Abstract
The ability of Aspergillus niger GH1 in converting creosote bush ellagitannins into ellagic acid (EA) was evaluated in solid state culture. Creosote bush leaves were used to extract the ellagitannins fraction, which was impregnated in polyurethane foam used as support of solid state culture. Ellagitannins content, EA accumulation, and the related enzymatic activities were evaluated. A. niger GH1 was able to completely degrade creosote bush ellagitannins with an EA yield of 23.1% at 36 h of culture. The ability to degrade creosote bush ellagitannins exhibited by A. niger GH1 was clearly associated to an ellagitannin-hydrolysing enzyme with a maximum activity of 43 U/l, while that ability was not associated to tannase activity that was detected in the culture extract. This study demonstrated the great ability of A. niger GH1 to hydrolyze ellagitannins and the potential of solid state culture to produce the antioxidant EA by degradation of creosote bush ellagitannins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ellagic acid (EA) is a phenolic compound naturally occurring in several plants, such as oak tree, eucalyptus, pomegranate, strawberry, raspberry, blueberry, blackberry, cranberry, gooseberry, grape, pecan, walnut, valonea, and creosote bush (Seeram et al. 2004; Koponen et al. 2007). In recent years, EA has been reported as a potent antioxidant, anticarcinogenic, antiviral, antimutagenic, and antiparasitic molecule and have shown attractive alternatives of applications (Martens-Talcott et al. 2003; Ruibal et al. 2003; Vattem and Shetty 2003; Fukuda et al. 2003; Olsson et al. 2004; Okuda 2005; Huetz et al. 2005; Divipriya et al. 2007).
EA is usually present in plant sources as ellagitannins, which consist of glucose esterified with hexahydroxydiphenic acid, gallic acid, and their derivatives (Fig. 1). To obtain EA from plants, some studies on hydrolyzing ellagitannins with acid or base have been reported (Daniel et al. 1991; Shi et al. 2005). Due to variation in plant sources, the differences of ellagitannins structure, and the difficulty of purification, these chemical methods often lead to low yields of EA and considerable impurities.
In this study, the biodegradation of creosote bush ellagitannins was studied. Creosote bush ellagitannins contain a large proportion of hexahydroxidiphenoyl groups, having the potential to yield a high amount of EA. However, about the enzyme(s) involved in the process of ellagitannins biodegradation, the information is scarce and confuse. Shi et al. (2005) hypothesized the role of typical tannase (tannin acyl hydrolase, TAH) and polyphenol oxidase in this biochemical event. Some reports of tannin biodegradation have been published (Bath et al. 1998; Aguilar et al. 2007), and the role of a nontypical tannase (ellagitannase) has been seriously considered and partially demonstrated in the studies carried out by Huang et al. (2007a, b, c, d).
In this study, the ability of A. niger GH1 to convert creosote bush ellagitannins into EA was evaluated. The degradation of ellagitannins and the related enzymes produced in solid state culture (SSC) were estimated and associated with EA accumulation.
Materials and Methods
In this study, the creosote bush ellagitannins biodegradation was evaluated in SSC. For that culture, total creosote bush polyphenols (TCbP) were used as sole carbon source and inducer of related enzymes involved in biodegradation of ellagitannins.
Microorganism and Plant Material
A. niger strain GH1 (culture collection of the DIA-UADEC, Saltillo, Coahuila, Mexico) was used in this study because of its potential to degrade polyphenols. Fungal strain was previously isolated, identified, and characterized (Cruz-Hernández et al. 2005).
Creosote bush shrubs were collected from the desert region of Coahuila State in North Mexico and transported to the Microbiology Laboratory of the Food Research Department, where the material was cleaned, dried at 60 °C during 48 h, pulverized in a homogenizer LP12 series 600 (Maquinaria®, Monterrey, Nuevo León, Mexico), and stored at room temperature in black bags.
Polyphenols Extraction and Ellagitannins Fractionation
Creosote bush powder was refluxed with acetone at 70% in the ratio of 1:4 (w/v) by 12 h at 60 °C. Then, it was filtered through Whatman no. 41 filter. Solvent was eliminated in a rotovapor system (model RE 540, Yamato Scientific America, Inc., Santa Clara, CA, USA.). The aqueous extract was lyophilized in a vacuum system (Labconco, FreeZone Dry system, Kansas City, MO, USA) until obtaining a green powder considered as TCbP. Based on the ellagitannins fractionation protocol reported by Seeram et al. (2005), it was possible by it to obtain the substrate used in the enzymatic assay for ellagitannin-hydrolysing activity. Briefly, the extraction of ellagitannins consisted in a depigmentation of TCbP using hexane, sugars content was removed with ethanol, and finally, a column packed with amberlite lipophilic resin preequilibrated with water and methanol and eluted with increasing amounts of methanol was used to obtain the ellagitannins. Content of ellagitannins was quantified by the method reported by Ascacio et al. (2007), employing the high-performance liquid chromatography (HPLC) method and using as standard punicalagin extracted from pomegranate peels supplied by the Bioprocess Laboratory of the Food Research Department (School of Chemistry, Universidad Autónoma de Coahuila).
Culture Medium
The culture medium used for EA production was previously reported by Robledo et al. (2008), and it contained (g/l) NaNO3 (6.0), KH2PO4 (1.520), KCl (0.52), MgSO4*7H2O (0.52), ZnSO4 (0.0010), FeCl3, (0.00085), 0.05% of yeast extract, and 1.0 ml trace metals solution. Trace metal solution was composed of (mg/l) Na2B4O7·10H2O (100.0), MnCl2·4H2O (50.0), Na2MoO4·2H2O (50.0), and CuSO4·5H2O (250.0). The pH of the medium was adjusted to 5.0, and then, the medium was autoclaved (1.1 kg/cm3; 121 °C) for 15 min. When the medium temperature was 35 °C, TCbP were added at a final concentration of 12 g/l. The pH was 5.5. The medium was filtered–sterilized through nylon membranes of 25-mm diameter and 0.45-μm pore size.
Solid State Culture
The EA production was evaluated in SSC employing polyurethane foam (PUF; 3 g) as support into 250 ml reactors. Support was impregnated with the culture medium at an initial moisture content of 70%. Inoculum was previously added to the culture medium at a level of 2 × 107 spores per gram of support. Wet PUF was carefully homogenized, incubated at 30 °C, and monitored during 96 h. All samples were analyzed in triplicates.
Crude Extract Recovery
A total of 20 ml of citrate buffer (pH 5.0, 25 mM) was added to each reactor, homogenized during 1 min and separated by filtration using Whatman no. 40 filter; later, the filtered extract was refiltered by 0.45-μm nylon membranes, and the new extract was used to analyze the ellagitannins and glycosides consumption, EA accumulation, TAH, and ellagitannin-hydrolyzing activity (EHA).
Analytical Determinations
Glycosides consumption was carried out by phenol-sulfuric method (Dubois et al. 1956). The TCbP concentration was determinated by the modified Folin–Ciocalteu method (Makkar et al. 1993). Ellagitannins content was quantified by the HPLC method reported by Ascacio et al. (2007). For EA recovering and quantification, the method employed was as follows: An aliquot of extract was added into 1.5-ml conic tubes and centrifuged at 6,000 rpm (3,600 g) during 20 min. The supernatant was decanted, and the precipitate was resuspended in ethanol. The sample was mixed into a sonic bath for 20 min. The sample with suspended material was filtered through 0.45-μm nylon membrane and injected into HPLC. The EA recovered was quantified by HPLC (Varian Pro Star, Palo Alto, CA, USA) using a photodiode array detector (PDA Pro Star 330) at 254 nm. Separation was carried out with a Prodigy ODS column (5 μm; 250 × 4.6 mm, Phenomenex) and temperature of 30 °C. A gradient profile of mobile phase, consisting of acetonitrile (solvent A) and 0.3% acetic acid in water (v/v; solvent B), 7–20% A (0–7 min), 20–30% A (7–12 min), 30% A (12–18 min), 30–60% A (18–20 min), 60–100% A (20–23 min), 100% A (23–30 min), 7% A (30–31 min), and 7 min for baseline stabilization was applied at a flow rate of 0.7 ml/min. The sample injection was of 10 μl. TAH activity was assayed using the HPLC method reported by Aguilar et al. (1999). A TAH unit was defined as the amount of enzyme required to release one micromole of gallic acid under assay conditions. EHA was assayed using the HPLC method reported by Aguilera-Carbo et al. (2007a). An EHA unit was defined as the amount of enzyme required to release one micromole of EA under assay conditions.
Results and Discussion
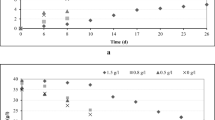
Figure 2 shows the glycosides consumption by Aspergillus niger GH1. The depletion of total glycosides was fast during the first 24 h of culture, and it permitted a rapid mycelial invasion on trabecules of PUF. Phenolics content was also reduced during the first 24 h of culture as result of consumption of free monomers present in the TCbP fraction (Robledo et al. 2008), and then, an increment of these compounds was detected as a result of enzymatic hydrolysis of TCbP (Fig. 2).
Ellagitannins content remained unchanged during the first 24 h of culture because the fungus grew by consuming glycosides and free phenolics. However, after this period, glycosides were completely consumed by the fungus during the next 48 h of culture (Fig. 2).
Maximum concentration of EA released was reached at 36 h of culture with a yield of 23.1% of the total content of ellagitannins (Fig. 3). However, the EA was decreased, possibly because EA represents one of the substrates generated by the action of ellagitannin-hydrolyzing enzymes produced in SSC (Aguilera-Carbo et al. 2008). For this reason, it is very important to stop the fermentation at this time. Pattern of EA released was clearly associated to evaluated EHA, which was detected at its maximum level at the same time (36 min) and decreased afterward (Fig. 4).
Typical tannase activity was not associated to EA released because it was detected after 48 h of culture, maybe as the result of the presence of galloyl residues that are inducers of this enzyme (Aguilar et al. 2007).
The obtained results suggest that creosote bush ellagitannins are hydrolyzed by the ellagitanins-hydrolyzing enzymes, which has not been previously reported. In addition, these results demonstrated that typical tannase activity is not sufficient to hydrolyze ellagitannins as suggested by Shi et al. (2005) during their study of EA accumulation by the submerged co-culture of A. niger and Candida utilis. However, the EA yields obtained in both studies were very similar, 21% (Shi et al. 2005) and 23% (in our present study).
Important advances in EA production from ellagitannins have been reported in submerged co-cultures by Huang et al. (2007a, b, c). Recently, high EA yields (24%) were obtained after optimization of the co-culture of A. oryzae with Trichoderma reesei using acorn cups extract containing up to 62% ellagitannins as substrate (Huang et al. 2007c). However, in SSC, the information is limited to those studies reported by Vattem and Shetty (2002, 2003), using cranberry pomace as support and source of ellagitannins with very low EA yields. Huang et al. (2007c) suggested, for the first time, the presence of ellagitannin acyl hydrolase as the enzyme responsible of the EA accumulation, which indicates that a new tannase is involved in the biodegradation ellagitannins. Also, they reported that such enzyme had an synergistic activity with other enzymes as xylanase and cellulase to enhance the EA accumulation. However, further studies are needed to define the catalytic role and properties of this new EHA or ellagitannin acyl hydrolase detected.
It is known that tannins have a range of effects on various organisms, from toxic effects on animals to growth inhibition of microorganisms. Some microbes are, however, resistant to tannins, and have developed various mechanisms and pathways for tannin degradation in their natural habitats. Tannases generally act on gallotannins. However, in the particular case of ellagitannins, the information is scarce and confuse (Vivas et al. 2004), mainly due to their chemical complexity and diversity. Saavedra et al. (2005) reported that the production of EA has not been explored due to its high production cost and the great amount of subproducts generated as result of ellagitannin biodegradation. This results in serious problems regarding the recovery and purification of EA. To date, there is no published data on ellagitannin degradation using biological methods (microbial or enzymatic). However, it is known that the selective hydrolysis of galloyl groups of the ellagitannin phyllianmblinin is catalyzed by tannase (Zhang et al. 2001). Vattem and Shetty (2002, 2003) reported on EA production from cranberry pomace fermented by a SSC using Lentinus edodes, attributing the catalysis to the enzyme β-glucosidase. Huang et al. (2005) described a new valonea tannin hydrolase as responsible for the biodegradation of valonea tannins. This enzyme is itself a TAH.
Recently, we have described relevant information about the advances and perspectives on microbial tannases (Aguilar et al. 2007), and also, we reported a review describing the advances on the microbial production of EA and the biodegradation of ellagitannins (Aguilera-Carbo et al. 2008).
Conclusions
The present research indicated that the SSC of A. niger GH1 using creosote bush ellagitannins impregnated in PUF could remarkably enhance EA accumulation, which was clearly associated to EHA. The EA yield of 23%, obtained under non-optimal conditions, suggests a new scheme for EA production from creosote bush, a yet poorly explored forest material.
References
Aguilar, C. N., Augur, C., Viniegra-González, G., & Favela, E. (1999). A comparison of methods to determine tannin acyl hydrolase activity. Brazilian Archives in Biology and Technology, 42, 355–361.
Aguilar, C. N., Rodríguez, R., Gutierrez-Sanchez, G., Augur, C., Favela-Torres, E., Prado Barragán, L. A., et al. (2007). Microbial tannases: Advances and perpectives. Applied Microbiology and Biotechnology, 76(1), 47–59.
Aguilera-Carbo, A. F., Augur, C., Prado-Barragan, L. A., Favela-Torres, E., & Aguilar, C. N. (2008). Microbial production of ellagic acid and biodegradation of ellagitannins. Applied Microbiology and Biotechnology (in press). DOI 10.1007/s00253-007-1276-2.
Aguilera-Carbo, A. F., Robledo-Olivo, A., Augur, C., Prado-Barragán, L. A., Favela-Torres, E., & Aguilar, C. N. (2007). Method for ellagic acid quantification in novel sources of this natural antioxidant. Proceedings of the 5th International Congress on Food Technology, Thessaloniki, Greece.
Ascacio, J., Prieto-Nieto, A., Hernández-Rivera, J. S., Aguilera-Carbo, A., & Aguilar, C. N. (2007). Determination of ellagitannins in native plants of Mexican semiarid zone, relationship between two analytical methods. Proceedings of the Third Mexican Congress on Biopolymers: Advances and perspectives (p. 25).
Bath, T. K., Singh, B., & Sharma, O. P. (1998). Microbial degradation of tannins—A current perspective. Biodegradation, 9, 345–357.
Cerdá, B., Cerón, J. J., Tomás-Barberán, F. A., & Espín, J. C. (2003). Repeated oral administration of doses of the pomegranate ellagitannins punicalagin to rats for 37 days is not toxic. Journal of Agricultural and Food Chemistry, 51, 3493–3501.
Cruz-Hernández, M., Contreras-Esquivel, J. C., Lara, F., Rodríguez, R., & Aguilar, C. N. (2005). Isolatation and evaluation of tannin-degrading fungal starins from the Mexican desert. Zeitschrift für Naturforschung, 60, 844–848.
Daniel, M. E., Ratnayake, S., Kinstle, T., & Stoner, G. D. (1991). The effects of pH and intestinal contents on the liberation of ellagic acid from purified and crude ellagitannins. Journal of Natural Products, 54, 946–952.
Divipriya, N., Srinivasan, M., Sudheer, A. R., & Menon, V. P. (2007). Effect of ellagic acid, a natural polyphenol, on alcohol-induced prooxidant imbalance: A drug dose dependent study. Singapore Medical Journal, 48(4), 311–318.
Dubois, M., Guiles, K. A., Hamiton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugar and related substances. Analytical Chemistry, 21, 145–149.
Fukuda, T., Ito, H., & Yoshida, T. (2003). Antioxidative polyphenols from walnuts (Junglans regia L.). Phytochemistry, 63, 795–801.
Huang, W., Ni, J., & Borthwick, A. G. L. (2005). Biosynthesis of valonia tannin hydrolase and hydrolysis of valonia tannin to ellagic acid by A.niger SHL 6. Process Biochemistry, 40, 1245–1249.
Huang, W., Niu, H., Gong, G., Lu, Y., Li, Z., & Li, H. (2007a). Individual and combined effects on physicochemical parameters on ellagitannin acyl hydrolase and ellagic acid production from ellagitannin by Aspergillus oryzae. Bioprocess Biosystems Engineering, 30, 281–288.
Huang, W., Niu, H., Li, Z., He, Y., Gong, W., & Gong, G. (2007c). Optimization of ellagic acid production from ellagitannins by co-culture and correlations between its yield and activities of relevant enzymes. Bioresource Technology, 99, 769–775.
Huang, W., Niu, H., Li, Z. m., Lin, W., Gong, G., & Wang, W. (2007b). Effect of ellagitannin acyl hydrolase, xylanase and cellulase on ellagic acid production from cups extract of Valona acorns. Process Biochemistry, 42, 1291–1295.
Huang, W., Niu, H., Li, Z., & Wang, W. (2007d). Ellagic acid from acorn fringe by enzymatic hydrolysis and combined effects of operational variable and enzymes on yield of the production. Bioresource Technology, 99, 1518–1525.
Huetz, P., Mavaddat, N., & Mavri, J. (2005). Reaction between ellagic acid and ultimate carcinogen. Journal of Chemical Information and Modeling, 45, 1564–1570.
Koponen, J. M., Happonen, A. M., Mattila, P. H., & Törrönen, A. R. (2007). Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. Journal of Agricultural and Food Chemistry, 55, 1612–1619.
Makkar, H. P. S., Blümmel, M., Borowy, N. K., & Becker, K. (1993). Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. Journal of Science and Food Agriculture, 61, 161–165.
Martens-Talcott, S. U., Talcott, S. T., & Percival, S. S. (2003). Low concentrations of quercitin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLT-4 human leukemia cells1–3. Journal of Nutrition, 133, 2669–2674.
Okuda, T. (2005). Systematics and Health effects of chemically distinct tannins in medicinal plants. Phytochemistry, 66, 2012–2031.
Olsson, M. E., Ekvall, J., Gustavsson, T.-E., Nilsson, J., Pillai, D., Sjöholm, I., et al. (2004). Antioxidants, low molecular weight carbohydrates, and total antioxidant capacity in strawberries (Fragaria x annanassa): Effects of cultivar, ripening, and storage. Journal of Agricultural and Food Chemistry, 52, 2490–2498.
Robledo, A., Aguilera-Carbo, A., Rodríguez, R., Martinez, J. L., Garza, Y., & Aguilar, C. N. (2008). Ellagic acid production by Aspergillus niger in solid state fermentation of pomegranate residues. Journal of Industrial Microbiology and Biotechnology (in press). DOI 10.1007/s10295-008-0309-x.
Ruibal, B. I. J., Dubed, E. M., Martínez, L. F., Noa, R. E., Vargas, G. L. M., & Santana, R. J. L. (2003). Inhibición de la replicación del virus de inmunodeficiencia humana por extractos de Pinus caribaea Morelet. Revista Cubana de Farmacia, 37(2), 1–8.
Saavedra, P. G. A., Couri, S., Ferreira, L. S. G., & de Brito, E. S. (2005). Tanase: Conceitos, produçao e aplicaçao. B. CEPPA. Curitiba, 23, 435–462.
Seeram, N. P., Lee, R., Hardy, M., & Herber, D. (2005). Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Separation and Purification Technology, 41, 49–55.
Seeram, N. P., Lee, R., & Herber, D. (2004). Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clinical Chimica Acta, 348, 63–68.
Shi, B., He, Q., Yao, K., Huang, W., & Li, Q. (2005). Production of ellagic acid from degradation of valonea tannins by Aspergillus niger and Candida utilis. Journal of Chemical Technology and Biotechnology, 80, 1154–1159.
Vattem, D. A., & Shetty, K. (2002). Solid-state production of phenolic antioxidants from cranberry pomace by Rhizophus oliogosporus. Food Biotechnology, 16(3), 189–210.
Vattem, D. A., & Shetty, K. (2003). Ellagic acid production and phenolic antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus edodes using a solid-state system. Process Biochemistry, 39, 367–379.
Vivas, N., Laguerre, M., Pianet de Boissel, I., Vivas de Gaulejac, N., & Nonier, M. F. (2004). Conformational interpretation of vascalagin and castalagin physicochemical properties. Journal of Agricultural and Food Chemistry, 52, 2073–2078.
Wang, C. C., Chen, L. G., & Yang, L. L. (1999). Antitumor actitvity of four macrocyclic ellagitannins from Cuphea Hyssopifolia. Cancer Letters, 140, 195–200.
Zhang, Y. J., Abe, T., Tanaka, T., Yang, C. R., & Kouna, I. (2001). Phyllanemblinins A-F, nre ellagitannins from Phyllanthus emplica. Journal of Natural Products, 64, 1527–1532.
Acknowledgment
Financial support of this research was from the National Council of Science and Technology of Mexico with grant no. SEP-CONACYT-J-51360. Authors A. Aguilera-Carbo and J. S. Hernández-Rivera are graduate students supported by CONACYT, Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguilera-Carbo, A., Hernández, J.S., Augur, C. et al. Ellagic Acid Production from Biodegradation of Creosote Bush Ellagitannins by Aspergillus niger in Solid State Culture. Food Bioprocess Technol 2, 208–212 (2009). https://doi.org/10.1007/s11947-008-0063-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0063-0