρ s), our results showed that when ρ s increases, the thickness increases (p < 0.001, F = 208) but, as may be observed, film thickness does not vary linearly with ρ s (Fig. 1). Because in a previous work (Giancone et al. 2008) we demonstrated that on increasing the ρ s the number of cross-links does not change, this result suggests that by increasing the ρ s, a denser structure is obtained due to the higher pectin concentration. This hypothesis was indeed supported by our microscopy results (Fig. 2). Pectin films seem to be characterized by the absence of a homogeneous structure that appears to be the result of pectin clusters packed more or less tightly. Moreover, it may be observed that by varying the ρ s the microstructure of the film does not change in agreement with the results reported in a previous work (Giancone et al. 2008).
Film Solubility
TSM is a measure of water resistance and the integrity of film. ANOVA analysis of TSM data obtained at different pH from films made at different ρ s (Table 1) showed that the effect of ρ s and pH was statistically significant (p < 0.001; F 5,54 = 29; p < 0.001, F 2,54 = 395, respectively) on film TSM (%), whereas the effect of the interaction between ρ s and pH was not statistically significant (p = 0.06). In Table 1, the results of Duncan’s test are also reported: Film solubility was negatively related to ρ s for all pH tested. This confirms the hypothesis that by increasing ρ s, the film becomes denser. To test film solubility, samples of constant weight were prepared. The difference in density between the samples explains the different solubility: by increasing film density, at constant weight, the contact surface is reduced and the film thickness is increased; thus, it becomes more difficult for water to penetrate samples. Moreover, results showed that film solubility was greater at pH 4.0 and 6.0 than at pH 8.0. The lower solubility observed at pH 8.0 can be explained by the stabilization of the network due to the formation of hydrogen bonds between the hydrogen of methoxyl groups and the dissociated carboxyl groups according to the model proposed by Okenfull (1991).
Thermogravimetric Analysis
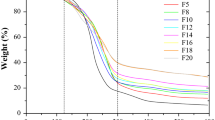
The films formulated with different ρ s (from 2.5 to 5.8 mg cm−2) were submitted to thermogravimetric analysis to determine the sample moisture content and the film decomposition temperature. As the films were submitted to heating, weight loss (WL) was observed as a function of temperature, T (Fig. 3). Curves present the same two-step behavior: the first, between 30°C and 150°C, depends on water loss; the second, above 250°C, is determined by thermal degradation of the samples. No effect of surface density was observed (p > 0.05), implying no difference in the structure of samples. The same behavior was observed by Maior et al. (2008) who studied unmodified and modified low methoxyl (LM) pectin. By calculating the derivative of weight (%) as a function of temperature, it was found that the highest rate of water loss (~2%/min) occurs at 62–65°C, whereas pectin degradation occurs at temperatures of around 260°C (Td). Moisture content, calculated at 200°C, was around 12–13% for all samples.
Mechanical Analysis
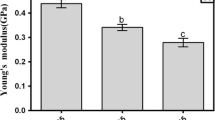
As shown by the mechanical properties of HM pectin film at three different ρ s (Fig. 4), no significant variation in elastic modulus (E), TS, or elongation to break (e%) were observed as the ρ s increased (p > 0.05). These results confirm that on increasing the ρ s, link density does not change but only a more tightly packed structure is obtained. It may be explained by the fact that during drying of the film solution, moisture evaporates, allowing the formation of a polysaccharide network, and during this stage, the proximity of pectin chains is favored by higher pectin surface density, thus facilitating the formation of a more compact matrix. TS is a measure of integrity and heavy-duty use potential of films (Gennadios et al. 1994), and E represents the intrinsic stiffness of the film. HM pectin-based film had a TS of about 20 ± 7 MPa and an E of 2,400 ± 200 MPa. However, pectin films were quite brittle as the elongation to break (e) was close to 1%.
On the whole, the mechanical properties of pectin-based film were of the same order of magnitude of those pertaining to several edible polysaccharide films. On this point, Fishman and Coffin (1998) reported that HM pectin-based edible film had initial modulus of about 1,500 MPa and elongation to break close to 1%. Di Pierro et al. (2006) reported that chitosan films, prepared in the presence of glycerol, have an elastic modulus equal to 1,200 MPa, tensile stress at break of 14 MPa, and elongation to break of 23%. Starch-based edible film is characterized by poorer mechanical properties than pectin- or chitosan-based edible films as reported by Gaudin et al. (1999). The latter found that starch film had a maximum stress of 50 MPa and an elongation to break equal to 9%. In conclusion, HM pectin forms self-supported film with good mechanical properties but of extreme brittleness. Thus, addition of plasticizer is recommended to improve film performance, though it can have a detrimental effect on the film barrier function. From the present results, it can be concluded that ρ s does not contribute to changing the mechanical properties of film.
Permeability Analysis
One of the primary functions of an edible film or coating is to restrict the moisture transfer between the food and the surrounding atmosphere or between two components of a heterogeneous food product. As regards to that, the WVP of HM pectin-based films at different ρ s was influenced by ρ s, increasing from 18 to 54 pg m−1 s−1 Pa−1 as ρ s increased from 2.5 to 5.8 mg cm−2 (p < 0.001, F = 19; Fig. 5). Duncan’s test results are also plotted (Fig. 5), showing that the main significant difference within samples occurs between films at ρ s values of 2.5 and 4.5 mg cm−2 and between films at ρ s of 4.5 and 5.8 mg cm−2. An increase in WVP as ρ s increases was also reported by Maftoonazad et al. (2007). This effect could be attributed to the higher number of free hydroxyl groups, enhancing interaction with water and favoring water vapor transmission through the films (Miller and Krochta 1997). Similar behavior was previously reported for chitosan–whey protein films (Di Pierro et al. 2006), for chitosan–ovoalbumin films (Di Pierro et al. 2007), and for LM pectin modified film (Maior et al. 2008).
a Water vapor permeability (WVP), b oxygen and carbon dioxide permeability (\( {\text{kP}}_{{{\text{O}}_2 }} \) (open squares), \( {\text{kP}}_{{{\text{CO}}_2 }} \) (filled squares)) of HM pectin-based films at different pectin surface densities (ρs). Means with different letters are significantly different (upper letters refer to \( {\text{kP}}_{{{\text{O}}_2 }} \) and lower letters to \( {\text{kP}}_{{{\text{CO}}_2 }} \); Duncan test, p ≤ 0.05)
Actually, by increasing the ρ s, as reported in Fig. 1, the thickness of the films increased. Thus, a positive correlation was observed between WVP and thickness. Similar results were observed by Banker (1966) who reported that the water vapor permeability of hydrophilic films increased linearly with film thickness. Theoretically, permeability is independent of film thickness but several explanations have been provided for such an anomalous thickness effect. McHugh et al. (1993) observed that, as film thickness increased, the film provided an increased resistance to mass transfer across it; consequently, the equilibrium water vapor partial pressure at the inner film surface increased. This caused an increase in permeability due to the higher RH gradient between film and environment. Recently, Bertuzzi et al. (2007) investigated the effect of thickness and relative humidity on high amylose corn starch film WVP and showed that when a w > 0.60, the increase in film thickness is significantly dependent upon relative humidity and original film thickness. Thus, under high relative humidity, high amylose corn starch film, full of almost liquid water, offers high water molecule mobility resulting in a sharp increase in the rate of diffusion. Since in this work the WVP was determined by ASTM methods, which entails the presence of high relative humidity conditions on one side of the film, the effect of swelling can explain the anomalous effect of thickness on WVP. Further investigation will be carried out using Permatram-W methods, where the a w on one side of the film can be set between 0.3 and 1, to better understand whether pectin surface density has a significant effect on WVP. On the whole, the WVP of HM pectin-based edible films at low ρ s is of the same order of magnitude as biodegradable films (Mater-Bi) which, as reported in a previous work (Di Pierro et al. 2006), have a WVP equal to 22.6 pg m−1 s−1 Pa−1. With respect to other polysaccharides or protein-based edible films, HM pectin-based edible films showed approximately the same WVP as sodium caseinate–starch film (Arvanitoyannis et al. 1996), edible film made of natural resources (Psomiadou et al. 1996), chitosan-based edible films (Di Pierro et al. 2006, 2007; Chillo et al. 2008), and caseinate-based edible films (Bruno et al. 2008) and almost two orders of magnitude lower than the WVP of starch-based edible films (Bertuzzi et al. 2007; Flores et al. 2007; Garcia et al. 2000) and WPI films (Reinoso et al. 2008). Moreover, the WVP of HM pectin-based films was of the same order of magnitude as that (66–90 pgm−1 s−1 Pa−1) of quite hydrophobic films such as the composite chitosan and metylcellulose films (García et al. 2004), but about two orders of magnitude greater than that (0.55 or 0.23 pg m−1 s−1 Pa−1) of low- or high-density polyethylene (LDPE or HDPE) films, respectively (Siew et al. 1999).
Figure 5b shows the effect of ρ s on \( {\text{kP}}_{{{\text{O}}_2 }} \) and \( {\text{kP}}_{{{\text{CO}}_2 }} \). Contrasting with WVP, \( {\text{kP}}_{{{\text{O}}_2 }} \) and \( {\text{kP}}_{{{\text{CO}}_2 }} \) are negatively correlated with ρ s, with a statistically significant effect of ρ s (p < 0.001, F = 147; p < 0.001, F = 315, respectively). The reduction in film permeability to O2 and CO2 as the ρ s increased can be explained by the occurrence of free volume reduction due to more compact microstructure of the films at higher ρ s (Miller and Krochta 1997; Arvanitoyannis et al. 1997). More investigation on the relation between gas permeability and film crystallinity could help a better interpretation of the reported results (Arvanitoyannis et al. 1996, 1997; Psomiadou et al. 1996). The barrier properties of HM pectin films against O2 and CO2 at high ρ s are of the same orders as magnitude of LDPE (21 and 94 pg m−1 s−1 Pa−1, respectively, for O2 and CO2) films (Garcia et al. 2000), but one order of magnitude higher than that of Mater-bi biodegradable films (\( {\text{kP}}_{{{\text{O}}_2 }} \) = 8 pg m−1 s−1 Pa−1) and about two orders of magnitude greater than that of HDPE films (0.7 and 1.9 pg m−1 s−1 Pa−1, respectively, for O2 and CO2). What appears to be in contrast with respect to some edible films and with respect to almost all synthetic films is the HM-based edible film selectivity (\( {\text{kP}}_{{{\text{CO}}_2 }} \)/\( {\text{kP}}_{{{\text{O}}_2 }} \)). In fact, as may be seen from Fig. 5b, \( {\text{kP}}_{{{\text{CO}}_2 }} \) and \( {\text{kP}}_{{{\text{O}}_2 }} \) are almost equal, with a selectivity lower than one. Similar results were also obtained for chitosan–whey protein film (Di Pierro et al. 2006). Packaging film with a selectivity equal to one is required to package fresh-cut produce (with a respiratory quotient equal to one) by using equilibrium-modified atmosphere packaging technology (Torrieri et al. 2009). Thus, HM pectin-based films can be desirably used as packaging material or as coating for fresh-cut produce.
Conclusion
In this work, the engineering properties of HM pectin-based film were studied to better understand the role of pectin surface densities on film structure and functional properties. HM pectin-based edible films at different ρ s showed similar thermal and mechanical properties, confirming that the film structure does not change as ρ s increases. HM pectin-based films were found to have a tensile strength of the order of 20 ± 7 MPa, and an elastic modulus of 2,400 ± 200 MPa. However, they are quite brittle as the elongation to break (e) is close to 1%. Although film structure was unaffected by ρ s, barrier properties change according to ρ s. In particular, water vapor permeability increased as ρ s increased and \( {\text{kP}}_{{{\text{O}}_2 }} \) and \( {\text{kP}}_{{{\text{CO}}_2 }} \) decreased as ρ s increased. Although the detrimental effect of ρ s on WVP may be explained by the hydrophilic nature of pectin, further investigation has to be performed in order to better understand the role of a w on WVP. On the whole, HM pectin-based film showed barrier properties comparable to biodegradable commercial film and a low selectivity that may be promising for their application to equilibrium modified atmosphere packaging of fresh-cut produce.
References
Arvanitoyannis, I., Psomiadou, E., & Nakayama, N. (1996). Edible films made from sodium caseinate, starches, sugar or glycerol, Part I. Carbohydrate Polymers, 31, 179–192. doi:10.1016/S0144-8617(96)00123-3.
Arvanitoyannis, I., Psomiadou, E., & Nakayama, N. (1997). Edible films made from gelatine, soluble starch and polyols. Part 3. Food Chemistry, 60(4), 593–604. doi:10.1016/S0308-8146(97)00038-1.
ASTM. (1993). E96-93: Annual book of ASTM standards. Philadelphia: American Society for Testing and Materials.
ASTM. (2001). D882-00: Annual book of ASTM standards. West Conshohocken: American Society for Testing and Materials.
Banerjee, R., & Chen, H. (1995). Functional properties of edible films using whey protein concentrate. Journal of Dairy Science, 78, 1673–1683.
Banker, G. S. (1966). Film coating theory and practice. Journal of Pharmaceutical Sciences, 55, 81–89. doi:10.1002/jps.2600550118.
Bertuzzi, M. A., Castro Vidaurre, E. F., Armada, M., & Gottifredi, J. C. (2007). Water vapour permeability of edible starch based films. Journal of Food Engineering, 80, 972–978. doi:10.1016/j.jfoodeng.2006.07.016.
Bruno, M., Giancone, T., Torrieri, E., Masi, P., & Moresi, M. (2008). Engineering properties of edible transglutaminase cross-linked caseinate-based films. Food and Bioprocess Technology, 1, 393–404. doi:10.1007/s11947-007-0031-0.
Chen, H. (1994). Functional properties and applications of edible films made of milk proteins. Journal of Dairy Science, 78, 2563–2583.
Chillo, S., Flores, S., Mastromatteo, M., Conte, A., Gerschenson, L., & Del Nobile, M. A. (2008). Influence of glycerol and chitosan on tapioca starch-based edible film properties. Journal of Food Engineering, 88, 159–168. doi:10.1016/j.jfoodeng.2008.02.002.
Clark, A. H., & Farrer, D. B. (1996). Shear modulus-concentration relationships for low DE pectin-calcium gels in the temperature range 20–85°C. Food Hydrocolloids, 10, 31–39.
Clark, A. H., Evans, K. T., & Farrer, D. B. (1994). Shear modulus-temperature meltdown profiles of gelatine and pectin gels. A cascade theory description. International Journal of Biological Macromolecules, 15, 125–130. doi:10.1016/0141-8130(94)90038-8.
Coffin, D. R., & Fishman, M. L. (1993). Viscoelastic properties of pectin/starch blends. Journal of Agricultural and Food Chemistry, 41, 1192–1197. doi:10.1021/jf00032a005.
Di Pierro, P., Mariniello, L., Giosafatto, C. V. L., Masi, P., & Porta, R. (2005). Solubility and permeability properties of edible pectin-soy flour film obtained in the absence or presence of transglutaminase. Food Biotechnology, 19, 37–49. doi:10.1081/FBT-200049059.
Di Pierro, P., Chico, B., Villalonga, R., Mariniello, L., Damiao, A. E., Masi, P., et al. (2006). Chitosan-whey protein edible films produced in the absence or presence of transglutaminase: Analysis of their mechanical and barrier properties. Biomacromolecules, 7, 744–749. doi:10.1021/bm050661u.
Di Pierro, P., Chico, B., Villalonga, R., Mariniello, L., Masi, P., & Porta, R. (2007). Transglutaminase-catalyzed preparation of chitosan–ovoalbumin films. Enzyme and Microbial Technology, 40, 437–441. doi:10.1016/j.enzmictec.2006.07.017.
Fishman, M. L., & Coffin, D. R. (1998). Mechanical, microstructural and solubility properties of pectin/poly (vinyl alcohol) blends. Carbohydrate Polymers, 35, 195–203. doi:10.1016/S0144-8617(97)00245-2.
Fishman, M. L., Coffin, D. R., Konstance, R. P., & Onwulata, C. I. (2000). Extrusion of pectin/starch blends plasticized with glycerol. Carbohydrate Polymers, 41, 317–325. doi:10.1016/S0144-8617(99)00117-4.
Fishman, M. L., Coffin, D. R., Onwulata, C. I., & Konstance, R. P. (2004). Extrusion of pectin and glycerol with various combinations of orange albedo and starch. Carbohydrate Polymers, 57, 401–413.
Flores, S., Fama, L., Rojas, A. M., Goyanes, S., & Gerschenson, L. (2007). Physical properties of tapioca-starch edible films: Influence of filmmaking and potassium sorbate. Food Research International, 40, 257–265. doi:10.1016/j.foodres.2006.02.004.
Garcia, M. A., Martino, M. N., & Zaritzky, N. E. (2000). Lipid addition to improve barrier properties of edible starch-based films and coatings. Journal of Food Science, 65(6), 941–947. doi:10.1111/j.1365-2621.2000.tb09397.x.
García, M. A., Pinotti, A., Martino, M. N., & Zaritzky, N. E. (2004). Microstructure, mechanical and barrier properties of composite chitosan and methylcellulose biofilms. Proceedings of the 9th International Conference on Engineering and Food (ICEF9), Montpellier, France, March 7–9, 2004.
Gaudin, S., Lourdin, D., Le Botlan, D., Forssell, P., Ilari, J. L., & Colonna, P. (1999). Effect of polymer-plasticizer interactions on the oxygen permeability of starch-sorbitol-water films. Macromolecular Symposia (Polymer–Solvent Complexes and Intercalates II), 138, 245–248.
Gennadios, A. (2002). Protein-based films and coating. Boca Raton: Dekker.
Gennadios, A., McHugh, T. H., Weller, C. L., & Krochta, J. M. (1994). Edible coatings and films based on proteins. In J. M. Krochta, E. A. Baldwin & M. Nisperos-Carriedo (Eds.), Edible coatings and films to improve food quality, Chap. 9, pp. 201–277. Lancaster: Technomic.
Giancone, T., Torrieri, E., Di Pierro, P., Mariniello, L., Moresi, M., Porta, R., et al. (2008). Role of constituents on the network formation of hydrocolloid edible films. Journal of Food Engineering, 89, 195–203. doi:10.1016/j.jfoodeng.2008.04.017.
Giancone, T., Torrieri, E., Masi, P., & Michon, C. (2009). Protein-polysaccharide interactions: Phase behaviour of pectin-soy flour mixture. Food Hydrocolloids, 23, 1263–1269. doi:10.1016/j.foodhyd.2008.09.001.
Giosafatto, C. V. L., Mariniello, L., & Ring, S. (2007). Extraction and characterization of Foeniculum vulgare pectins and their use for preparing biopolymer films in the presence of phaseolin protein. Journal of Agricultural and Food Chemistry, 55, 1237–1240. doi:10.1021/jf062725d.
Guilbert, S. (1986). Food packaging and preservation. London: Elsevier Applied Science.
Iijima, M., Nakamura, K., Hatakeyama, T., & Hatakwyama, H. (2000). Phase transition of pectin with sorbed water. Carbohydrate Polymers, 41, 101–106. doi:10.1016/S0144-8617(99)00116-2.
Kester, J. J., & Fennema, O. R. (1986). Edible films and coatings: A review. Food Technologist, 40, 47–59.
Krochta, J. M., & De Mulder-Johnston, C. (1997). Edible and biodegradable polymer films: Challenges and opportunities. Food Technologist, 51(2), 61–74.
Löfgren, C., & Hermansson, A. M. (2007). Synergistic rheological behaviour of mixed HM/LM pectin gels. Food Hydrocolloids, 21, 480–486. doi:10.1016/j.foodhyd.2006.07.005.
Lootens, D., Capel, F., Durand, D., Nicolai, T., Boulenguer, P., & Langendorff, V. (2003). Influence of pH, Ca concentration, temperature and amidation on the gelation of low methoxyl pectin. Food Hydrocolloids, 17, 237–244. doi:10.1016/S0268-005X(02)00056-5.
Maftoonazad, N., Ramaswamy, H. S., & Marcotte, M. (2007). Evaluation of factors affecting barrier, mechanical and optical properties of pectin-based film using response surface methodology. Journal of Food Process Engineering, 30, 539–563. doi:10.1111/j.1745-4530.2007.00123.x.
Maior, J. F. A. S., Reis, A. V., Muniz, E. C., & Cavalcanti, O. A. (2008). Reaction of pectin and glycidyl methacrylate and ulterior formation of free films by reticulation. International Journal of Pharmaceutics, 335, 184–194. doi:10.1016/j.ijpharm.2007.12.006.
McHugh, T. H., Avena-Bustillos, R., & Krochta, J. M. (1993). Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. Journal of Food Science, 58(4), 899–902. doi:10.1111/j.1365-2621.1993.tb09387.x.
Miller, K. S., & Krochta, J. M. (1997). Oxygen and aroma barrier properties of edible films: A review. Trends in Food Science & Technology, 8, 226–237.
Nisperos-Carriedo, M. O. (1994). Edible coating and films based on polysaccharides. In J. M. Krochta, E. A. Baldwin & M. O. Nisperos-Carriedo (Eds.), Edible coatings and films to improve food quality. Lancaster: Technomic.
Okenfull, D. G. (1991). The chemistry and technology of pectin. In R. H. Walter (Ed.), The chemistry and technology of pectin, pp. 87–108. New York: Academic.
Park, S. K., Rhee, C. O., Bae, D. H., & Hettiarachchy, N. S. (2001). Mechanical properties and water-vapor permeability of soy-protein films affected by calcium salts and glucono-δ-lactone. Journal of Agricultural and Food Chemistry, 49, 2308–2312. doi:10.1021/jf0007479.
Psomiadou, E., Arvanitoyannis, I., & Yamoto, N. (1996). Edible films made from natural resources; microcrystalline cellulose (MCC), methylcellulose (MC) and corn starch and polyols; part 2. Carbohydrate Polymers, 31, 194–204. doi:10.1016/S0144-8617(96)00077-X.
Reinoso, E., Mittal, G. S., & Lim, L. T. (2008). Influence of Whey protein coatings on Plum (Prunus Domestica L.) fruit quality. Food and Bioprocess Technology, 1, 314–325. doi:10.1007/s11947-007-0014-1.
Siew, D. C. W., Heilmann, C., Easteal, A. J., & Cooney, R. P. (1999). Solution and film properties of sodium caseinate/glycerol and sodium caseinate/polyethylene glycol edible coating systems. Journal of Agricultural and Food Chemistry, 47, 3432–3440. doi:10.1021/jf9806311.
Sothornvit, R., & Pitak, N. (2007). Oxygen permeability and mechanical properties of banana films. Food Research International, 40, 365–370. doi:10.1016/j.foodres.2006.10.010.
Sriamornsak, P., & Kennedy, R. A. (2006). A novel gel formation method, microstructure and mechanical properties of calcium polysaccharide gel films. International Journal of Pharmaceutics, 323, 72–80. doi:10.1016/j.ijpharm.2006.05.045.
Stuchell, Y. M., & Krochta, J. M. (1994). Enzymatic treatment and thermal effects on edible soy protein films. Journal of Food Science, 59, 1332–1337. doi:10.1111/j.1365-2621.1994.tb14709.x.
Torrieri, E., Mahajan, P. V., Cavella, S., De Sousa Gallagher, M., Oliveira, F. A. R., & Masi, P. (2009). Mathematical modelling of modified atmosphere package: an engineering approach to design packaging systems for fresh-cut produce. In P. J. Papajorgji, & P. M. Pardalos (Eds.), Advances in modelling agricultural systems (pp. 455–484). US: Springer.
Villalobos, R., Chanona, J., Hernández, P., Gutiérrez, G., & Chiralt, A. (2005). Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocolloids, 19, 53–61. doi:10.1016/j.foodhyd.2004.04.014.
Voragen, A. G. J., Pilnik, W., Thibault, J. F., Axelon, M. A. V., & Renard, C. M. G. C. (1995). Pectins. In A. M. Stephan (Ed.), Food polysaccharides and their functional applications, pp. 287–339. New York: Marcel Dekker.
Walkinshaw, M. D., & Arnott, S. (1981). Conformations and interactions of pectin. II. Models for junction zone in pectinic acid and calcium pectate gels. Journal of Molecular Biology, 53, 1075–1085. doi:10.1016/0022-2836(81)90468-X.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giancone, T., Torrieri, E., Di Pierro, P. et al. Effect of Surface Density on the Engineering Properties of High Methoxyl Pectin-Based Edible Films. Food Bioprocess Technol 4, 1228–1236 (2011). https://doi.org/10.1007/s11947-009-0208-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-009-0208-9