Abstract
Purpose of review
Ischemic stroke is the fifth leading cause of death in the world with cardioembolic stroke (CES) causing a disproportional amount of the morbidity and mortality associated with stroke. Atrial fibrillation (AF) is the leading cause of CES, and as the population ages, the incidence of CES is anticipated to rise. The importance of proper diagnosis and treatment of patients with embolic-appearing stroke is significant due to the burden of disease and the severity of the illness.
Recent findings
The past decade has seen an explosion of treatment options for patients with CES related to AF as well as better mechanisms by which to monitor and diagnose patients with AF. While optimal secondary prevention of stroke with anticoagulation in the setting of AF is known, what remains to be defined is the appropriate treatment of other types of strokes that appear embolic, but no source of the embolism is discovered.
Summary
In this article, we will review what is known about the diagnosis and treatment of CES, discuss the emergence of novel therapeutics and emphasize what must be investigated in the future to move the field forward, such as the emerging concept of atrial cardiopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardioembolic stroke (CES) is devastating, leading to poor functional outcomes for patients, with a higher risk of recurrent stroke and death compared to other stroke subtypes. Proper diagnosis of the ischemic stroke subtype is of the utmost importance as future testing and treatment stem from this important branch point in stroke medical decision making. CES in particular is a complex disease, representing one diagnostic entity but composed of many disparate conditions. Etiologies of cardiac embolism include, but are not limited to, atrial tachyarrhythmia, valvular disease, endocarditis, structural heart disease, acute myocardial infarction, and ventricular thrombus [1]. Other conditions that have been proposed as potential sources of cardiac embolism include intracardiac tumors, mitral valve strands, and the presence of spontaneous echocardiography contrast [2,3,4]. The majority of CES is caused by atrial fibrillation (AF), the single most common type of cardiac arrhythmia. AF leads to a fivefold increase in stroke risk, with no decline in risk with age, which stands in stark contrast to other stroke risk factors [5]. The literature to support anticoagulation (AC) in AF is deep, with the most recent American Heart Association guidelines stating that the best timing of initiation of AC for most stroke patients was 4–14 days post-stroke [class IIa; level of evidence B] [6••]. While proper treatment for these embolic stroke patients (known AF) should remain a public health concern, there are patients with other forms of cardiac disease, or with cardiac disease in whom the best treatment remains unclear, and who, by default, are currently treated with antiplatelet medications. A growing burden of evidence suggests that a dichotomous consideration of this paradigm (presence/absence of AF) may not be sufficient when determining either the source of the embolism (cardiac-related or not) or the initiation of AC. As CES is known to occur independently of AF, there might be a subset of CES patients without AF who would be best treated with AC? Unless new strategies are proposed and investigated, the potential for under-diagnosis and under-treatment of CES remains.
Diagnostic evaluation
Determining stroke etiology
The traditional approach to classifying stroke etiology has divided stroke into either hemorrhagic or ischemic, with five ischemic stroke-subtypes: (1) large-artery atherosclerosis, (2) cardioembolism, (3) small-vessel occlusion, (4) stroke of other determined etiology, and (5) stroke of undetermined etiology. Such an approach, known as the TOAST classification system, is a longstanding diagnostic algorithm that has reasonably high interrater reliability (Table 1) [7]. It importantly enabled categorization of every patient into one of these five stroke subtypes.
This approach has been expanded upon by different groups with algorithms that try to decrease the number of patients classified as “undetermined.” The Causative Classification System (CCS) is web based and further divides each TOAST subtype into three subcategories of evident, probable, and possible based upon the recognition that there might be multiple competing mechanisms in the same patient (Table 1) [8]. The ASCO phenotyping system, recently expanded to the ASCOD system, suggests five categories (Table 1) with a numeric grading scale for the grade of disease. For example, if a disease state is present but a causal link to ischemic stroke is unlikely, it is designated a 2. Therefore, a patient with a stroke with apical akinesia of the left ventricle and a reduced ejection fraction is graded a C2 (cardiac embolism, unlikely causal link). The benefit of ASCOD per the investigators is removal of the undetermined or cryptogenic category contained in other grading systems [9].
Regardless of the algorithm used, there are challenges implicit in the diagnosis of etiological stroke subtype. Common risk factors such as hypertension, diabetes, and smoking are known to be associated with stroke in general, but the exact subtype remains unclear [10]. Additionally, the diagnosis inevitably depends on the risk factors or pathology that are uncovered by diagnostic testing, such as cerebral imaging, echocardiography, and long-term cardiac monitoring. Notably, if certain diagnostic tests are not performed, then it is not possible to state conclusively that disease does not exist.
Which diagnostic tests should be performed in the early workup of a patient with acute ischemic stroke remains unclear. The latest American Stroke Guidelines, now under revision, suggests that there is not enough evidence to pursue routine echocardiography or cardiac monitoring in patients [11••, 12].
Implicit in the workup of stroke etiology is the assumption that what is likely to have a causal link with certain stroke etiologies has already been defined, with no remaining knowledge gaps. For CES in particular, there are cardiac conditions that may be identified in the workup, but the likelihood of embolism associated with those conditions remains unclear, which has reduced enthusiasm for including these components in the workup.
When considering treatment of CES, it becomes apparent that having a standard approach is essential for assuring uniform subclassification of stroke. Such standardization is critical in not only the diagnostic algorithm, but also evaluating treatment outcomes in clinical trials. The best evidence suggests the following characteristics as suspicious for the embolism being cardiac in etiology: a history of cardiac disease, reduced consciousness, and non-fractionated arm weakness, where fractionation refers to the ability to move one segment of the arm independently of other segments voluntarily, abrupt onset of symptoms, and systemic embolism [13, 14]. Even in the absence of a clear cardiac source, certain imaging findings raise suspicion for a cardioembolic etiology. An imaging pattern of diffuse ischemia involving multiple branches of different arterial territories, for example, is strongly suggestive of a cardiac embolic shower [15]. Embolism can also be suggested by early hemorrhagic conversion of the ischemic infarct or maximal deficits at onset with rapid improvement, representing clot breakdown [16].
As physicians consider treatment options for CES, it is important to understand the limitations of any subtype classification system. The need for further research becomes apparent as identifying the etiologic mechanism of stroke is essential for secondary stroke prevention.
Cardioembolic stroke
The most common cause of CES is non-valvular atrial fibrillation (AF), with an estimated incidence in 2010 of 77.5 per 100,000 in men and 59.5 in women [17••]. It is anticipated that the prevalence of AF will only increase as the population ages, so timely diagnosis and treatment is important. The stroke risk in atrial fibrillation is believed to stem from uncoordinated myocyte activity that then leads to irregular atrial contraction, formation of thrombus and subsequent embolus [18].
The standard of CES treatment is anticoagulation (AC), with AF being one of the few cardiac etiologies where the evidence supports lifelong treatment. There are several risk prediction scores used in determining utility of AC once AF is discovered, with the most widely used being the CHA2DS2-VASc score [19]. According to the algorithm, patients with no other risk factors except a recent stroke or transient ischemic attack should be placed on anticoagulation to prevent recurrent stroke (scores ≥ 2 necessitate AC). Despite the clear guidelines dictating treatment, data suggests that a large proportion of patients remain underdiagnosed and undertreated, with only 39% of highest risk AF patients (CHADS2 3–6) taking warfarin at the time of stroke [20]. AF can be difficult to diagnose as many patients do not manifest the typical symptoms of palpitations, dyspnea, and fatigue. Silent AF is considered a major healthcare problem as the risk of stroke in symptomatic or asymptomatic AF has been shown to be the same [21].
Prolonged cardiac monitoring can be used to decrease the proportion of patients who may be underdiagnosed due to occult AF. The use of implantable cardiac monitoring devices, which allow for continuous cardiac monitoring, has demonstrated the benefit of prolonged monitoring in capturing and characterizing more patients who may have paroxysmal or occult AF. Recent trials have shown a steady increase in percentage of AF captured as the duration of monitoring increases, with 40% detection rate reported after 30 months of monitoring [22].
Current treatments
Pharmacologic treatment
For many years, warfarin was the only option available for secondary stroke prevention for patients with AF. The most recent guidelines recommend that for patients with acceptably low risk of hemorrhagic complications, long-term oral anticoagulant therapy with warfarin be initiated with a target INR of 2.0 to 3.0 [6].
With the development of the non-vitamin K antagonist oral anticoagulant drugs, however, the treatment decision as to which agent to initiate has become more complicated, but also more convenient for patients, with several options now available. The discussion here will be limited to FDA-approved drugs in this category.
Dabigatran is a competitive direct thrombin inhibitor while rivaroxaban, apixaban, and edoxaban inhibit factor Xa and prothrombinase activity, thus preventing the conversion of prothrombin to thrombin. Rivaroxaban first received FDA approval to prevent strokes in those with non-valvular AF in 2011. In a head-to-head trial (ROCKET AF), rivaroxaban demonstrated non-inferiority compared to warfarin with less intracranial and fatal bleeding [23••]. Dabigatran, approved in 2010, also showed non-inferiority to warfarin [24]. Apixaban, approved in 2012, demonstrated both non-inferiority and superiority to dose adjusted warfarin [25]. Subsequent meta-analyses that included the latest drug, edoxaban, have shown a reduced risk of stroke or systemic embolic events (RR 0.81, 95% CI 0.73-0.91) with reduction in all-cause mortality and intracranial hemorrhage, but increased gastrointestinal bleeding [26].
These DOACs (direct oral anticoagulants) have distinct mechanisms of actions, dosing frequencies, and half-lives (Table 2). Kidney function is an important consideration as dabigatran, rivaroxaban, and edoxaban dosing vary depending on the patient’s creatinine clearance and are not appropriate for use in patients with end stage renal diseae. Some agents are dosed twice daily (dabigatran or apixaban) which may be more difficult for some patients versus once a day rivaroxaban. Patients on enzyme-inducing antiepileptic drugs or protease inhibitor-based antiretroviral therapy should not be placed on DOAC drugs.
The benefits over warfarin that are shared by DOACs include a rapid onset of action, a shorter half-life, and a more predictable pharmacokinetic profile, which enables the drug to be taken without monitoring. A prevalent fear when DOACs first appeared on the market was, should the patient develop an intracranial hemorrhage, there would be no mechanism by which to reverse the medications’ anticoagulant effect. Dabigatran was the first to have a specific reversal agent (idarucizumab, FDA-approved 2015), which was followed in May 2018 with FDA approval of andexanet for factor Xa inhibitors. This has lessened some of these fears as more hospitals now have access to these agents.
Adherence to any kind of therapy is of utmost importance as AF patients who either are not treated or have sub-therapeutic INRs have at a minimum twice the risk of stroke compared to those with INRs from 2 to 3 [27]. In the most recent guidelines [28], warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban all have a class 1 recommendation for prevention of thromboembolism.
Interventional procedures
As cerebrovascular physicians are acutely aware, there are patients for whom long-term AC after AF may be contraindicated. One of the best recognized non-pharmacological options that is utilized in this patient population is placement of a percutaneous left atrial appendage (LAA) occlusion device. The LAA is a leading cause of the thrombogenic risk among patients with AF, and devices have been developed that enable LAA occlusion in patients who are not undergoing cardiac surgery. The WATCHMAN device and the AMPLATZER are the two leading devices used in the USA, with the WATCHMAN the only device FDA approved for this indication.
The two pivotal trials that used the WATCHMAN (PROTECT AF, PREVAIL) demonstrated that for stroke or systemic embolism, the device was non-inferior to warfarin [29, 30]. Real-world data from prospective registries has shown an ischemic stroke rate of 1.1% and demonstrated that the device is safe with high rates of procedural success and low rates of post procedure complications [31]. It is important to note that the patients who are deemed eligible for WATCHMAN implantation must be able to tolerate warfarin for approximately six weeks. In the primary trial, implantation was accompanied by warfarin for 45 days followed by dual antiplatelet therapy for 6 months.
Observational registries for the AMPLATZER Cardiac Plug have shown a procedural success rate of 97.3% [32]. Enrollment in clinical trials is ongoing (Amulet IDE, STROKECLOSE) with the primary effectiveness endpoint a composite of ischemic stroke or systemic embolism through 18 months [32]. The AMPLATZER Amulet will be compared to the WATCHMAN in a 1:1 ratio representing the first head-to-head comparison of two occlusion devices [33].
The LARIAT Suture Delivery Device, another procedure used in LAA occlusion in an off-label indication in the USA, has been used in patients who are deemed ineligible for anticoagulation. The LARIAT suture seals off the LAAA from the rest of heart, preventing material exposure on the endocardial side so that AC is not indicated [34]. The LARIAT registry study showed successful deployment in 95.5% of participants, but its safety profile is not as favorable as the other devices, with 9.1% of cases resulting in major bleeding [35]. In 2015, the FDA also issued a safety statement expressing concern over complications arising after LARIAT implantation such as laceration or perforation of the heart that has hampered enthusiasm for the device [36].
Acute treatment decisions
Thrombolysis and endovascular therapy
Intravenous thrombolysis (IV tPA) is the bedrock of acute stroke treatment within the first 4.5 h for patients who meet criteria [11]. The benefits of administration of alteplase or IV tPA declines the further out from onset of stroke symptoms that the drug is administered, emphasizing the importance of rapid identification of eligible patients. The immediate goal of reperfusion therapy is restoration of blood flow. The most recognized complications of IV tPA include intracerebral hemorrhage (ICH), systemic bleeding, and angioedema. The quoted risk of symptomatic ICH from IV tPA is 5–7% [37], but concerns for ICH alone should not delay administration as this risk is outweighed by the net clinical benefit [38,39,40].
When considering CES, there are conflicting data as to whether IV tPA administration is more or less effective in this stroke subtype when compared to the others. One large registry study reported that CES patients were less likely to have ICH after IV tPA [41]. Another multicenter stroke registry study did not find a difference in complication rates or outcome at 3 months [42]. Additional studies have confirmed the latter, which reflects the original conclusion of the National Institute of Neurological Disorders and Stroke trial [43, 44].
The field of acute stroke care was changed dramatically in 2015 with the release of several concurrent clinical trials which demonstrated that acute clot retrieval in the form of endovascular therapy (ET) dramatically improved patient outcomes when compared to standard therapy [45,46,47,48]. The patients enrolled in these trials were those who had evidence of a large vessel occlusion (internal carotid artery or proximal middle cerebral artery), a reassuring noncontrast head CT, minimal pre-stroke disability, and a National Institutes of Health stroke scale score ≥ 6 and could be treated within 6 h of last known normal. Subsequent years have led to the release of more trials that have expanded the time window, and used imaging criteria, rather than last known well, to determine eligibility for this life-saving procedure [49, 50]. These guidelines should be followed for CES patients.
Additional considerations
Once a decision to initiate thrombolysis has been made, a unique consideration for CES patients is that they may already be on AC. Therapeutic AC is a contraindication to IV tPA. Coumadin use can be rapidly assessed in the emergency room with a point of care INR (tPA use safe if ≤ 1.7). Determining DOAC use presents a challenge due to lack of a similar test by which to reliably assess the drug’s therapeutic effect. While the PT and APTT are modified by DOACs, the levels vary widely depending on the test reagent and individual patient. In patients with normal renal function, with confirmation that no medication was taken in the past 48 h, IV tPA is safe to administer. In contrast, when the timing of the last dose of DOAC is unclear, then IV tPA administration is contraindicated.
Left ventricle (LV) intracavitary thrombus is a potential complication after ST-segment elevation anterior myocardial infarction (STEMI), leading to an increased risk of ischemic strokes and systemic embolism, with an estimated incidence ranging from 3 to 15% [51]. Due to the risk of hemorrhagic events associated with anticoagulation in this clinical setting, the best treatment regimen has yet to be defined, especially regarding the amount (dual versus triple therapy), and type (vitamin K inhibitors versus DOAC) of anticoagulant used [52]. Given that LV thrombus is a potential etiology of CES, the evidence supports administration of IV tPA acutely for these patients. Under current IV tPA guidelines, the patient should not receive any additional thrombolytic therapy for at least 24 h from the time of IV tPA bolus, although case reports have suggested earlier may be safer [53].
Acute severe LV dysfunction with apical ballooning pattern and increased troponin levels are common findings in the acute phase of stress-induced cardiomyopathy (takotsubo syndrome) (TCM), increasing the risk of LV thrombus formation and consequent ischemic stroke and systemic embolism [54]. The prevalence of thromboembolic events associated with TCM ranges from 1.3 to 9.2%, with the majority of the thrombi discovery in the first 2 weeks of disease onset [55]. The literature regarding AC for TCM to prevent stoke is not robust. Prophylactic anticoagulation treatment has been suggested in patients with reduced LV ejection fraction, hemodynamic instability, LV outflow obstruction, and older age (> 75 years old) [56].
Although the details are outside the scope of this review, another potential cause of CES is low EF or heart failure with reduced left ventricular ejection fraction (LVEF). The best evidence, from the WATCH and WARCEF trials, did not demonstrate a difference in prophylactic antiplatelet versus AC (warfarin) in patients with LVEF ≤ 35 for the primary outcome of all-cause mortality [57, 58]. Warfarin did significantly reduce the rate of incident stroke compared to aspirin, but this benefit was tempered by the increased risk of major hemorrhage with AC. As a result, guidelines do not recommend AC in patients with systolic HF without AF [59].
Future directions
Atrial cardiopathy
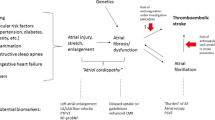
Major research efforts for effective secondary prevention in CES have targeted AF, given the high proportion of CES referable to this etiology and the clear evidence regarding treatment. A growing body of evidence has suggested that considering CES in terms of AF alone may not fully capture heart-brain stroke risk. For example, there is no direct causal link between the onset of AF and occurrence of stroke, with the ASSERT trial demonstrating only 8% of patients had AF within 30 days of their embolic event [60]. As such, there has been increasing interest in capturing patients at high embolic risk who may never manifest AF [61].
The American Stroke Association (ASA) recently released a statement emphasizing the importance of further research in this area, postulating that stroke might occur at any stage along the pathway that leads to embolism from the left atrium and that AF is simply one marker of this disease process [62]. It becomes important then to develop new mechanisms by which the cardiovascular health of a patient with an acute ischemic stroke can be assessed with the purpose of identifying individuals who may be at an earlier or non-AF stage along the pathway to embolism.
The earliest studies that considered markers of embolism outside of AF considered atrial anatomy, particularly the size of the left atrium (LA). The literature has supported that in both men and women, the greater the size of the left atrium, the greater the risk of stroke, independent of AF [63]. LA enlargement on TTE predicts LA thrombus on transesophageal echocardiogram, as well as stroke recurrence [64].
Electrocardiogram parameters have also been considered in hopes of better characterizing an atrial cardiopathy. A recent meta-analysis suggests that abnormalities in P-wave terminal force, with the p-wave reflecting atrial depolarization, P-wave duration, and the maximum P-wave area could be used for determining the risk of incident ischemic stroke [65].
It is becoming more apparent that there may be multiple mechanisms converging to lead to pathology of the atrium that subsequently leads to a predisposition to form thrombus, which subsequently embolizes. AF is one pathway, but not the only pathway that leads to an atrial cardiopathy. Atrial cardiomyopathy has been best defined as any complex of structural, architectural, contractile, or electrophysiological changes affecting the atrial with a potential to produce clinically relevant results [66]. The importance of recognizing this concept model has implications for not only diagnostic algorithms but also treatments. If consideration of rhythm alone is not appropriate, then it would follow, for example, that cardiac ablation of AF is not sufficient treatment for future stroke prevention [67]. An important next step in defining this atrial cardiopathy is the development of appropriate imaging strategies and biomarkers by which to further define the abnormal substrate.
Cardiac imaging in ischemic stroke
The role of cardiac evaluation in stroke management remains controversial, with no agreement on what, if any, assessment should be routinely performed. Although previously part of a standard clinical stroke evaluation, as stated above, recent ASA guidelines questioned the use of routine transthoracic echocardiography (TTE) in ischemic stroke cardiovascular evaluation, citing lack of evidence [11]. These guidelines were then retracted with portions currently under revision, emphasizing the lack of consensus in the field and the need for improvement in cardiac imaging approaches in the stroke patient [68].
TTE has limitations in assessing potential sources of cardiac emboli such as aortic plaque, the LAA, or smaller valvular abnormalities [69]. Advances in echocardiography now enable direct assessment of atrial function using speckle tracking or strain analysis (sTTE). The ability to define phases of the LA cycle using sTTE has shown benefit in diseases such as hypertension, diabetes, heart failure, and atrial fibrillation [70, 71]. It has also shown incremental value for embolic risk stratification above and beyond the CHA2DS2-VASc score in patients with AF [72]. sTTE is not routinely performed in stroke care despite its low cost and the ability to perform the analysis even after image acquisition.
Two other imaging modalities that hold promise are cardiac computed tomography angiography (C-CTA) and cardiac magnetic resonance imaging (C-MRI). C-CTA has been shown to be feasible to perform in stroke patients [73], and description of LAA shape has added value when considering AC in patients with low CHA2DS2-VASc scores [74]. A benefit of C-CTA compared to transesophageal echocardiography (TEE), which is more commonly performed for evaluation of the LAA, is that C-CTA is noninvasive. It also offers a simultaneous evaluation of the coronary arteries for the presence of atherosclerotic disease. Atherosclerotic plaque is important to identify in stroke as it represents another substrate that can embolize, but is treated differently, relying on statin and antiplatelet medications, rather than anticoagulation that is prescribed for AF-associated emboli. Considerations when using C-CTA is the use of iodinated contrast, and radiation exposure, which can be a concern to some patients.
Similarly, C-MRI has certain advantages over other forms of cardiac imaging that might have a role in identifying an atrial cardiopathy. Atrial fibrosis is widely regarded as one of the underlying mechanisms behind the development of AF (Fig. 1). Delayed enhancement MRI enables assessment of scar burden and residual fibrosis after AF ablation, but there is also new evidence that atrial fibrosis is also present in non-AF patients, with a higher percentage of LA fibrosis found among those with a previous stroke [75, 76]. C-MRI also has excellent spatial resolution and does not expose the patient to radiation like C-CTA. However, gating (timing to the ECG) is always required and longer acquisition time increases susceptibility to patient movement [69]. While the prospective work in stroke patients with C-MRI is limited, a recent study with 85 consecutive patients found that addition of C-MRI to standard diagnostic evaluation decreased the percentage of patients classified as cryptogenic or of unknown etiology, from 27 to 20% [77]. The importance of these technologies in acute stroke care is becoming increasingly recognized and undoubtedly will be even more frequently employed in future research efforts [76].
Assessment of left atrial fibrosis utilizing cardiac MRI (C-MRI). Figure showing late-gadolinium enhancement (LGE) cardiac magnetic resonance processing for left atrial subtract mapping. A Step 1: LGE cardiac magnetic resonance axial view images are acquired. B Step 2: epicardial and endocardial contour are manually drawn around the left atrial myocardial wall. C Step 3: the image intensity ratio is used to delineate myocardial late-gadolinium enhancement (in red). D 3D shell with LGE distribution is generated for detail characterization of the left atrial arrhythmogenic substrate.
Emerging therapies
As has been reiterated, stroke subtype is essential in choosing the right treatment paradigm for secondary stroke prevention. But what is the best approach when a subtype, despite a thorough workup, cannot be determined? The standard of care has remained antiplatelet therapy, but are there patients who might benefit from more aggressive therapies, such as anticoagulation? Stroke classification systems (Table 1) frequently include cryptogenic, or etiology unknown, and are frustrating diagnoses for both the patient to receive as well as the treating physician to give. The ESUS (Embolic Stroke of Unknown Source) classification defines ESUS as (1) stroke detected by CT or MRI that is not lacunar, (2) absence of extracranial or intracranial atherosclerosis causing ≥ 50% luminal stenosis in arteries supplying the area of ischemia, (3) no other cause of stroke identified, and (4) no major risk cardioembolic source of embolism [78].
Twenty-five percent of ischemic strokes are classified as cryptogenic, and ESUS represents a high proportion of these patients. It may be that ESUS patients would benefit from anticoagulation, since the mechanisms by which AF leads to embolism might be similar in patients with ESUS [79]. Three recent clinical trials have investigated, or are actively investigating, the use of DOACs for stroke prevention after ESUS. In one of two recently completed trials, rivaroxaban (NAVIGATE ESUS) was not found to be superior to aspirin in preventing recurrent stroke and was associated with a higher risk of bleeding [23••]. The RE-SPECT ESUS investigators have yet to publish results, but presented their findings at the World Stroke Congress in Montreal, Canada (October, 2018). After a mean follow-up of 19 months, the rate of recurrent stroke was 4.1% per year with dabigatran and 4.8% per year with aspirin, a non-significant difference (HR 0.85; p = 0.1). There was no difference in major bleeding between the two groups [80]. The ATTICUS trial is still ongoing and designed to determine whether apixaban, administered within 7 days after ESUS, is superior to aspirin for secondary stroke prevention at 12 months [81].
As the risk of recurrent stroke after cryptogenic stroke is at least as high as stroke from other causes, future research is needed in this area to facilitate the development of clear, evidenced-based guidelines. Recent initiation of clinical trials, such as AtRial Cardiopathy and Antithrombotic Drugs in Prevention After Cryptogenic Stroke (ARCADIA), that use a biomarker-driven approach to define atrial cardiopathy and randomize treatment are an important first step [82••]. Treatment of all patients with embolic-appearing strokes with anticoagulation appears to only increase risk of bleeding without any gains in stroke secondary prevention. However, developing strategies to define atrial pathology outside of AF will help elucidate the potential cardiac mechanisms contributing to embolism in some cases of ESUS. Such a mechanistic understanding will enable the clinician to more effectively risk-stratify patients and discover which patients might benefit from more aggressive therapies.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
O’Carroll CB, Barrett KM. Cardioembolic stroke. Continuum (Minneap Minn). 2017;23(1, Cerebrovascular Disease):111–32.
Kasarskis EJ, O’Connor W, Earle G. Embolic stroke from cardiac papillary fibroelastomas. Stroke. 1988;19(9):1171–3.
Tice FD, Slivka AP, Walz ET, Orsinelli DA, Pearson AC. Mitral valve strands in patients with focal cerebral ischemia. Stroke. 1996;27(7):1183–6.
Castello R, Pearson AC, Labovitz AJ. Prevalence and clinical implications of atrial spontaneous contrast in patients undergoing transesophageal echocardiography. Am J Cardiol. 1990;65(16):1149–53.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983–8.
•• Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45(12):3754–832 Most recent guidelines addressing primary stroke prevention for patients with atrial fibrillation.
Gordon DL, Bendixen BH, Adams HP Jr, Clarke W, Kappelle LJ, Woolson RF. Interphysician agreement in the diagnosis of subtypes of acute ischemic stroke: Implications for clinical trials. TOAST Investig Neurol. 1993;43(5):1021–7.
Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: the causative classification of stroke system. Stroke. 2007;38(11):2979–84.
Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36(1):1–5.
Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58(5):688–97.
•• Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49(3):e46–e110 Most recent guidelines regarding early management and workup for patients with ischemic stroke, with sections under revision.
Furie KL, Jayaraman MV. 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49(3):509–10.
Timsit SG, Sacco RL, Mohr JP, Foulkes MA, Tatemichi TK, Wolf PA, et al. Early clinical differentiation of cerebral infarction from severe atherosclerotic stenosis and cardioembolism. Stroke. 1992;23(4):486–91.
Timsit SG, Sacco RL, Mohr JP, Foulkes MA, Tatemichi TK, Wolf PA, et al. Brain infarction severity differs according to cardiac or arterial embolic source. Neurology. 1993;43(4):728–33.
Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol. 2003;60(12):1730–4.
Beghi E, Bogliun G, Cavaletti G, Sanguineti I, Tagliabue M, Agostoni F, et al. Hemorrhagic infarction: Risk factors, clinical and tomographic features, and outcome. A case-control study. Acta Neurol Scand. 1989;80(3):226–31.
•• Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–47 Important statement summarizing the evidence that supports consideration of a new causal model for cardioembolic stroke.
Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47(3):895–900.
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72.
Nattel S, Guasch E, Savelieva I, Cosio FG, Valverde I, Halperin JL, et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J. 2014;35(22):1448–56.
Barbarossa A, Guerra F, Capucci A. Silent atrial fibrillation: a critical review. J Atr Fibrillation. 2014;7(3):1138.
Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R, et al. Investigators. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the REVEAL AF study. JAMA Cardiol. 2017;2(10):1120–7.
•• Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191–201 Clinical trial investigating use of a DOAC in patients with ESUS.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. Lancet. 2014;383(9921):955–62.
Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335(8):540–6.
January CT, Wann LS, Calkins H, Field ME, Chen LY, Furie KL, Cigarroa JE, Heidenreich PA, Cleveland JC Jr, Murray KT, Ellinor PT, Shea JB, Ezekowitz MD, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society. Heart Rhythm. 2019 23.
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial. Lancet. 2009;374(9689):534–42.
Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64(1):1–12.
Boersma LV, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, et al. EWOLUTION investigators. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37(31):2465–74.
Tzikas A. Left atrial appendage occlusion with Amplatzer cardiac plug and amplatzer amulet: a clinical trials update. J Atr Fibrillation. 2017;10(4):1651.
St. Jude Medical. AMPLATZER™ Amulet™ LAA Occluder Trial (Amulet IDE).; 2016 https://clinicaltrials.gov/ct2/show/NCT02879448.
Lakkireddy D, Afzal MR, Lee RJ, Nagaraj H, Tschopp D, Gidney B, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: results from a US multicenter evaluation. Heart Rhythm. 2016;13(5):1030–6.
Price MJ, Gibson DN, Yakubov SJ, Schultz JC, Di Biase L, Natale A, et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. transcatheter LAA ligation consortium. J Am Coll Cardiol. 2014;64(6):565–72.
FDA. Safety Alerts for Human Medical Products [Internet]. www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm454660.htm.
Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomized trials. Lancet. 2014;384(9958):1929–35.
Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–703.
Lees KR, Emberson J, Blackwell L, Bluhmki E, Davis SM, Donnan GA, et al. Stroke Thrombolysis Trialists’ Collaborators Group. Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke. 2016;47(9):2373–9.
Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480–8.
Vaclavik D, Vilionskis A, Jatuzis D, Karlinski MA, Gdovinova Z, Korv J, et al. Clinical outcome of cardioembolic stroke treated by intravenous thrombolysis. Acta Neurol Scand. 2018;137(3):347–55.
Fuentes B, Martinez-Sanchez P, Alonso de Lecinana M, Egido J, Reig-Rosello G, Diaz-Otero F, et al. Efficacy of intravenous thrombolysis according to stroke subtypes: the Madrid Stroke Network Data. Eur J Neurol. 2012;19(12):1568–74.
Hsia AW, Sachdev HS, Tomlinson J, Hamilton SA, Tong DC. Efficacy of IV tissue plasminogen activator in acute stroke: does stroke subtype really matter? Neurology. 2003;61(1):71–5.
Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke RT-PA Stroke Study Group. N Engl J Med [Internet]. 1995;333(24):1581–7.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med [Internet]. 2015;372(1):11–20.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med [Internet]. 2015;372(11):1019–30.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med [Internet]. 2015;372:2296–306.
Albers GW, Lansberg MG, Kemp S, Tsai JP, Lavori P, Christensen S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke. 2017;12(8):896–905.
Chen CJ, Ding D, Starke RM, Mehndiratta P, Crowley RW, Liu KC, et al. Endovascular vs medical management of acute ischemic stroke. Neurology. 2015;85(22):1980–90.
McDaniel MC. Anticoagulation after anterior myocardial infarction: Primum non nocere, or first do no harm. JACC Cardiovasc Interv. 2015;8(1 Pt B):163–5.
Moulson N, LaHaye SA, Bertrand OF, MacHaalany J. Prophylactic warfarin post anterior ST-elevation myocardial infarction: a systematic review and meta-analysis. Cardiovasc Revasc Med. 2017;18(8):559–64.
Gill R, Donahey E, Ruland S. Early administration of therapeutic anticoagulation following intravenous thrombolysis for acute cardiogenic embolic stroke caused by left ventricular thrombus: case report and topic review. Front Neurol. 2015;6:9.
Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39(22):2047–62.
Heckle MR, McCoy CW, Akinseye OA, Khouzam RN. Stress-induced thrombus: prevalence of thromboembolic events and the role of anticoagulation in takotsubo cardiomyopathy. Ann Transl Med. 2018;6(1):4.
Santoro F, Stiermaier T, Tarantino N, De Gennaro L, Moeller C, Guastafierro F, et al. Left ventricular thrombi in takotsubo syndrome: incidence, predictors, and management: Results from the GEIST (german italian stress cardiomyopathy) registry. J Am Heart Assoc. 2017;6(12):e006990. https://doi.org/10.1161/JAHA.117.006990.
Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859–69.
Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, et al. WATCH Trial Investigators. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the warfarin and antiplatelet therapy in chronic heart failure (WATCH) trial. Circulation. 2009;119(12):1616–24.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College Of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52.
Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, et al. ASSERT Investigators. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129(21):2094–9.
Kamel H, Bartz TM, Elkind MSV, Okin PM, Thacker EL, Patton KK, et al. Atrial cardiopathy and the risk of ischemic stroke in the CHS (cardiovascular health study). Stroke. 2018;49(4):980–6.
Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, et al. Atrial fibrillation burden: Moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137(20):e623–44.
Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. Framingham Heart Study Circ. 1995;92(4):835–41.
Anaissie J, Monlezun D, Seelochan A, Siegler JE, Chavez-Keatts M, Tiu J, et al. Left atrial enlargement on transthoracic echocardiography predicts left atrial thrombus on transesophageal echocardiography in ischemic stroke patients. Biomed Res Int. 2016;2016:7194676.
He J, Tse G, Korantzopoulos P, Letsas KP, Ali-Hasan-Al-Saegh S, Kamel H, et al. P wave indices and risk of ischemic stroke: a systematic review and meta-analysis. Stroke. 2017;48(8):2066–72.
Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14(1):e3–e40.
Al-Khatib SM, Allen LaPointe NM, Chatterjee R, Crowley MJ, Dupre ME, Kong DF, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160(11):760–73.
Fisher M. Update on the early management of patients with acute ischemic stroke guidelines. Stroke. 2018;7:STROKEAHA118023990.
Pagan RJ, Parikh PP, Mergo PJ, Gerber TC, Mankad R, Freeman WD, et al. Emerging role of cardiovascular CT and MRI in the evaluation of stroke. AJR Am J Roentgenol. 2015;204(2):269–80.
Liu Y, Wang K, Su D, Cong T, Cheng Y, Zhang Y, et al. Noninvasive assessment of left atrial phasic function in patients with hypertension and diabetes using two-dimensional speckle tracking and volumetric parameters. Echocardiography. 2014;31(6):727–35.
Mochizuki A, Yuda S, Oi Y, Kawamukai M, Nishida J, Kouzu H, et al. Assessment of left atrial deformation and synchrony by three-dimensional speckle-tracking echocardiography: comparative studies in healthy subjects and patients with atrial fibrillation. J Am Soc Echocardiogr. 2013;26(2):165–74.
Obokata M, Negishi K, Kurosawa K, Tateno R, Tange S, Arai M, et al. Left atrial strain provides incremental value for embolism risk stratification over CHA(2)DS(2)-VASc score and indicates prognostic impact in patients with atrial fibrillation. J Am Soc Echocardiogr. 2014;27(7):709–716.e4.
Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY, et al. Left atrial appendage thrombi in stroke patients: detection with two-phase cardiac CT angiography versus transesophageal echocardiography. Radiology. 2009;251(3):683–90.
Lee JM, Kim JB, Uhm JS, Pak HN, Lee MH, Joung B. Additional value of left atrial appendage geometry and hemodynamics when considering anticoagulation strategy in patients with atrial fibrillation with low CHA2DS2-VASc scores. Heart Rhythm. 2017;14(9):1297–301.
Gal P, Marrouche NF. Magnetic resonance imaging of atrial fibrosis: redefining atrial fibrillation to a syndrome. Eur Heart J. 2017;38(1):14–9.
Yaghi S, Liberman AL, Atalay M, Song C, Furie KL, Kamel H, et al. Cardiac magnetic resonance imaging: a new tool to identify cardioaortic sources in ischaemic stroke. J Neurol Neurosurg Psychiatry. 2017;88(1):31–7.
Baher A, Mowla A, Kodali S, Polsani VR, Nabi F, Nagueh SF, et al. Cardiac MRI improves identification of etiology of acute ischemic stroke. Cerebrovasc Dis. 2014;37(4):277–84.
Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429–38.
Yaghi S, Chang AD, Hung P, Mac Grory B, Collins S, Gupta A, et al. Left atrial appendage morphology and embolic stroke of undetermined source: a cross-sectional multicenter pilot study. J Stroke Cerebrovasc Dis. 2018;27(6):1497–501.
TCTMD. [Internet].
Geisler T, Poli S, Meisner C, Schreieck J, Zuern CS, Nagele T, et al. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): rationale and study design. Int J Stroke. 2017;12(9):985–90.
•• National Library of Medicine. AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke (ARCADIA) [Internet]. ClinicalTrials.gov. Ongoing clinical trial that is using cardiac biomarkers to risk stratify patients with ESUS for treatment with apixaban versus aspirin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michelle C. Johansen reports grants from American Heart Association Mentored Clinical and Population Research Award and grants from NIH/ICTR KL2 Early Career Investigator Award, outside the submitted work. Henrique Doria De Vasconcellos declares no potential conflict of interest. Rebecca F. Gottesman is an Associate Editor for the journal Neurology.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cerebrovascular Disorders
Rights and permissions
About this article
Cite this article
Johansen, M.C., De Vasconcellos, H.D. & Gottesman, R.F. Understanding Atrial Cardiopathy: an Under-Recognized Contributor to Cardioembolic Stroke. Curr Treat Options Neurol 21, 32 (2019). https://doi.org/10.1007/s11940-019-0571-4
Published:
DOI: https://doi.org/10.1007/s11940-019-0571-4