Abstract
The objective of this work was to study the mobility of water and sucrose during osmotic dehydration and storage of apple tissue and to conduct an analysis of the behavior of the effective diffusion coefficients determined from concentration profiles. Osmotic dehydration (OD) of apple was carried out at 40°C for 1 h, and the solution: sample ratio was 20:1 (w/w). Samples of 20-mm diameter were extracted from the dehydrated apple immediately after the OD process and after 4 and 24 h of storage at 25 °C. Moisture of these samples and soluble solids content were analyzed. Our results showed, after 1 h of OD, the outer layer of the apple sample lost 0.37 kg water/kg apple and gained 0.30 kg sucrose/kg apple. These values decreased toward the internal layers of the apple. A fine layer of greatly dehydrated cells was formed on the surface around the sample, which determined the mass transfer rate in the whole tissue. Smaller mass transport rates were observed in the development of concentration profiles during storage. Diffusion coefficients obtained for the outer layer after 1 h of OD were 1.53 × 10−10 and 1.05 × 10−10 m2/s for water and sucrose, respectively. The analysis of compositional profiles developed during osmodehydration was a useful tool to get a better understanding of the changes in the water activity of the outer layer of the apple tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osmotic dehydration involves immersing a biological material in a concentrated solution of higher osmotic pressure. Water is released from the product, and the biological material is simultaneously impregnated with the solute of the osmotic solution. Osmotic dehydration has been found especially suitable for the production of minimally processed fruits of improved quality and extended shelf-life. Foods with high a w are suitable habitats for the growth of bacteria, yeasts, and molds. But high acid contents determine an unsuitable environment for the growth of most bacteria. So the low pH of these minimally processed fruits establishes a potential type of spoilage comprised of yeasts, molds, and acid tolerant bacteria. Considering that a slight reduction of pH below the optimum increases the lower limit of a w for bacterial growth and, vice versa, a slight reduction of a w (0.94–0.98) diminishes the range of pH that permits growth, it is expected that the interaction pH–a w in those ranges will be enough to suppress the growth of most bacteria of concern in fruit preservation (Alzamora et al. 1995; Welti-Chanes and Vergara-Balderas 1995). The combined factor approach has resulted in extensive research and development of shelf-stable foods with a w between 0.90 and 0.95 supplemented by additional factors to ensure microbial stability (Chirife and Favetto 1992). It was shown that a reduction in a w to about 0.93 would be sufficient to suppress the growth of most pathogenic bacteria, and the remaining ones could be effectively dealt with using the other factors such as reduced pH and pasteurization (Jayaraman 1995). However, depending on the technique of water activity measurement, the result may be different. It is necessary for a slow equilibrium between the product and air surrounding it that this equilibrium is reached. The obtaining of equilibrium is asymptotic. One has to inform about the time needed to reach such equilibrium. Rapid measurement of a w using the dew point technique only gives the surface equilibrium relative humidity of the product, which may not be sufficient for shelf-life prediction for example (Mathlouthi 2001).
Mass transfer in osmotic dehydration of tissue foods, such as fruit and vegetables, produces the establishment of water and solutes concentration profiles and significant structural changes in the tissue. The analysis of compositional profiles developed during osmodehydration is a useful tool to get a better understanding not only of the mechanisms promoting the mass transfer within the plant tissue but also of the process kinetics and the stability of the foodstuff during storage (Atarés et al. 2004). The phenomena of osmotically induced mass transfer in plant tissues have been studied for decades. Several approaches for modeling these phenomena have been proposed. The simplest approach is to use the traditional Fick’s law of diffusion. Crank (1975) made a detailed theoretical description of this approach for non-cellular materials. For cellular materials, an effective or apparent diffusion coefficient is usually obtained to account for the effect of void fraction and tortuosity (Floros and Chinnan 1989). However, due to the complexity of cellular materials, empirical and semi-empirical expressions pertaining to the observed systems must be used (Hawkes and Flink 1978; Conway et al. 1983; Biswal and Bozorgmehr 1992; Azuara et al. 1992).
There are a number of works reported in the literature for the calculation of effective diffusion coefficients for water and solute during osmotic dehydration based on moisture and solute concentration profiles. Salvatori et al. (1999a, b) determined water and sugar content profiles and proposed an “advancing disturbance front” (ADF) mechanism. The results showed that full development of concentration profiles takes several hours depending on temperature and sample thickness. Rastogi and Raghavarao (2004) estimated the effective diffusion coefficients of potatoes from the solution of Fick’s law for unsteady-state mass transfer developed by Crank (1975) for relative distance. The values of moisture and solute diffusion coefficients remained constant up to a relative distance of 0.5, beyond which it increased with increase in relative distance. Other authors (Mauro and Menegalli 2003; Monnerat et al. 2005; Monnerat et al. 2006) developed a complete model that takes into account the simultaneous resolution of diffusing species and global flux of mass in a shrinkage solid matrix. The mathematical model considered a ternary system of species k (A, B, and C). The species A and B constitute a solution (water and solute), and they can move in and out of the system. Species C represent the solids that are permanently in the system, forming a fixed matrix that delimits the boundaries of the system. The model was applied to the osmotic dehydration of potatoes and apples, obtaining effective diffusion coefficients for sucrose and water one or even two orders of magnitude lower than those for the pure solutions and presented unusual concentration dependence.
The objective of this work was to study the mobility of water and sucrose during osmotic dehydration and storage of apple tissue and to conduct an analysis of the behavior of the effective diffusion coefficients determined from concentration profiles.
Materials and Methods
Process Conditions and Analytical Methods
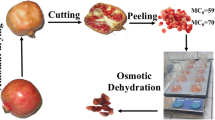
Apples of the “Golden Delicious” variety were purchased from a local supermarket. The osmotic solution (60% w/w) was prepared by dissolving sucrose in deionized water at 40 °C up to complete dissolution. A 20:1 weight ratio (osmotic solution: apple slices) avoided changes in the solution concentration due to sample withdrawal. Apple halves were obtained by cross-sectionally cutting the fruit without removing the skin, so that only the cut surface was available for mass transfer (Fig. 1). The system was maintained at 40 °C in a water bath, and the osmotic dehydration (OD) of apple was carried out without agitation during 1 h. At the end of the osmosis period, the apple half was removed from the solution, and its surface was gently blotted with tissue paper. A sharp tubular cork borer (20-mm internal diameter) was used to extract three cylindrical samples. The apple cylinders were analyzed immediately after the OD process and then after 4 and 24 h of storage at 25 °C.
The moisture loss to the environment during the storage was prevented covering over the apple cylinders with a plastic adhesive film. Starting at the surface exposed to the osmotic solution, each sample cylinder was sliced into 1.5-mm thick disks (Fig. 1).
Three disks from the same level in the cores were used for each determination. The concentrations of osmotic medium and of soluble solids of apple disks were monitored at 20.5 °C using a bench-top refractometer (Atago Model 1T, Japan) equipped with an Atago digital thermometer. Each apple disk was crushed, and a drop of filtered juice was placed in the refractometer to determine the concentration of soluble solids. Moisture and total solids measurements were done by the vacuum oven method at 60 °C for 74 h. Water activity was measured at 25 °C using an Aqualab hygrometer model Series 3, Decagon Devices Inc., Pullman, WA, USA. The density was calculated using the mass of the apple slice and the volume determined with an electronic digital caliper (Truper CALDI-6MP). The concentration C (kg/m3) used to determine the diffusion coefficients from Eqs. 6 and 7 was calculated by multiplying the water and sucrose contents by the density of each apple slice (Fig. 2).
The precision or the magnitude of the differences between replicates, of the water content, sucrose content, and a w by disk were studied using the standard deviation of the mean of a set of three replicates.
Determination of the Diffusion Coefficients
The diffusion coefficients for water and sucrose were determined by using the procedure developed by Crank (1975). A case of great practical interest is that in which the diffusion coefficient depends only on the concentration of diffusing substance. Such a concentration-dependence exists in most systems, but often, e.g., in dilute solutions, the dependence is slight and the diffusion coefficient can be assumed constant for practical purposes. In other cases, however, the concentration dependence is very marked. A number of methods have been used to obtain numerical solutions, some applicable to any type of concentration-dependent diffusion coefficient, and others restricted to particular types, e.g., exponential or linear dependence. In other cases, algebraic solutions have been expressed in terms of a single integral, and these will be referred to as formal solutions even though the integral has to be evaluated numerically (Crank 1975). There are a number of optical methods for observing how either the refractive index or its gradient depends on distance measured in the direction of diffusion at a given time (Crank 1975). If two infinite media are brought together at t = 0, the diffusion coefficient and its concentration-dependence can readily be deduced from the concentration distribution observed at some known subsequent time. The conditions of the experiment are:
where C is the concentration of the component in which we are interested, and z = 0 is the position of the initial interface between the two components at time t = 0. The equation for one-dimensional diffusion when the diffusion coefficient D is a function of concentration C is:
We may use the Boltzmann variable \(\eta = {z \mathord{\left/ {\vphantom {z {2t^{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} }}} \right. \kern-\nulldelimiterspace} {2t^{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} }}\), and obtain the ordinary differential equation:
On integration with respect to η becomes:
Finally, by rearrangement of Eq. 5 and introducing z and t, we have:
where: C 1 (kg /m3) is any value of concentration between 0 and C s , C s is the concentration in the interface, z (m) is distance from the surface, t (s) is the time, and D (m2/s) is the diffusion coefficient. The procedure consists in plotting concentration vs distance for a defined time to locate the plane z = 0 by Eq. 7 and later D is obtained by Eq. 6.
The conditions 1 and 2 can be satisfied for both sucrose and water by introducing the follow definitions:
-
(a)
For sucrose, C is the concentration of sucrose at a given time, \(C_s = C_{\infty s} \) and \(C_{\infty s} \) is the initial concentration of sucrose in the osmotic solution.
-
(b)
For water, \(C = C* - C_{\infty w} ,C_s = C_{wa} - C_{\infty w} \), C* is the concentration of water at a given time, C wa is the initial concentration of water in the apple tissue, and \(C_{\infty w} \) is the initial concentration of water in the osmotic solution.
Results and Discussion
The concentration profiles for water immediately after the OD process and after 4 and 24 h of storage at 25 °C are shown in Fig. 3. It can be observed that water content increased from the surface exposed to the osmotic solution until 4 mm from the interface, where the water concentration was similar to that of the fresh tissue. The same figure shows different concentration profiles for apple cylinders stored for 4 and 24 h due to water migration from internal layers to the apple surface. This resulted in higher moisture content at the surface of apple tissue stored for 4 and 24 h.
Sucrose concentration profiles inside the apple are presented in Fig. 4. As it can be noted, the extent of penetration by the sucrose was about 4 mm in osmodehydrated apple samples for 1 h at 40 °C (see the arrow in the Fig. 4). Monnerat et al. (2005, 2006) reported smaller depth of penetration for sucrose, probably because their experiments were carried out at lower sucrose solution concentration and temperature (50% w/w at 27 °C). Other authors presented experimental results of sucrose and water content in apple slices 20 and 30 mm thick and at 20, 30, 40, and 50 °C after different times of exposure to a 65% w/w sucrose solution (Salvatori et al. 1999a). The data analysis showed a quickly equilibrated thickness close to the surface and a constant advancing rate of a disturbance front, defined by the authors as an imaginary plane locate between the more external zone affected by the mass transfer and the other (internal) without concentration changes. The results, determined by these authors, for slices 30 mm thick, treated in 65% w/w sucrose solution at 30 °C up to 1 h, could be compared to our results, for apple tissue, treated in 60% sucrose solution at 40 °C. During storage (4 and 24 h), the penetration of solids was higher, and the sucrose migrated from the apple surface toward the internal layers, decreasing the sucrose concentration in the zone of the apple near the interface. The mobility of water and sucrose during storage produced important changes in the water activity of the outer layer of apple product. Figure 5 shows that the water activity of the outer layer of apple measured immediately after the OD process is lower than the water activity of the samples stored 4 and 24 h. This result is a consequence of the counter diffusion of water and sucrose during storage. At equilibrium, all layers of the apple sample have the same water activity. However, the time to reach the equilibrium during storage is a function of several parameters as size and shape of the foodstuff, molecular weight of gained solids, food microstructure, storage temperature, etc. These observations indicate that the analysis of compositional profiles developed during osmotic treatment is a useful tool to get a better understanding of not only the mechanisms promoting the mass transfer within the plant tissue but also of the food stability throughout the storage. A slight increase in the water activity of the outer layer of minimally processed fruits can permit the growth of pathogenic bacteria. When water activity is measured, it is generally required to know if the product has reached the equilibrium. That is why accuracy within 0.01 a w unit is sufficient for most food related applications (Mathlouthi 2001).
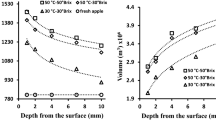
Figures 6 and 7 depict the estimated diffusion coefficients for water and sucrose as a function of the distance from the surface. Diffusion coefficients for water and sucrose were in the range of 1.53 × 10−10 to 7.89 × 10−10 m2/s and 1.05 × 10−10 to 3.1 × 10−10 m2/s, respectively. These results were similar to those obtained by other authors (Azuara et al. 2003; Warin et al. 1997; Regier et al. 2004; Telis et al. 2004; Jena and Das 2005; Khin et al. 2007). The variation of the diffusion coefficients values with respect to the distance from the surface lead to the conclusion that the higher diffusion coefficients in the internal layers of apple tissue are probably due to the constant advancing rate of a disturbance front. The position of the ADF is a function of time of treatment and temperature (Salvatori et al. 1999a). This behavior influences the position of the maximum of the curves represented in Figs. 6 and 7, where the highest value the diffusion coefficient corresponded to the ADF. Diffusion coefficients calculated from experimental concentration–distance curves were of the same order of magnitude for water and sucrose at the apple surface but, for the internal layers, were higher for water than for sucrose. The conjunction of these factors leads to higher effective diffusion coefficients in the surface region and lower coefficients in the inner region only for short processing times. For longer processing times, the constant advancing rate of a disturbance front will lead to higher diffusion coefficients in the inner region of apple tissue as a result of the preferential movement of ADF toward deeper layers (Fig. 8). Monnerat et al. (2005, 2006) estimated effective diffusion coefficients for sucrose and water in treated apple tissue one or even two orders of magnitude lower than those for the pure solutions reported by Henrion (1964). Moreover, their results for 50% sucrose solution showed an unusual behavior when compared with the curve for binary coefficients of pure sucrose solutions. The diffusion coefficients obtained in this work were one order of magnitude higher than those estimated by Monnerat et al. (2005, 2006) but one order lower than those reported by Rastogi and Raghavarao (2004), probably because of the differences in sucrose solution concentration, temperature, and raw material.
For comparison purposes, the values of diffusion coefficients obtained from experimental concentration–distance curves were plotted with the diffusion coefficients determined by Henrion (1964) for pure solution as a function of sucrose concentration (Figs. 9 and 10). In Fig. 9, the curves obtained for the diffusion coefficients of the apple cylinders analyzed immediately after 1 h of osmotic dehydration at 40 °C showed the same behavior as the sucrose–water binary coefficients found in the literature (Henrion 1964), i.e., higher diffusion coefficients correspond to lower sucrose concentrations or deeper layers. Furthermore, it can be observed at the lowest sucrose concentrations an inverse behavior corresponding to the zone with no significant concentration changes and therefore without any significant mass fluxes, where water and sucrose diffusion coefficients decay quickly to zero. Figure 10 shows a comparison of binary coefficients of pure sucrose solutions with the values calculated for apple tissue stored 4 and 24 h at 25 °C. As can be seen, the coefficients obtained for apple tissue storage are lower than those for pure solutions and present an inverse but much smoother behavior at very low sucrose concentrations. Consequently, during storage, water and sucrose can move inside of apple tissue producing lower concentration gradients and lower diffusion coefficients until the equilibrium is reached.
Diffusion coefficients for water and sucrose as a function of the sucrose concentration (osmotic solution at 60% w/w; 40 °C). Binary diffusion coefficients at 50 °C determined by Henrion (1964) for pure solutions as a function of sucrose concentration
Diffusion coefficients for water and sucrose as a function of the sucrose concentration (storage at 25 °C, during 4 and 24 h). Binary diffusion coefficients at 25 °C determined by Henrion (1964) for pure solutions as a function of sucrose concentration
Conclusions
The values of diffusion coefficients obtained from experimental concentration–distance curves showed the same behavior as the sucrose–water binary coefficients found in the literature. Diffusion coefficients for water and sucrose were higher in the internal layers of the apple cylinders than in the layer near the surface exposed to the osmotic solution. The higher diffusion coefficients in the internal layers of apple tissue are probably due to the constant advancing rate of a disturbance front. The sucrose resistance to diffusion was higher than water resistance, which explains the dehydration efficiency of osmotic process for fruits. The counter diffusion of water and sucrose during storage produced important changes in the water activity of the outer layer of apple cylinders.
References
Alzamora, S. M., Cerrutti, P., Guerrero, S., & López-Malo, A. (1995). Minimally processed fruits by combined methods. In G. V. Barbosa-Cánovas, & J. Welti-Chanes (Eds.), Food preservation by moisture control: Fundamentals and applications—ISOPOW practicum II (pp. 463–492). Lancaster, USA: Technomic Publishing.
Atarés, S. L., González, M. C., & Chiralt, A. (2004). Development of concentration profiles during storage of osmodehydrated apple slices. In: Proceedings of the International Congress on Engineering and Food (ICEF9), 7–11 March 2004, Montpellier, France (CD-ROM).
Azuara, E., Cortes, R., García, H. S., & Beristain, C. I. (1992). Kinetic model for osmotic dehydration and its relationship with Fick’s second law. International Journal of Food Science and Technology, 27(4), 409–418.
Azuara, E., Gutiérrez, G. F., & Beristain, C. I. (2003). Mass transfer description of the osmodehydration of apple slabs. In J. Welti-Chanes, J. F. Vélez-Ruiz, & G. V. Barbosa-Cánovas (Eds.), Transport phenomena in food processing (pp. 95–107). Florida, USA: CRC.
Biswal, R. N., & Bozorgmehr, K. (1992). Mass transfer in mixed solute osmotic dehydration of apple rings. Transactions of American Society of Agricultural Engineers, 35(1), 257–262.
Chirife, J., & Favetto, G. J. (1992). Some physicochemical basis of food preservation by combined methods. Food Research International, 25(5), 389–396.
Conway, J., Castaigne, G., Picard, G., & Vovan, X. (1983). Mass transfer considerations in the osmotic dehydration of apple. Canadian Institute of Food Science and Technology Journal, 16(1), 25–29.
Crank, J. (1975). The mathematics of diffusion (2nd ed.). London, UK: Clarendon.
Floros, J. D., & Chinnan, M. S. (1989). Determining the diffusivity of sodium hydroxide through tomato and capsicum skins. Journal of Food Engineering, 9(2), 129–141.
Hawkes, J., & Flink, J. M. (1978). Osmotic concentration of fruit slices prior to freeze dehydration. Journal of Food Processing and Preservation, 2(4), 265–284.
Henrion, P. N. (1964). Diffusion in the sucrose+water system. Transaction Faraday Society, 60, 72–74.
Jayaraman, K. S. (1995). Critical review on intermediate moisture fruits and vegetables. In G. V. Barbosa-Cánovas, & J. Welti-Chanes (Eds.), Food preservation by moisture control: Fundamentals and applications-ISOPOW Practicum II (pp. 411–441). Lancaster, USA: Technomic Publishing.
Jena, S., & Das, H. (2005). Modelling for moisture variation during osmo-concentration in apple and pineapple. Journal of Food Engineering, 66(4), 425–432.
Khin, M. M., Zhou, W., & Perera, C. O. (2007). Impact of process conditions and coatings on the dehydration efficiency and cellular structure of apple tissue during osmotic dehydration. Journal of Food Engineering, 79(3), 817–827.
Mathlouthi, M. (2001). Water content, water activity, water structure and the stability of foodstuffs. Food Control, 12(7), 409–417.
Mauro, M. A., & Menegalli, F. C. (2003). Evaluation of water and sucrose diffusion coefficients in potato tissue during osmotic concentration. Journal of Food Engineering, 57(4), 367–374.
Monnerat, S. M., Pizzi, T. R. M., Mauro, M. A., & Menegalli, F. C. (2005). Spatial distribution of solutes and water in sucrose solution dehydrated apples. Drying Technology, 23(9–11), 2289–2299.
Monnerat, S. M., Pizzi, T. R. M., Mauro, M. A., & Menegalli, F. C. (2006). Concentration profiles and effective diffusion coefficients of sucrose and water in osmo-dehydrated apples. Food Research International, 39(6), 739–748.
Rastogi, N. K., & Raghavarao, K. S. M. S. (2004). Mass transfer during osmotic dehydration determination of moisture and solute diffusion coefficients from concentration profiles. Transactions of the Institution of Chemical Engineers, Part C, Food and Bioproducts Processing, 82(C1), 44–48.
Regier, M., Knörzer, K., Schubert, H., & Schuchmann, H. (2004). Water distribution and mobility in food during microwave vacuum drying studied by magnetic resonance. In: Proceedings of the International Congress on Engineering and Food (ICEF9), 7–11 March 2004, Montpellier, France (CD-ROM).
Salvatori, D., Andrés, A., Chiralt, A., & Fito, P. (1999a). Osmotic dehydration progression in apple tissue I: spatial distribution of solutes and moisture content. Journal of Food Engineering, 42(3), 125–132.
Salvatori, D., Andrés, A., Chiralt, A., & Fito, P. (1999b). Osmotic dehydration progression in apple tissue II: Generalized equations for concentration prediction. Journal of Food Engineering, 42(3), 133–138.
Telis, V. R. N., Murari, R. C. B. D. L., & Yamashita, F. (2004). Diffusion coefficients during osmotic dehydration of tomatoes in ternary solutions. Journal of Food Engineering, 61(2), 253–259.
Warin, F., Gekas, V., Voirin, A., & Dejmek, P. (1997). Sugar diffusivity in agar gel/milk bilayer systems. Journal of Food Science, 62(3), 454–456.
Welti-Chanes, J., & Vergara-Balderas, F. (1995). Fruit preservation by combined methods: an Ibero-American Research Project. In G. V. Barbosa-Cánovas, & J. Welti-Chanes (Eds.), Food preservation by moisture control: Fundamentals and applications-ISOPOW Practicum II (pp. 449–462). Lancaster, USA: Technomic Publishing.
Acknowledgments
Authors acknowledge the support received from CONACYT-Mex., projects 25773 and 53014. Flores E. thanks CONACYT for their study grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azuara, E., Flores, E. & Beristain, C.I. Water Diffusion and Concentration Profiles During Osmodehydration and Storage of Apple Tissue. Food Bioprocess Technol 2, 361–367 (2009). https://doi.org/10.1007/s11947-008-0077-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0077-7