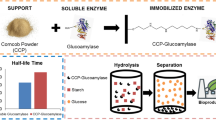
Abstract
This research assesses the bench-scale application of a non-conventional support, bone particles, for glucoamylase (GA) immobilization and its subsequent use in cassava starch hydrolysis. Upon determining the appropriate conditions to immobilize GA onto chicken bone particles, such as pH, ionic strength, particle size, and enzyme load, bench-scale immobilization of commercial GA without further purification was performed. Under the selected conditions, 270 GA units per gram of support were adsorbed. Optimal temperature and thermal stability of immobilized GA were only slightly different from those of the free enzyme, while optimal pH became more acidic by about one unit. The feasibility of the use of this immobilized biocatalyst for high glucose syrup production from liquefied cassava starch, at bench scale in batch process using a stirred-tank reactor, was demonstrated. Repeated use of the GA-bone derivative showed that similar conversions to those achieved with soluble enzyme (dextrose equivalent = 98) were reached until the third batch and over 90% until the 25th batch.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch constitutes the main carbohydrate reserve for all the higher plants. In the food industry, starch is used extensively to obtain hydrolysis products, especially sweeteners such as glucose, maltose, and fructose. Furthermore, nowadays, the higher cost of producing ethanol from biomass makes starch an important source of fermentable sugars (Bhargava et al. 2005).
The major sources of starch are cereal grains, legumes, roots, tubers, and some unripe fruits. Maize has been the main raw material for conversion of starch to sugars because of its high productivity (Schenck and Hebeda 1992). However, when compared with root starches, corn starch is not optimal for hydrolysis. The low content of lipids (0.1%) in cassava starch ensures that amylose–lipid complex formation be negligible; consequently, good liquefaction can be achieved at a lower temperature (Waliszweski et al. 1992), and retrogradation problems are less severe. An additional advantage of cassava starch is its low protein content (<0.1%; Swinkels 1985); consequently, less color is developed during hydrolysis, and refining requirements are reduced (Reeve 1992). All these factors make cassava starch a suitable raw material for producing syrups.
High glucose syrups are used mainly as a source of crystalline dextrose or as a substrate for fructose production. These glucose hydrolyzates are produced exclusively by enzymatic processes, using first heat-stable α-amylase to liquefy starch and glucoamylase for the subsequent conversion.
Industrial glucose production uses soluble GA, and enzyme concentration is selected so that the maximal conversion is achieved after 48–96 h, but soluble enzyme is lost in the product stream. Afterwards, it must be removed at additional cost or be destroyed at the end of each process, depending on its application (Atia et al. 2003). Enzyme immobilization allows ease of removal from the product and further reuse, so it lowers the cost. In some cases, enzymes are stabilized by immobilization, and the immobilized biocatalysts can be used in continuous processes.
Several immobilization techniques such as cross-linking, covalent binding, and adsorption have been investigated to couple GA to insoluble supports (Arica et al. 1998; Bahar and Çelebi 1998; Kovalenko et al. 2006; Miller 1998; Rani et al. 2000; Roig et al. 1995; Sasvári and Asbóth 1999; Silva et al. 2005; Tanriseven et al. 2002; Torres et al. 2004; Varavinit et al. 2001). Although covalent coupling usually leads to very stable preparations compared to those obtained by physical adsorption and ionic binding, adsorption is selected for large-scale applications mainly because of its comparative simplicity and economy (Kierstan and Coughlan 1991).
The most important components contributing to the performance of an immobilized biocatalyst are the support and the method of immobilization. Judicious selection of the support, based on surface characteristics, morphology, and composition, is critical to improving the properties of an immobilized biocatalyst (Cabral and Kennedy 1991). Bone is a resistant, porous, insoluble material obtained as a byproduct of meat processing. It is a natural, non-toxic material (Findlay et al. 1986) composed mainly of the protein collagen and the mineral hydroxyapatite. In prior studies, some enzymes have been immobilized onto bone particles at laboratory scale for food applications (Carpio et al. 2000; Mukataka et al. 1993; Negishi et al. 1989; Schafhauser and Storey 1992b), but there are no reported studies for bench scale or industrial bone utilization. The relatively economical application of enzyme immobilization onto bone particles makes it an attractive option for industrial application.
The aim of this work is to assess the bench-scale application of a non-conventional support, bone, for glucoamylase immobilization and its subsequent use in cassava starch hydrolysis. It includes the determination of optimum conditions to immobilize GA on to chicken bone particles. Some properties of the insoluble biocatalyst, such as optimum temperature and pH, kinetic parameters (K M, V max and K M app, V max app), and stability to heat denaturation, are determined. Furthermore, the operational stability of bone derivative to produce dextrose hydrolyzates from liquefied cassava starch, at bench scale, in batch processes was assessed.
Materials and Methods
Materials
Cassava starch was isolated by wet extraction (Corbishley and Miller 1984). Aspergillus niger amyloglucosidase AMG 300L also named glucoamylase (GA) (EC 3.2.1.3) and Bacillus licheniformis α-amylase Termamyl 120L (EC 3.2.1.1) were obtained from Novo Industry, Bagsvaerd, Denmark. PD-10 Sephadex G-25 columns were purchased from Pharmacia BTG, Uppsala, Sweden. Bovine serum albumin (BSA) and Coomassie Plus reagent were obtained from Pierce, Rockford, IL, USA. The glucose determination kit was purchased from Reacur, Montevideo, Uruguay; 3,5-dinitrosalicylic acid (DNS), and papain (EC 3.4.22.2) were bought from Merck, Darmstadt, Germany; glucose was obtained from Sigma Chemical, St. Louis, MO, USA. Soluble potato starch was purchased from Panreac Química S.A., Barcelona, Spain. All other chemicals used were of reagent grade.
Preparation of Bone Particles
Chicken necks, a poultry industry byproduct, were used to prepare the support. Vertebrae were partially cleaned manually, followed by proteolytic treatment with 0.1% papain solution in 0.02 M phosphate buffer pH 5.7, at 60 °C for approximately 1 h. The material obtained was treated with a boiling 1% (w/v) sodium hydroxide solution to remove residual proteins and fats (Nolsøe and Undeland 2008) from the bone surface and to obtain an easily breakable product, which was first neutralized with a dilute hydrochloric acid solution (1% w/v) and then washed with tap water until neutral pH. Finally, it was dried to a moisture content of about 9% and milled in an attrition disk mill. Particles ranging in size from 125 to 360 µm were obtained by sifting, using standard ASTM sieves; average particle size \(\mathop {d_{\text{p}} }\limits^ - \) for supports B 360, B 203, and B 125 was calculated using Eq. 1 (American Water Works Association 1991):
where \(\mathop {d_{\text{p}} }\limits^ - \) is the average particle size in microns (µm), W i is the weight of the sieve fraction in grams, and d i is the opening in microns that corresponds to the average of the openings in the two sieves that enclose that mesh fraction (Table 1).
GA Activity
The activity of soluble GA was determined according to the Bernfeld method (Bernfeld 1955), at 20 °C using 0.05 M sodium acetate buffer, pH 4.5 (activity buffer), and 5% soluble starch in activity buffer as substrate. A blank made with dinitrosalicylic (DNS) inactivated enzyme was used. The immobilized enzyme activity was assayed as described by Carpio et al. (2000). One GA unit (GA U) was defined as the amount of enzyme that catalyzes the release of reducing carbohydrates equivalent to 1 µmol of glucose per minute.
Determination of Proteins
The protein content in the enzyme solution, supernatant, and washings was determined by the Coomassie blue G-250 dye-binding method using Coomassie Plus® reagent and BSA as the standard (microassay procedure, Pierce Instructions booklet). The immobilized protein was estimated as the difference between the amount of protein applied to the support and the amount recovered in the supernatant and washings (Eq. 2):
where p is the amount of GA adsorbed onto the bone carrier in milligram protein per gram of support; C i, C s, and C wash j are the protein concentrations in the solution applied to the support, in the supernatant and washings, respectively, in milligram per milliliter; V is the immobilization volume; V j are buffer volumes used for each washing (ml); and W is the weight of the carrier in grams.
Determination of Adsorption Conditions
Several immobilization tests were carried out at laboratory scale to determine the conditions (ionic strength, pH, particle size, and initial load) for bench-scale immobilization.
The influence of ionic strength and pH on the GA adsorption was studied utilizing a two-factorial central composite rotatable design (CCRD) with an axial point (α = ±1.41) and three replicates at the center point. Each variable in the design was studied at five different levels, namely, −α, −1, 0, +1, and +α (Table 2; Montgomery 2001). A set of 11 experiments was carried out (Table 3) by applying approximately 700 GA U per gram of support [bone particles with an average size of 360 µm (B360)]. The relationship of the independent variables and the response was calculated by using Eq. 3:
where Y (expressed activity) is the predicted response, β 0, β i , β ii , and β ij were constant coefficients of intercept, linear, squared, and interaction terms, respectively; k is the number of factors, and X i and X j are independent variables. Results were analyzed by Statgraphics plus version 5.1 software. Duncan’s multiple range test was applied to determine significant differences among the responses, at a level of p < 0.05 (Montgomery 2001).
The effect of particle size on adsorption was studied using bone particles with an average size of 360 µm (B360) and 203 µm (B203).
GA Immobilization
A standard immobilization procedure was carried out as follows: 0.2 g of bone particles were equilibrated in 2.0 ml of the activity buffer or one of the above-mentioned buffers (Table 3), for 1.5 h under stirring, and then incubated at 20 °C for 20 h under gentle agitation with 4 ml of enzyme solutions with or without prior gel-filtration. The excess enzyme was removed by filtration and washings of the derivative with activity buffer until no more enzyme was released. Enzyme activity and protein content in supernatant and washings were quantified.
Determination of the Maximal Load Capacity of the Support with the Selected Particle Size
To determine the maximal load capacity (q max) of the selected support, increasing amounts of GA in activity buffer (2.0–40.6 mg protein per gram of support, corresponding to 73–1,291 GA U per gram of support) were applied to the matrix, at 20 °C. The amount of enzyme in the solid phase, q*, in milligram protein per gram of support, was calculated as the total amount of enzyme present at the beginning of the experiment (C i V) less the amount still in the bulk liquid phase (supernatant) at equilibrium (C*V; Chase 1984; Soriano et al. 1999):
The influence of temperature on adsorption was determined in the same way by incubating enzyme solutions and the support at 51.5 °C.
Bench-Scale Immobilization
Bench-scale immobilization was carried out under the selected conditions as follows: enzyme solution (175 ml diluted ten times with the selected buffer) was incubated with previously equilibrated bone particles (620 g), yielding an enzyme activity to support ratio of 506 GA U per gram of support. The mixture was gently agitated for 20 h, at 20 °C. Excess enzyme was removed by filtration and the derivative washed as described previously.
Determination of Kinetic Parameters
The K M and V max values of soluble GA were determined by measuring the initial rates of the reaction with soluble starch at concentrations in the range 0.04–1.60 mg ml−1 in activity buffer at 20 °C. The apparent kinetic parameters (K M app and V max-app) of immobilized GA were determined in a batch system by varying the starch concentration from 0.41 to 16.43 mg ml−1. The kinetic parameters were calculated by non-linear regression of Michaelis–Menten equation using Statgraphics Plus, version 5.1.
Optimum pH and Temperature
The effect of pH on the activity of soluble and immobilized GA was determined at 20 °C. For free GA, the substrate was 5% (w/v) soluble starch in citrate-phosphate buffer, pH range 2.6–7.0, and for GA-bone derivative, 5% (w/v) soluble starch in 0.05 M sodium acetate buffer pH 3.6–5.6 was used as the substrate. The effect of temperature on the enzyme activity was studied by determining the initial reaction rate at different temperatures in the range of 20–70 °C. The relative activity versus pH or temperature was plotted.
Thermal Inactivation Kinetics
The inactivation kinetics of free and immobilized GA at 55 °C were monitored for 12 h. For free GA, 1 ml of an enzyme solution containing 3 GA U was incubated at 55 °C in capped tubes. At predetermined intervals, the marked tubes were brought to room temperature (20 °C) and the residual activity determined. For GA bone derivative, aliquots of the filter-dried immobilized biocatalyst containing 3 GA U were added to 1 ml activity buffer, and the assay was performed as described before. Based on the studies of Polakovič and Bryjak (2002) for soluble A. niger GA, Sadana and Henley model (1987) (Eq. 5) was used to describe inactivation kinetics. The parameters α 1, k 1, and k 2 of free and immobilized GA were calculated according to the bi-exponential decay by using Eqs. 6 and 7, using the Statgraphics Plus version 5.1.
where A (%) is the residual activity of GA at a specific time t; α 1 (%) and α 2 (%) are the relationships between the activities of the final states E 1 or E 2 to the initial state E, respectively. It was assumed that E 2 has no residual activity, so α 2 = 0; k 1 (h−1) and k 2 (h−1) are the first-order inactivation constants and A 1 and A 2 are coefficients described by the following relationships:
The half-lives (t 1/2), time at which A is 50%, was calculated by using Eq. 6 after A 1, A 2, k 1, k 2 were determined.
Pilot-Scale Liquefaction of Cassava Starch
The cassava starch (120 kg) was liquefied by jet cooking a 30% (w/w) starch slurry containing thermostable α-amylase (0.05% (w/w) enzyme/cassava starch), at 105°C for 5 min and subsequent dextrinization at 90°C for approximately 1.5 h. The liquefied cassava starch solution, dextrose equivalent (DE) 20, was then cooled down, its pH adjusted to 4.5 with concentrated HCl and finally spray-dried to be stored until used for saccharification experiments.
Saccharification of Liquefied Cassava Starch at Bench Scale
The reconstituted 30% w/w liquefied cassava starch (8 l) was mixed with the GA bone derivative (6.7 GA U per gram of starch) and incubated at 55 °C in a stirred-tank reactor (Microferm Fermentor, New Brunswick Scientific) with a nominal volume of 14 l, to produce high glucose syrup. The reaction mixture was stirred at 400 rpm. During the reaction, reducing sugars were monitored with DNS solution (Miller 1959) and the released glucose by an enzymatic method (Trinder 1969) using the Reacur kit.
Operational Stability of Immobilized Glucoamylase
The stability of the GA bone derivative during use was estimated at bench scale by its repeated usage (up to 25 cycles) for the saccharification of liquefied cassava starch at 55 °C. Saccharification was carried out as described before. At the end of each batch, the derivative was separated from the reaction mixture by sifting. The half-life (t 1/2), k 1 and k 2 were calculated by using Eq. 6 from the hydrolysis data of each batch [plot of the starch conversion (DE) after a 3-h reaction period versus total hydrolysis time].
Results and Discussion
Parameters Influencing the Adsorption of GA onto Chicken Bone
Several assays were carried out to determine the optimal parameters for bench-scale immobilization of glucoamylase onto bone particles. These assays assessed the effects of (a) stabilizers (sugars) existing in the commercial GA, (b) ionic strength and pH, (c) bone particle size, and (d) enzyme load on both the amount of enzyme adsorbed on the matrix, and the activity of the derivatives (expressed activity).
Effect of stabilizers on the GA immobilization
The effect of stabilizers (14.7% reducing sugars) contained in commercial GA on the adsorption and on the activity of immobilized GA was determined by adsorbing a gel-filtered and a non gel-filtered enzyme solution (553 GA U per gram of support) onto bone particles with an average size of 125 µm (B125). The results showed that the sole parameter affected by the utilization of gel-filtered GA was the amount of immobilized enzyme, which was 10% higher than that obtained with non gel-filtered GA (412 ± 2 and 373 ± 15.2 GA U per gram of support, respectively) while expressed activity was similar for both derivatives (87 ± 5.7 and 83 ± 2.9 GA U per gram of support) (p > 0.05). Therefore gel-filtration of the commercial enzyme preparation used is not necessary to adsorb GA onto chicken bone; this represents an important saving for industrial applications.
Influence of ionic strength and pH on GA adsorption onto chicken bone
Adsorption of GA onto bone is an easy immobilization method that does not require the introduction of reactive species on the matrix or the utilization of any crosslinking agent after adsorption. The mechanism of GA binding to bone by adsorption apparently depends on the ionic interactions with both the hydroxyapatite analogue and the amino acid side chains of collagen; in addition, hydrophobic interactions with other amino acid side chains of collagen may also be involved (Schafhauser and Storey 1992a).
Due to the nature of the forces involved in the adsorption of proteins onto solid surfaces (van der Waals forces, hydrogen bonding, ionic, and hydrophobic interactions), GA adsorption can be expected to depend on variables such as ionic strength, pH, enzyme load, and temperature (Kennedy and Cabral 1987; Kierstan and Coughlan 1991). Thus, evaluating the influence of these parameters on the activity of the derivatives is of paramount importance to reach an optimal adsorption.
The effect of ionic strength and pH on the expressed activity of GA derivatives is shown in Table 3. Statistical analysis showed that, in the range studied, pH has the strongest effect on expressed activity which diminished as the pH of the immobilization buffer increased (trials: 1 and 2, 3 and 4, and 6, 8, 9–11). The linear effect of ionic strength was also within the range of significance (95%) although the increase in the expressed activity of the derivatives, at constant pH, showed a limited variation (trials: 1 and 3, 2 and 4, and 5, 7, 9, 10, and 11). The interaction and the square of the parameters were not significant (p < 0.05).
The predicted values of the response are closely correlated to the experimental data. The correlation coefficient (R 2 = 0.97) indicates that the model is suitable to represent the relationship between the parameters studied, ionic strength, and pH. Typical response surface is shown in Fig. 1, and the response model for expressed activity was given by Eq. 9 for actual parameters.
The maximum activity would be attained using 0.195 M sodium acetate buffer, pH 3.6, for GA adsorption. However, other considerations to be borne in mind are the pH of the end product, which should be 4.5–5.5 (Howling 1992), and the possibility of d-glucose polymerization to form di- and higher saccharides (Mulvihill 1992) under acidic conditions, thus reducing the glucose yield. By immobilizing at pH 4.5, the optimal pH for soluble GA, derivatives with an expressed activity equivalent to 90% of the maximal value were obtained (Table 3). The usage of pH 4.5 for adsorption could minimize the risk of GA desorption that could be expected if immobilization is carried out at pH 3.6, and the immobilized derivative is later used at higher pH (4.5). Based on these considerations, immobilization was performed using the 0.05 M sodium acetate buffer pH 4.5 (activity buffer).
Influence of bone particle size on GA immobilization
The support (matrix, carrier) is the most important component affecting the performance of an immobilized system, with the exception of the enzyme (Kennedy and Cabral 1987). A high specific surface area is needed to allow the immobilization of elevated enzyme loads (Kierstan and Coughlan 1991); this can be achieved utilizing small particles (Bahar and Çelebi 2000; Emnéus and Gorton 1993). Therefore, the influence of particle size on GA adsorption was studied using carriers with \(\mathop d\limits^ - _{\text{p}} \) of 203 (B203) and 360 µm (B360), with an initial load of 223 GA U per gram of support. Comparison of the GA immobilization on both matrixes showed that the enzyme adsorption enhanced with the increase in the specific area of the support exposed to the enzyme (19.8 to 25.5 m2 per gram of support) as the particle size decreased from 360 to 203 µm (Table 4). This 1.3-fold increase in surface area produced not only a 1.1-fold incremental increase of GA adsorption but also a 1.8-fold improvement in expressed activity. Therefore, B203 was chosen for subsequent studies.
Effect of enzyme load on the adsorption of GA onto bone \(\left( {\mathop {d_p }\limits^ - = 203\;{\text{ $ \mu $ m}}} \right)\) at 20 and 51.5 °C
The amount of immobilized enzyme increases with the application of higher enzyme loads until a limit value (q max) is reached (Kennedy and Cabral 1987). The function that relates the amount of adsorbed protein to the solution concentration of protein at equilibrium is an isotherm. In the present study, the isotherms follow a marked nonlinear behavior within the concentration range studied, at both temperatures, 20 and 51.5 °C (Fig. 2). The best correlation between the experimental results and data predicted by the models was found for the Langmuir equation (Eq. 10), which corresponds to the formation of a monolayer on the adsorbent. Similar correlation has been reported by Kovalenko et al. (2002) and Soriano et al. (1999) for GA adsorption onto graphite and graphite-like carbon prepared on the surface of monolithic support (Kovalenko et al. 2002) and GA I isozyme on the anionic exchanger DEAE-Toyopearl (Soriano et al. 1999).
Although adsorption and deposition of proteins and other biomaterials are considered an irreversible (Adamczyk et al. 2004) or a complex process (Fang and Szleifer 2001), the Langmuir model, initially developed to describe adsorption of gases onto solid adsorbents in a monolayer fashion, is used to explain the behavior of protein adsorption on different sorbent types like ion exchangers (Soriano et al. 1999), graphite (Kovalenko et al. 2002), or some affinity adsorbents (Chase 1984; Velayudhan and Horváth 1994). The selection of the Langmuir model was based on either the interactions that can occur in these systems (Chase 1984) or the shape of the plot of q* versus C*, which reflects the behavior of adsorbent and adsorbate interaction (Kovalenko et al. 2002).
The adsorption capacity of GA onto bone increased from 7.73 to 34.3 mg protein per gram of support, when immobilization temperature increased from 20 to 51.5 °C. The increment of enzyme adsorption at 51.5 °C may have been due to the decrease in the viscosity of the enzyme solution. This enabled the diffusion of GA molecules toward the internal surface of the matrix as evidenced by the higher difference in immobilized enzyme as the initial GA load increased (Fig. 3). However, this increment did not improve the expressed activity, which remained relatively unchanged for derivatives obtained at both immobilization temperatures; the only exception was observed for the expressed activity of biocatalyst produced by applying the lowest initial load, which was 60% of that obtained for the derivative prepared at 20 °C, probably because of the higher instability of proteins as its concentration diminishes (Klibanov and Ahern 1987).
For an appropriate design of an immobilization process, it is also important to know if the specific activity (SA) is reduced when the enzyme bound to the matrix increases. Table 5 shows that SA diminished from 15.2 to 6.7 GA U mg−1 of protein as the amount of enzyme adsorbed on the support increased threefold. Decrease of SA would mean that GA is less efficient. This inefficiency may be attributed to the crowding of protein on the support, which reduces the accessibility of substrate molecules to the GA active site (Kennedy and Cabral 1987; Woodward 1985).
Although the highest SA (15.2 GA U mg−1 of protein) was attained by applying the lowest enzyme load (73 GA U per gram of support), the expressed activity of this derivative was the lowest (29 GA U per gram of support). The expressed activity doubled (57 GA U per gram of support) when the initial load was increased 14.7 times (1,025 GA U per gram of support). It was also observed that derivatives with an average expressed activity of 51 GA U per gram of support, equivalent to 90% of the maximal value, were obtained when enzyme loads ranging from 382 to 540 GA U per gram of support were used. Therefore, for an efficient utilization of GA, the optimal enzyme load to be applied for bench-scale immobilization could vary between 382 and 540 GA U per gram of matrix.
Bench-Scale GA Immobilization onto Chicken Bone Particles
From the previous studies, the following conditions were determined as optimal for GA immobilization: support made by the mixture of bone particles retained on number 70, 80, and 100 ASTM sieves with an average particle size of 203 µm (B203); 0.05 M sodium acetate buffer, pH 4.5; enzyme loads from 382 to 540 GA U per gram of support; and 20 °C as the immobilization temperature.
In this study, 506 GA U per gram of support were applied to the matrix. The amount of GA adsorbed onto B203 was 171 GA U per gram of support and the expressed activity 34 GA U per gram of support, which was 20% of the total immobilized activity. Immobilization efficiency (20%) was similar to that reported for GA adsorption on synthetic carbon material (Kovalenko and Perminova 2008). Both values (adsorbed and expressed activity) were lower than those attained, at laboratory scale, for tests performed to determine maximal support capacity in which adsorbed GA varied from 226 to 258 GA U per gram of support and expressed activity between 49 and 52 GA U per gram of support (Table 5). These differences may be due to the lower ratio of GA solution volume to support weight (2.8:1) used for bench-scale immobilization in order to avoid handling large volumes of enzyme solution that would have been produced if the laboratory scale ratio (20:1) had been used.
Properties of GA bone Derivative
The GA bone derivative and the free GA were compared to assess the effects of immobilization on enzyme properties (kinetic parameters, optimum pH and temperature, and thermal stability). This evaluation would allow the proper utilization of the insoluble biocatalyst.
The kinetic parameters of free enzymes are generally changed by immobilization. The results demonstrated that free and immobilized GA followed Michaelis–Menten kinetics; however, adsorption onto bone support increased the K M about nine times (from 0.08 to 0.71 mg soluble starch ml−1). Different K M variations ranging from a 10% reduction to a 69% increment were reported for biocatalysts produced by GA adsorption onto active carbon (Rani et al. 2000), bone particles of 600–2,000 µm (Schafhauser and Storey 1992b), gelatinized corn starch plus subsequent trapping in alginate fibers (Tanriseven et al. 2002), and synthetic carbon material Sibunit® (Kovalenko and Perminova 2008). The K M increment determined in this study could be due to changes in GA three-dimensional conformation produced by immobilization and to the limited access of the macromolecular substrate (maltodextrin) to the active site of the enzyme (Bahar and Çelebi 1998; Brena 1996; Trevan 1980). This K M increment would not significantly affect the performance of the insoluble GA since the substrate concentration used at industrial scale is higher than 320 mg ml−1.
For V max values, the above-mentioned researchers found that adsorption produced a decrease in its value; for GA-B203 derivative, V max decreased 4.4 times, from 3.23 to 0.73 mg starch min−1 mg−1 prot.
Glucoamylase exhibited different optimum pH and pH activity profiles after immobilization on bone particles (Fig. 4). Optimum pH ranges for soluble and immobilized GA were 4.5–5.5 and 3.6–4.0, respectively. Changes in the optimum pH depend on the charge of enzyme and/or of the carrier (Rani et al. 2000). This shift to the acid side of immobilized GA may be due to changes in the microenvironment of the GA adsorbed on bone, a matrix composed mainly of protein and hydroxyapatite, which becomes more basic than the bulk solution so that optimal pH of the immobilized GA turns more acidic (Atkinson and Mavituna 1991; Kierstan and Coughlan 1991). Immobilized GA also showed maximum activity in a narrower pH range than the soluble GA; consequently, the activity of immobilized GA is more sensitive to pH changes than the free enzyme.
Optimum temperature of GA adsorbed onto chicken bone remained unchanged, around 60–65 °C (data not shown). Similar results were reported by Tanriseven et al. (2002) for GA immobilized by adsorption to gelatinized corn starch and subsequent alginate fiber entrapment.
Thermal stability is an important property of a biocatalyst that determines its possible application. It was defined in this study as the half-life (t 1/2), since it includes the contribution of the deactivation rate constants k 1 and k 2 and the residual activity α 1. As it was the case for inactivation of peroxidase at temperatures lower than 90 °C (Rudra et al. 2008), inactivation of soluble and immobilized GA followed the series type inactivation model (R 2 > 0.99, Eq. 5), and the kinetic constant k 1 was significantly larger than k 2. The thermal stability of GA (4.3 h), at 55 °C in a 0.05 M sodium acetate buffer pH 4.5, remained unchanged after its adsorption on B203 (4.4 h) (Table 6). This result can be expected since insoluble biocatalysts prepared by physical or ionic adsorption are less stable than those produced by covalent binding (Flynn and Johnson 1978; Kennedy and Cabral 1987).
Thermostability of immobilized enzymes depends on the nature of the soluble enzyme used, the support, the method of immobilization (Kierstan and Coughlan 1991), and the presence of substrate and/or product during the incubation; however, there are no defined rules about the relationship between these parameters and the thermal stability. It is known that substrate stabilizes GA (Kovalenko et al. 2007). The thermostability of bone adsorbed GA in the presence of substrate (t 1/2 = 293 h) was estimated from its operational stability in the hydrolysis of 30% w/w cassava maltodextrin (Fig. 5), and it proved to be 66.5 times higher than that in the buffer solution (4.4 h).
Operational Stability of GA bone Derivative, Determined at 55 °C
Operational stability is one of the most important characteristics to be assessed in an immobilized enzyme system, since it measures the capacity of a derivative to withstand continuous use for prolonged periods (Adlercreutz 1993). For the GA bone derivative, this property was evaluated at 55 °C in batch system using a stirred tank reactor. This type of reactor was chosen for this study instead of a packed bed reactor, the preferred option for continuous processing, because final starch conversions obtained with packed bed reactors (DE, 93.0–93.5) were lower than those attained with soluble GA (DE, 96; Lee et al. 1976; Rugh et al. 1979). Furthermore, our experimental results at laboratory scale demonstrated that stirring produced a 3.6-fold increase of the initial reaction rate at 55 °C.
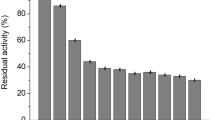
The hydrolysis percentages obtained with the stirred tank reactor, DE 99.8 and 96, for 24- and 72-h usage of the derivative, respectively, demonstrated that similar conversions to those produced with the soluble GA can be obtained initially. The final DE after using the derivative for 25 batches was 90.3 and the glucose concentration 88% (Fig. 6).
Kinetics of 30% (w/w) cassava maltodextrin hydrolysis with GA-bone derivative (d p, 203 µm, 6.7 GA U g−1 of cassava maltodextrin) to produce glucose syrups in first and 25th batches, evaluated at 55 °C and pH 4.5. Empty triangles and circles represent glucose concentration (%) in the first and 25th batches, respectively, and filled diamonds and squares represent DE in the first and 25th batches, respectively
Under the reaction conditions used in this study, half-life of the GA bone derivative (293 h), was similar to that obtained by Rugh et al. (1979) in a fixed bed reactor (300 h) for the hydrolysis of raffinate (a glucose-rich stream produced during the purification of fructose syrups) with the commercial immobilized glucoamylase (Dextrozyme®) produced by Novo but lower than the t 1/2 attained by Lee et al. (1976) in a fixed bed reactor with a derivative produced by covalent binding of GA to glutaraldehyde activated silica (581 h).
The main drawback of non-covalent immobilization by adsorption and ionic binding is the possible leakage of enzymes from the carrier to the reaction media (Kierstan and Coughlan 1991). However, a test performed to assess enzyme release at 55 °C by determining protein concentration in the centrifuged substrate (30% w/w cassava maltodextrin), before and after 1 and 39 h of reaction, proved that there was no detectable GA leakage. The activity loss of bone-bound GA could be attributed instead to the interplay of the following factors: (a) gradual fragmentation of the support, (b) biocatalyst losses during its separation from the reaction media and later washing, (c) adsorption of minute substrate particles on the derivative as evidenced by the slow color change of the biocatalyst. Factors b and c could be prevented at industrial scale by suppressing washing of the derivative after each batch and by the selection of finer filtering media or by more careful centrifugation of the substrate.
Conclusions
Bone proved to be an alternative matrix for bench-scale GA adsorption, since the derivatives obtained displayed good operational stability for the production of high-glucose syrups at 55 °C. The final DE of these syrups ranged from 90% to 99% for 25 batches. Bone-bound GA generates a relatively clean glucose hydrolyzate and, thus, expands the possible application of glucose syrups derived from liquefied cassava starch in the thriving ethanol production industry.
It was demonstrated that commercial GA can be successfully immobilized at 20 °C without any additional purification. Optimal conditions for glucoamylase adsorption on to bone were determined to be the following: bone particles with an average size of 203 µm, enzyme loads varying from 367 to 533 GA U per gram of support and 0.195 M sodium acetate buffer, pH 3.6. However, to minimize the risk of GA desorption when using the insoluble derivative at higher pH (4.5) for saccharification, 0.05 M sodium acetate buffer, pH 4.5, was used for GA adsorption. This allows the production of glucose syrups at pH 4.5, the value required for the final product, without further neutralization.
It was determined that the kinetic parameters K M and V max and optimum pH were the only enzyme properties affected by the immobilization process. It has been found that thermal stability of the immobilized GA is significantly improved by the substrate.
References
Adamczyk, Z., Jaszczólt, K., Siwek, B., & Weronski, P. (2004). Irreversible adsorption of particles at random-site surfaces. Journal of Chemical Physics, 120(23), 11155–11162. doi:10.1063/1.1712967.
Adlercreutz, P. (1993). Immobilized enzymes. In T. Nagodawithana, & G. Reed (Eds.), Enzymes in food processing (pp. 103–119, 3rd ed.). New York, USA: Academic.
American Water Works Association (1991). AWWA standard for granular activated carbon p. 11. Denver, USA: American Water Works Association.
Arica, M. Y., Alaeddinoğlu, N. G., & Hasirci, V. (1998). Immobilization of glucoamylase onto activated phema/egdma microspheres: Properties and application to a packed-bed reactor. Enzyme and Microbial Technology, 22(3), 152–157. doi:10.1016/S0141-0229(97)00139-7.
Atia, K. S., Ismail, S. A., & Dessouki, A. M. (2003). Immobilization of β-amylase using polyacrylamide polymer derivatives. Journal of Chemical Technology & Biotechnology, 78(8), 891–898. doi:10.1002/jctb.875.
Atkinson, B., Mavituna, F. (Eds.) (1991). Biochemical engineering and biotechnology handbook: Enzymes (2nd edn., pp. 521–605). Mexico: Stockton.
Bahar, T., & Çelebi, S. S. (1998). Characterization of glucoamylase immobilized on magnetic poly(styrene) particles. Enzyme and Microbial Technology, 23(5), 301–304. doi:10.1016/S0141-0229(98)00048-9.
Bahar, T., & Çelebi, S. S. (2000). Performance of immobilized glucoamylase in a magnetically stabilized fluidized bed reactor (MSFBR). Enzyme and Microbial Technology, 26(1), 28–33. doi:10.1016/S0141-0229(99)00129-5.
Bernfeld, P. (1955). Amylases, alpha and beta. In S. P. Colowick, & N. O. Kaplan (Eds.), Methods in enzymology, vol. 1 (pp. 149–158). USA: New York.
Bhargava, S., Frisner, H., & Bisgard-Frantzen, H. (2005). A process of producing a fermentation product. United States patent WO2005/113785 A2 (in English).
Brena, B. M. (1996). Reversible immobilization of enzymes using agarose-bound group-specific ligands. Ph. D. thesis. Faculty of Science and Technology, Uppsala University, Uppsala, Sweden. pp. 8.
Cabral, J. M. S., & Kennedy, J. F. (1991). Covalent and coordination immobilization of proteins. In R. F. Taylor (Ed.), Protein immobilization fundamentals and applications (pp. 73–138). USA: New York.
Carpio, C., González, P., Ruales, J., & Batista-Viera, F. (2000). Bone-bound enzymes for food industry application. Food Chemistry, 68(4), 403–409. doi:10.1016/S0308-8146(99)00193-4.
Chase, H. A. (1984). Prediction of the performance of preparative affinity chromatography. Journal of Chromatography A, 297, 179–202. doi:10.1016/S0021-9673(01)89041-5.
Corbishley, D. A., & Miller, W. (1984). Tapioca, arrowroot, and sago starches production. In R. L. Whistler, J. N. BeMiller, & E. F. Paschall (Eds.), Starch: Chemistry and technology (pp. 469–478, 2nd ed.). Orlando, USA: Academic.
Emnéus, J., & Gorton, L. (1993). Comparison between different inorganic supports for the immobilizaton of amyloglucosidase and α-amylase to be used in enzyme reactors in flow-injection systems. Analytica Chimica Acta, 276(2), 303–318. doi:10.1016/0003-2670(93)80398-5.
Fang, F., & Szleifer, I. (2001). Kinetics and thermodynamics of protein adsorption: A generalized molecular theoretical approach. Biophysical Journal, 80, 2568–2589.
Findlay, C. J., Parkin, K. L., & Yada, R. Y. (1986). Bone as a solid support for the immobilization of enzymes. Biotechnology Letters, 8(9), 649–652. doi:10.1007/BF01025975.
Flynn, A., & Johnson, D. B. (1978). Some factors affecting the stability of glucoamylase immobilized on hornblende and other inorganic supports. Biotechnology and Bioengineering, 20(9), 1445–1454. doi:10.1002/bit.260200909.
Howling, D. (1992). Glucose syrup: Production, properties and applications. In F. W. Schenck, & R. E. Hebeda (Eds.), Starch hydrolysis products (pp. 277–317). New York, USA: VCH.
Kennedy, J. F., & Cabral, J. M. S. (1987). Enzyme immobilization. In H. J. Rehm, & G. Reed (Eds.), Biotechnology, vol. 72 (pp. 347–404). Weinheim: VCH Verlagsgesellschaft.
Kierstan, M. P. J., & Coughlan, M. P. (1991). Immobilization of protein by noncovalent procedures: Principles and applications. In R. F. Taylor (Ed.), Protein immobilization, fundamentals and applications (pp. 13–71). New York, USA: Marcel Dekker.
Klibanov, A. M., & Ahern, T. J. (1987). Thermal stability of proteins. In D. L. Oxender, & C. F. Fox (Eds.), Protein engineering (pp. 213–218). New York, USA: Liss.
Kovalenko, G. A., & Perminova, L. V. (2008). Immobilization of glucoamylase by adsorption on carbon supports and its application for heterogeneous hydrolysis of dextrin. Carbohydrate Research, 343, 1202–1211. doi:10.1016/j.carres.2008.02.006.
Kovalenko, G. A., Komova, O. V., Simakov, A. V., Khomov, V. V., & Rudina, N. A. (2002). Macrostructured carbonized ceramics as adsorbents for immobilization of glucoamylase. Journal of Molecular Catalysis. A, Chemical, 182–183, 73–80. doi:10.1016/S1381-1169(01)00479-4.
Kovalenko, G. A., Perminova, L. V., Plaksin, G. V., Chuenko, T. V., Komova, O. V., & Rudina, N. A. (2006). Immobilized glucoamylase: A biocatalyst of dextrin hydrolysis. Applied Biochemistry and Microbiology, 42(2), 145–149. doi:10.1134/S0003683806020050.
Kovalenko, G. A., Perminova, L. V., Terent’eva, T. G., & Plaksin, G. V. (2007). Catalytic properties of glucoamylase immobilized on synthetic carbon material sibunit. Applied Biochemistry and Microbiology, 43(4), 374–378. doi:10.1134/S0003683807040023.
Lee, D. D., Lee, Y. Y., Reilly, P., Collins, E. J., & Tsao, G. T. (1976). Pilot plant production of glucose with glucoamylase immobilized to porous silica. Biotechnology and Bioengineering, 18(2), 253–267. doi:10.1002/bit.260180210.
Miller, E. (1998). Immobilization of glucoamylase on polyamide nets. Acta Biotechnologica, 18(2), 135–146. doi:10.1002/abio.370180208.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. doi:10.1021/ac60147a030.
Montgomery, D. C. (2001). Design and analysis of experiments pp. 21–54, 427–510. New York, USA: Wiley.
Mukataka, S., Negishi, S., Sato, S., & Takahashi, J. (1993). Effect of substrate presoaking treatment of support materials on the activity of immobilized glucoamylase. Enzyme and Microbial Technology, 15(3), 229–233. doi:10.1016/0141-0229(93)90142-O.
Mulvihill, P. J. (1992). Crystalline and liquid dextrose products: Production, properties and applications. In F. W. Schenck, & R. E. Hebeda (Eds.), Starch hydrolysis products worldwide technology, production, and applications (pp. 121–176). New York, USA: VCH.
Negishi, S., Sato, S., Mukataka, S., & Takahashi, J. (1989). Utilization of powdered pig bone as a support for immobilization of lipase. Journal of Fermentation and Bioengineering, 67(5), 350–355. doi:10.1016/0922-338X(89)90254-7.
Nolsøe, H., & Undeland, I. (2008). The acid and alkaline solubilization process for the isolation of muscle proteins: State of the art. Food Bioprocess Technol. doi:10.1007/s11947-008-0088-4
Polakovič, M., & Bryjak, J. (2002). Modelling of the kinetics of thermal inactivation of glucoamylase from Aspergillus niger. Journal of Molecular Catalysis. B, Enzymatic, 19–20, 443–450. doi:10.1016/S1381-1177(02)00197-2.
Rani, A. S., Das, M. L. M., & Satyanarayana, S. (2000). Preparation and characterization of amyloglucosidase adsorbed on activated charcoal. Journal of Molecular Catalysis. B, Enzymatic, 10(5), 471–476. doi:10.1016/S1381-1177(99)00116-2.
Reeve, A. (1992). Starch hydrolysis: Processes and equipment. In F. W. Shenck, & R. E. Hebeda (Eds.), Starch hydrolysis products worldwide technology, production, and applications (pp. 79–120). New York, USA: VCH.
Roig, M. G., Slade, A., Kennedy, J. F ., Taylor, D. W., & Garaita, M. G. (1995). Investigations of stabilities, ph, and temperature profiles and kinetic parameters of glucoamylase immobilized on plastic supports. Applied Biochemistry and Biotechnology, 50(1), 11–33. doi:10.1007/BF02788037.
Rudra, S. G., Shivhare, U. S., Basu, S., & Sarkar, B. C. (2008). Thermal inactivation kinetics of peroxidase in coriander leaves. Food Bioprocess Technol, 1(2), 187–195. doi:10.1007/s11947-007-0013-2.
Rugh, S., Nielsen, T., & Poulsen, P. B. (1979). Application possibilities of a novel immobilized glucoamylase. Starch/Stärke, 31(10), S 333–337.
Sadana, A., & Henley, J. P. (1987). Single-step unimolecular non-first-order enzyme deactivation kinetics. Biotechnology and Bioengineering, 30(6), 717–723. doi:10.1002/bit.260300604.
Sasvári, Z., & Asbóth, B. (1999). Crosslinking of glucoamylases via carbohydrates hardly affects catalysis but impairs stability. Biotechnology and Bioengineering, 63(4), 459–463. doi:10.1002/(SICI)1097-0290(19990520)63:4<459::AID-BIT9>3.0.CO;2-I.
Schafhauser, D. Y., & Storey, K. B. (1992a). Coimmobilized amyloglucosidase, pululanase and glucose isomerase on biobonetm. Applied Biochemistry and Biotechnology, 36, 63–74. doi:10.1007/BF02950775.
Schafhauser, D. Y., & Storey, K. B. (1992b). Immobilization of amyloglucosidase onto granular chicken bone. Applied Biochemistry and Biotechnology, 32(2), 89–109. doi:10.1007/BF02922151.
Schenck, F. W., & Hebeda, R. E. (1992). Starch hydrolysis products: An introduction and history. In F. W. Schenck, & R. E. Hebeda (Eds.), Starch hydrolysis products worldwide technology production and applications (pp. 1–21). New York, USA: VCH.
Silva, R. N., Asquieri, E. R., & Fernandes, K. F. (2005). Immobilization of Aspergillus niger glucoamylase onto a polyaniline polymer. Process Biochemistry, 40(3), 1155–1159. doi:10.1016/j.procbio.2004.04.006.
Soriano, R., Bautista, L. F., Martínez, M., & Aracil, J. (1999). Adsorption isotherms of the Aspergillus niger glucoamylases i and ii on the anionic exchanger deae-toyopearl 650+. Journal of Chemical Technology & Biotechnology, 74(3), 199–203. doi:10.1002/(SICI)1097-4660(199903)74:3<199::AID-JCTB42>3.0.CO;2-B.
Swinkels, J. J. (1985). Sources of starch, its chemistry and physics. In G. M. A. Van Beynum, & J. A. Roels (Eds.), Starch conversion technology (pp. 15–46). New York, USA: Marcel Dekker.
Tanriseven, A., Uludağ, Y. B., & Doğan, Ş. (2002). A novel method for the immobilization of glucoamylase to produce glucose from maltodextrin. Enzyme and Microbial Technology, 30(3), 406–409. doi:10.1016/S0141-0229(02)00004-2.
Torres, R., Pessela, B. C. C., Mateo, C., Ortiz, C., Fuentes, M., Guisán, J. M., & Fernandes-Lafuente, R. (2004). Reversible immobilization of glucoamylase by ionic adsorption on sepabeads coated with polyethylenimine. Biotechnology Progress, 20(4), 1297–300. doi:10.1021/bp049943g.
Trevan, M. D. (1980). Immobilized enzymes. An introduction and applications in biotechnology p. 20. New York, USA: Wiley.
Trinder, P. (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annals of Clinical Biochemistry, 6(1), 24–28.
Varavinit, S., Chaokasem, N., & Shobsngob, S. (2001). Covalent immobilization of a glucoamylase to bagase dialdehyde cellulose. World Journal of Microbiology & Biotechnology, 17(7), 721–725. doi:10.1023/A:1012984802624.
Velayudhan, A., & Horváth, C. (1994). Adsorption and ion-exchange isotherms in preparative chromatography. Journal of Chromatography A, 663(1), 1–10. doi:10.1016/0021-9673(94)80490-7.
Waliszweski, K. N., Garcia-Alvarado, M., & Medina, J. C. (1992). Kinetics of enzyme hydrolysis of cassava flour starch-optimization and modelling. International Journal of Food Science & Technology, 27(4), 465–472.
Woodward, J. (1985). Immobilised enzymes: Adsorption and covalent coupling. In J. Woodward (Ed.),Immobilised cells and enzymes: A practical approach (pp. 3,13–15). Washington, DC, USA: IRL.
Acknowledgments
This research was supported by the International Program in the Chemical Sciences (IPICS), Uppsala University, Sweden (project ECU-01). The support of LATSOBIO and LANFOOD-IPICS networks is specially acknowledged. The authors thank to Engrs. N. Espín, O. Proaño and P. Polit from Escuela Politécnica Nacional for their valuable contribution to the development of experimental work. Prof. Dr. Jan D. Miller, Chair Department of Metallurgy, College of Mines and Earth Science, University of Utah and Dr. Ximena Díaz are kindly acknowledged for the surface area analysis of the supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant: International Program in the Chemical Sciences (IPICS), Uppsala University, Sweden (project ECU-01).
Rights and permissions
About this article
Cite this article
Carpio, C., Escobar, F., Batista-Viera, F. et al. Bone-Bound Glucoamylase as a Biocatalyst in Bench-Scale Production of Glucose Syrups from Liquefied Cassava Starch. Food Bioprocess Technol 4, 566–577 (2011). https://doi.org/10.1007/s11947-008-0164-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0164-9