Opinion statement
Fabry disease is an X-linked, lysosomal storage disorder caused by a mutation in the GLA gene leading to a deficiency in alpha-galactosidase A enzyme (α-Gal A) activity, which in turn results in accumulation of globotriaosylceramide in the vascular endothelium and smooth muscle cells of different organs, including kidney and heart, finally leading to impairment or failure of organ function. The central and peripheral nervous systems are also affected leading to neurological manifestations such as cerebrovascular diseases, small fiber neuropathy (SFN), and dysautonomic disorders that may be the presenting clinical features in a proportion of patients. This review offers a complete update of all neurological aspects of Fabry disease and therapeutic options. The rarity of disease, as well as the incomplete knowledge regarding natural history, pathogenic mechanisms, and the uncertain efficacy of available therapies, make imperative the acquisition of standardized data on natural disease course. These data are fundamental for the development of new treatments better able to access the central nervous system, to bypass the neutralizing antibodies and to improve the heart and kidney function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fabry disease (FD) was classified as a sphingolipidosis in 1963 [1]. Subsequently, the lysosomal nature of the disease was recognized [2], and a deficient enzyme was identified [3]. Thus, FD (Anderson-Fabry disease, Online Mendelian Inheritance in Man OMIM 301500) is an inborn error of glycosphingolipid metabolism due to mutations in the GLA gene on chromosome Xq22, providing a lack of alpha-galactosidase A (α-Gal-A). Alpha-galactosidase A deficiency leads to a failure in the metabolism of glycosphingolipids containing D-galactosyl moieties, particularly globotriaosylceramide (Gb3), resulting in accumulating in plasma and lysosomes of all tissue [4] but particularly within the vascular endothelium and smooth muscle cells, where the progressive accumulation leads to vessel occlusion, ischemia and, in later disease stages, organ failure [5••].
The disease is caused by mutations, mainly missense and nonsense, but also small and large deletions [6–11]. Disease severity is related to residual α-Gal A enzymatic activity, whereas a clear genotype-phenotype correlation has not been demonstrated given the high disease inter but also intrafamilial variability. However, mutations leading to a complete loss of function are thought to be associated with the “classical disease phenotype,” characterized by childhood onset with burning distal pain and poor growth followed by middle age life-threatening cardiac, cerebrovascular, or renal complications. Conversely, residual enzyme activity might lead to slow progression of the disease leading to the cardiac or renal variants with delayed onset.
Fabry disease is a relatively rare condition, although it is probably underdiagnosed given the heterogeneous phenotype. The reported incidence ranges from 1:40,000 to 1:117,000 worldwide [12]. However, definitive prevalence data are lacking since FD has been identified in different patient populations with cardiac, renal, or cerebrovascular diseases [5••]. An ethnic predisposition has never been assessed, but areas of increased incidence have been identified because of founder effects in Nova Scotia, Canada, and West Virginia (USA).
The average age of presentation is 6–8 years in males and 9 years in females. Age at onset, clinical features, and course are extremely variable even within the same family.
Fabry disease primarily manifests among hemizygous males in whom the disease displays multiple organ involvement. Gb3 storage begins in the prenatal age, but clinical symptoms do not manifest until childhood. The first disease symptoms are distal neuropathic pain (acroparesthesia), hypohydrosis, and the skin lesions known as angiokeratomas. Learning and growth delay are reported as well [5••]. During adolescence, ocular involvement (cornea verticillata) and autonomic dysfunction appear followed within the third decade by kidney and heart failure and cerebrovascular diseases (TIA/ischemic stroke or cerebral hemorrhage). Females generally present with a phenotype ranging from asymptomatic to organ failure [4] and generally with a later age of onset due to X inactivation mechanisms as well as other unknown genetic or environmental factors.
Given the availability of an enzyme replacement therapy, which can improve patient outcomes, FD should be considered in the differential diagnosis of some neurological disorders such as idiopathic stroke and painful neuropathies. This article is an updated review on neurological manifestations of Fabry disease aimed at helping clinicians to a prompt disease recognition and diagnosis.
FD etiology/pathophysiology
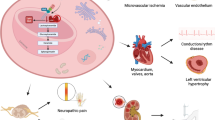
The lysosomal α-Gal A enzyme encoded by the GLA gene on Xq22 is responsible for the cleavage of two globotriaosylceramide galactose residues [13], the prominent globotriaosylceramide (Gb3; also named ceramide trihexoside: CTH) and the lesser galabiosylceramide (Gb2) (Fig. 1) [1, 14].
a Glycosphingolipid catabolism associated with FD. The schematic representation of the enzymes that catalyzes the catabolism of glycosphingolipids in Fabry disease. b FD pathophysiology. Fabry disease is an X-linked recessive inborn error of glycosphingolipid catabolism resulting from deficient activity of lysosomal enzyme α-Gal-A causing occlusive microvascular diseases affecting kidney, heart, peripheral nerves, and brain. ANS autonomic nervous system, α-Gal A α–galactosidase A, EC endothelial cells, ECM extracellular matrix, GalNAcβ3Galα4Galβ4GlcCer globotetraosylceramide/globoside, Galα4Galβ4GlcCer globotriaosylceramide, HEXA β-hexosaminidase A, HEXB β-hexosaminidase B, OS oxidative stress, SMC smooth muscle cells.
Deficiency of α-Gal A results in accumulation of Gb3 in endothelial, perithelial, and smooth muscle cells of the vascular system, as well as renal epithelial and autonomic nervous system cells (Fig. 1) [14, 15]. This causes progressive vascular occlusion, ischemia, and in the last disease stages organ failure. A complex mechanism in which cellular structural changes and functional failure due to Gb3 deposition induce inflammation and oxidative stress, which lead to hypertrophy, and extracellular matrix (ECM) remodeling have been implicated in the pathogenesis of FD. In particular, the ECM impairment would cause hypertrophic and fibrotic damage in cardiac and renal tissues and the increased vulnerability of cerebral vasculature, inducing the development of endothelial dysfunction. Also, smooth muscle cell proliferation is put forward as one possible initial step of the complex pathogenesis of FD. The intima-media vessel wall abnormalities, a hyperdynamic circulation and a less compliant vascular wall, as well as an up regulation of local renin angiotensin systems would result in increased reactive oxygen species (ROS), deregulation of the nitric oxide pathway and release of pro-thrombotic factors [4, 16–19].
Cerebrovascular involvement
Ischemic stroke and transient ischemic attacks are the most prevalent type of cerebrovascular complications in FD with up to 25 % of the patients suffering cerebrovascular events over their life course [20]. Hemorrhagic events, vascular dementia, cervical dissection, and thrombosis have also been reported in FD, with hemorrhagic strokes being more common among men than women (16.9 versus 6.9 %, respectively) [21].
Data from the Fabry Registry reported a prevalence of stroke in FD amounting to 6.9 % in men and 4.3 % in women with the median age at first stroke of 39 and 46 years, respectively [21, 22].
Fabry disease increases the relative risk of stroke in all age groups in comparison to controls and represents an important cause of cryptogenic stroke [21]. In 2005, Rolfs et al. analyzed the GLA gene in 721 patients affected by cryptogenic stroke (aged 18–55) and identified GLA gene mutation in 4.9 % of men and in 2.4 % of women [23]. However, no GLA gene mutations were found in a following study investigating the prevalence of FD in a young cryptogenic stroke cohort [24].
Two other studies investigating the prevalence of FD not only in a cohort of cryptogenic stroke but also in young patients with cerebrovascular disease revealed a GLA mutation prevalence of about 1 % [24, 25]. This rate increased to 4.6 % if ischemic stroke was associated with small vessel disease (SVD), to 7 % if SVD was associated with normal blood pressure (BP), and up to 12.5 % if the two conditions of SVD and normal BP coexisted with a vertebrobasilar stroke localization [26].
The most recent multicentric study evaluating the prevalence of FD in young stroke patients, aged 18–55 (SIFAP) revealed an incidence of definite and probable FD of 0.5 and 0.4 %, respectively, with a diagnosis of probable FD supported by the presence of at least two independent biochemical markers including Gb3, lyso-Gb3 o Gb3-24 in the urine [27•].
A recent meta-analysis showed a FD prevalence among young onset stroke patients ranging from 0.4 to 2.6 %, and the variability was even wider if cryptogenic stroke was included (0.6–11.1 %) [28].
Environmental, genetic, and epigenetic factors as well as common cerebrovascular risk factors have been identified to influence the risk of developing stroke in FD. An association between severity of cerebral white matter hyperintensity (CWMH) and lower estimated glomerular filtration rate has been also reported as a risk factor [29].
Different mechanisms underlying stroke are reported in FD. Large vessel stroke occurs because of the occlusion of large intracranial artery or embolism (from the heart or large vessels). Small vessel disease is instead strictly related to Gb3 deposits and results in subcortical stroke and cerebral white matter hyperinthensities (CWMHs). Stroke may occur both in the anterior and posterior circulation, although vessel ectasia more frequently involves the posterior circulation. Increased basilar artery diameter, which is probably due to an insufficient flow autoregulation leading to aberrant vascular remodeling [30••, 31], has been proposed as a sensitive indicator of FD.
CWMHs consist of usually symmetrical, periventricular, deep, and subcortical hyperintensities on T2-weighted or fluid-attenuated inversion recovery images and increase progressively during age. They may result from microvascular degeneration caused by Gb3 endothelial deposit and damage with chronic hypoperfusion.
The load and the distribution of white matter lesions in FD can radiologically mimic hypertensive encephalopathy or other diseases such as multiple sclerosis [32, 33].
A wider and diffuse CWMH load may be an indicator of progressive cerebrovascular disease in Fabry patients [32].
Other neurological manifestations
Neuropathy
Peripheral neuropathy in FD mainly involves small thin fibers in particular myelinated A-δ fibers, responsive to mechanical pain and pinprick stimuli. Different from other diseases associated with small thin fiber involvement (e.g., diabetes, amyloidosis) unmyelinated C fibers, which transmit warm sensation and pain sensitivity to heat, are less affected in FD. Small thin fiber impairment hence results in pain, sensory symptoms, and lower tolerance to cold.
Neuropathic involvement in FD may start in childhood and may represent an early red flag suggesting the diagnosis. The incidence of neuropathy is as high as 80 % in FD [34–36].
The most common presentation in FD is a distal length-dependent painful small fiber sensory neuropathy, characterized by acroparesthesia, burning dysesthesia, and sensory loss starting in the palms of the hands or in the soles of the feet.
Pain is a complaint of 60–80 % of FD patients and may be of two different types: chronic or episodic, the latter called “Fabry’s crises.” The former manifests as a constant, burning, shooting neuropathic pain with dysesthesias in the hands and feet. Fabry’s crises, instead, are characterized by severe and disabling paroxysmal pain starting at the hands and feet with centripetal radiation and dysautonomic features, lasting from hours to several days. Stress, physical exercise, fever, or fast temperature variation [34–38] can act as pain triggers. Chronic pain may induce psychiatric symptoms such as mood disorders, behavioral changes, and severe depression [34, 36–38], with a deleterious influence on health-related quality of life (HRQoL). A subset of FD patients (11 %) experiences a decrease in pain with aging. This may be due to small thin fiber damage that has become so extensive and severe leading to a complete loss of neurotransmission function.
Considering the particular features, course, and intensity of pain in FD patients, suffering from nociceptive pain episodes, a face-to-face Fabry Pain Questionnaire (FPQ) was developed in 2014 by Uceyler et al. to quantitatively and qualitatively investigate pain in FD adult patients [39]. The FPQ is an easy to apply instrument, consisting of 15 questions exploring the particular Fabry phenotype pain, characterized by triggerable pain attacks, evoked pain, pain crises, and chronic pain. This validated questionnaire represents a valuable tool for baseline and follow-up assessment of pain in FD patients and may guide treatment [39].
The pathophysiology of pain in FD is still debated. It seems to be related to Gb3 accumulation in dorsal root ganglion (DRG) neurons leading to neuronal apoptosis with dying back degeneration or in endothelial cells of the vasa vasorum leading to chronic nerve hypoxia and ischemia. Gb3 deposits may also interfere with cellular membrane proteins altering neuronal excitability and resulting in nerve fiber damage and death. With regard to triggerable pain attacks, low temperature may evoke pain probably by transient localized vasoconstriction causing fiber hypoperfusion [40].
Other molecular mechanisms such as nerve damage inducing overexpression and deregulation of sodium channels, central sensitization with hyperexcitability of nociceptive neurons, or loss of inhibition of peripheral neuronal activity have been postulated [40]. However, even if the involvement of small fibers is assumed to be responsible for pain in FD, some aspects are not completely in accord with the current concept of neuropathic pain. Particularly, the spreading of pain to the whole body during “Fabry’s crises,” some peculiar pain localizations (joints and teeth), and the reported benefit of non-steroidal anti-inflammatory drugs as acute pain treatment support the involvement of different pathogenic mechanisms.
A standard neurological examination usually reveals only a loss of temperature sensation in hands and feet and a reduced tolerance to low temperature exposure [41]. Nerve conduction studies, assessing only large myelinated fibers, are generally normal. For this reason, more sophisticated evaluation methods have been developed to assess the small fiber involvement. Quantitative sensory test (QST), a biophysical method based on computerized automated sensory testing to measure detection thresholds for warm and cold in the feet and hands, revealed significantly elevated detection thresholds for warm and cold stimuli in the foot and for cold in the hands of FD patients compared to controls. Warm sensation in hands was found to be normal [42, 43].
Pathological examination, usually from sural nerve biopsy, shows a significant loss of unmyelinated (C) and small myelinated fibers (A ∂) with normal density of large myelinated fibers. Ultrastructural examination reveals typical inclusions resulting from ceramide trihexoside and other glycolipid deposits in the perineurium and endothelial cells and groups of denervated Schwann cells [44–46].
A prospective single center study examined intraepidermal nerve fiber density (IENFD) in 120 FD patients and found a strong positive correlation in males between Glomerular filtration rate (GFR) and distal IENFD [46]. The IEFND deteriorated over time only in male but not female patients, decreasing to 46 % in the lower leg of FD compared to controls, and to 12.5 % in male with renal impairment [46].
Uceyler et al. concluded that small fiber neuropathy (SFN) in FD patients is both gender and renal function dependent and progresses, despite ERT [47].
Although clinicians should consider FD as a possible cause of idiopathic SFN, recent studies systematically screening for SFN did not find any associated GLA pathogenic mutations and have recommended screening for FD only in the presence of additional features [48].
Autonomic dysfunction
The autonomic nervous system (ANS) consists of myelinated preganglionic B fibers and small unmyelinated post ganglionic C fibers. The involvement of the ANS, and in particular of A ∂ unmyelinated fibers, is known in FD, and dysautonomic features such as hypo or anhidrosis, reduced salivation and lachrymation [49], gastrointestinal dysmotility, cardiac dysrhythmia, and reduced cutaneous flare after histamine injections are common manifestations of FD.
The quantitative sudomotor axon reflex test (QSART) [50] could represent a useful tool to assess sweating deficiency. Since sweat glands are normally innervated with no decrease in nerve fiber density on skin biopsy from patients with FD, anhidrosis is probably due to lamellar intracytoplasmic inclusions in myoepithelial cells and small vessels around eccrine glands. Also, the non-length distribution of hypohidrosis and the rapid benefit after a single enzyme infusion support this theory. The frequent association between anhidrosis and painful neuropathy can result in impaired physical exercise tolerance. Systemic levels of catecholamines were not found to be different between FD patients and controls supporting a vasogenic rather than neurogenic etiology of altered vessel response. Prospective studies on FD patients show a low prevalence of orthostatic hypotension, sexual dysfunction, and cardiovascular dysautonomia suggesting a non-severe autonomic neuropathy in FD even if the description of some cases of orthostatic hypotension and fainting supports the hypothesis of underlying cardiovascular autonomic dysfunction [49]. The lipid deposits in endothelial cells and vascular smooth muscle may explain end-organ failure resulting in clinical cardiovascular disorders.
Diagnostic assessment
Neuroradiological aspects
Diagnosis of FD is difficult given its variability in clinical presentation and heterogeneous phenotypes. Hence, possible neuroradiological clues of this disorder have been investigated. Pulvinar sign, extensive CWMH, stroke involving the posterior circulation, and an enlarged basilar artery have been proposed as findings highly suggestive for FD.
Hyperintensities on MRI T1-weighted images of the thalamic posterior region or the pulvinar sign were reported in FD by two different groups in 2003. Moore et al. [51] demonstrated pulvinar sign in 23 % of FD-affected patients but it was not detectable until the third decade and frequency increased with age. High intensity signal of the pulvinar on MRI T1-weighted images corresponds on computerized tomography (CT) scan to increased attenuation likely due to calcification. Mineralization could extend beyond the pulvinar as demonstrated by extrathalamic calcification on CT scan; hyperintensity was found to be strictly confined to the pulvinar.
Arterial spin tagging (AST) MRI images and positron emission tomographic (PET) studies disclosed increased cerebral blood flow (CBF) in the posterior circulation suggesting that hyperperfusion induced dystrophic posterior thalamic calcification, with a selective vulnerability of the putamen. Impairment of the vasoreactivity and autoregulation in the posterior circulation, probably due to pericytes, endothelial cells, or nitric oxide pathway dysfunction result in hyperperfusion followed by increasing capillary leakage.
An increased basilar diameter has been shown to represent another radiological finding suggestive of FD. Uceyler et al. [52] investigated a large cohort of FD patients and found that men with FD have larger arterial diameters of the posterior circulation than male controls, but not compared to stroke patients; hence, basilar diameter does not allow to distinguish FD patients from common stroke patients. Doppler sonography performed on the patients’ harboring enlarged vessel diameters did not find a change in cerebral blood flow velocities. Moreover the severity of disease, as measured by renal function, did not seem to influence basilar and posterior cerebral artery size. Therefore, this study suggests that a basilar diameter >3.2 mm could represent a tool to distinguish male FD patients from male controls, but not from non-FD stroke patients. Cerebral vessel diameter enlargement may result from reduced sympathetic innervation, nitric oxide over release, and glycosphingolipid deposition in vascular smooth muscle cells.
Hyperperfusion may lead to regional metabolic alterations with increased interstitial pressure, fluid shifts, demyelination (gliosis, leukoaraiosis), and subsequently, CWMHs.
Fazekas et al. have recently analyzed a cohort of unselected young patients affected with cerebrovascular events with 21 definite and 13 probable diagnoses of FD, concluding that brain MRI findings fail to distinguish FD patients from stroke patients [53•]. Rate of stroke in the posterior circulation, pulvinar signs, increased basilar diameter, or CWMH do not differ between FD and non-FD young stroke patients.
Biochemical diagnosis
If clinical examination raises a suspicion of FD, biochemical and/or genetic confirmation is needed [54]. In men with classical FD α-Gal-A activity is lower or virtually undetectable. In these patients, the measurement of α-Gal-A activity in cultivated fibroblasts (derived from skin biopsy), plasma, or peripheral blood leucocytes is the preferred method for the biochemical diagnosis [55, 56]. More recently, the α-Gal-A determination in dry blood spots (DBS) [57, 58] has been proposed as a cheaper, reliable, and very simple diagnostic tool [56]. However, this diagnostic methodology has been criticized due to its relatively low sensitivity and the risk of false-positive results [58].
Genetic testing
As already noted, FD is a rare X-linked, recessive, glycolipid storage disorder caused by the deficient activity of α-Gal-A. The enzymatic identification of female FD carriers, even obligate heterozygotes, is not certain with the application of the classical biochemical diagnosis (see above) due to random X-chromosomal inactivation. Moreover, only the identification of an α-Gal-A mutation in an at-risk female will provide precise carrier identification. Therefore, the molecular diagnosis of FD is important for detection of carrier status, genotype/phenotype correlation, and prenatal or early diagnosis [56].
To date, more than 400 FD mutations have been identified in the human gene mutation database (http://www.hgmd.cf.ac.uk/ac/index.php), most of which are missense or nonsense nucleotide substitutions. As de novo mutations have been documented, the absence of a FD family history does not rule out the diagnosis of the disease [59]. Some genotypes are also associated with different disease aspects. For example, the genotype Y222X was associated with classic FD, with unexpectedly rapid deterioration of visual acuity, while T410A has been associated with ventricular hypertrophy and neuropathic pain [60].
Identification of a specific point mutation in patient’s family members can be performed by means of restriction enzyme digestion methods, by probes for allele-specific synthetic oligonucleotides, or preferably through DNA sequencing of the gene fragment containing the alteration [61]. Multiplex ligation-dependent probe amplification (MLPA) has been demonstrated to be an efficient tool for discovering the deletions of one or more exons or the deletion encompassing the entire gene, especially in heterozygous females. In fact, in 2008 using MLPA, two novel deletions were detected in two FD patients, both had been negative by the common sequencing analyses. This type of screening should therefore be systematically included in genetic testing surveys of FD patients [62].
FD biomarkers
Candidate biomarkers for lysosomal storage disorders (LSDs) can be divided into two categories: (1) molecules that accumulate in tissue and body fluids due to the enzymatic defect and (2) molecules produced by storage cells that can be measured in plasma, urine, or cerebrovascular fluid (CSF) [63].
Nowadays, FD is considered a systemic vasculopathy due to Gb3 storage in endothelial cells [64]. Therefore, it is not surprising that considerable attention has been focused on identifying plasma protein abnormalities reflecting endothelial activation as candidate FD biomarkers [63]. Unfortunately, laboratory investigations conducted so far have not clarified the significance of endothelial plasma protein and of minimal abnormalities in indicators of coagulation, fibrinolysis, and platelet and endothelial activation [65]. For example, analysis of the entire plasma proteome, performed by liquid chromatography/mass spectrometry (LC-MS) analysis, showed only modest therapy-induced changes in a few proteins [66]. This was also confirmed by the Netherland systematic proteomics analysis of FD patients’ blood specimens [67].
Accurate methods have been developed to quantify the primary storage lipid Gb3 in plasma and urine specimens [68, 69], even if the use of these measurements as biomarkers to monitor the progression of FD is questionable. This is due to the poor capacity of plasma and urinary Gb3 to reflect FD manifestations and therapeutic outcome [69] and because the onset of clinical complications occurs several years after lipid deposition (e.g., in FD hemizygotes apparently occurring at or before birth, before prominent clinical symptoms). Moreover, the absence of infantile manifestations in FD patients, completely lacking α-Gal-A activity, also indicates that Gb3 accumulation does not appear immediately and may not be a direct sign of the disease [69].
Plasma of FD patients contains increased concentrations of deacylated globotriaosylceramide, globotriasylsphingosine (lysoGb3) [70], and independent investigations have confirmed that this specific metabolite may be a good diagnostic tool, particularly in female FD patients [71]. Therefore, LysoGb3 concentration has been evaluated in relation to clinical FD manifestations [72]. Not surprising, it was demonstrated that in female FD hemizygotes lysoGb3 is low at birth and increased gradually with age, and plasma levels seemed to be related to some disease manifestations. Consequently, some authors speculate that this specific metabolite could be implicated in the pathogenesis of FD [63].
Treatment of FD
Enzyme replacement therapy
Enzyme replacement therapy (ERT) for FD has been available since 2001 in Europe and 2003 in USA [73]. Agalsidase alfa (Replagal, Shire) and agalsidase beta (Fabrazyme, Genzyme) are the two different commercial ERT preparations available. Both enzymes are authorized in the EU, but only Fabrazyme is approved in the USA. ERT allows to slow the progression of the disease and to prevent its serious complications, in particular it contributes to decrease cardiac mass [73] and renal Gb3 deposits increasing glomerular filtration rate and lowering proteinuria in pediatric patients [74, 75], but its effects on central nervous system are not well established. Concerning peripheral and autonomic nervous system, the incidence of neuropathic pain crisis, as well as vestibular dysfunction, gastrointestinal symptoms, and hypo-/anhidrosis, has been found to improve after ERT, although there is not clear data regarding the long-term benefit for these disease manifestations.
Moreover, signs of intraepidermal nerve regeneration were not detected at 18-month follow-up [76].
Both formulations have the same amino acid sequence of the native enzyme but differ in the glycosylation pattern of the protein due to the originating cells. Different effects in reduction of Gb3 storage have been shown mostly due to the dosage administered.
A complete normalization of Gb3 levels in plasma, skin, renal, and cardiac tissue has been demonstrated with agalsidase beta, whereas agalsidase alfa provides only partial clearance in these tissues. However, a superiority of one of these enzymes has never been demonstrated, although few studies have compared the efficacy of the two enzyme formulations [75, 77–79].
The response to ERT is heterogeneous and difficult to predict. The most commonly used ERT efficacy parameter is evaluation of Gb3 accumulation in the kidney, heart, and skin as well as plasma levels. However, Gb3 is a subclinical marker, and to date, no direct correlation has been demonstrated between lipid storage levels at baseline and disease severity [80]. Thus, Gb3 should not be considered solely but in association with clinically relevant endpoints, such as premature mortality, major clinical events, and clinical symptoms.
Standard dosage
Both treatments are infused intravenously every 14 days and are lifelong. Agalsidase alfa, which is derived from a line of cultured human fibroblasts, is administered intravenously at a dosage of 0.2 mg/kg and with an infusion time of 40 min independently of body weight. Algasidase beta, which is produced by Chinese hamster ovary cells, is given as intravenous infusion at a dose of 1.0 mg/kg with an infusion time of 15 mg/h biweekly.
Controindications
ERT is not recommended or advised to be stopped if there are present clinical findings suggestive of irreversible organ damage. Extensive cardiac fibrosis when it represents the sole indication for ERT, end stage renal disease without an option for renal transplantation in combination with advanced heart failure (NYHA class IV), end stage FD or other comorbidities with a life expectancy of <1 year, and severe cognitive decline of any cause have been proposed as criteria for not starting ERT [81••]. ERT should be stopped if patients present life-threatening or severe infusion reactions that do not respond to prophylaxis (anaphylaxis) or in the case of lack of response except for neuropathic pain in male with classical FD who manifest a higher risk of developing organ involvement within a short period of time.
The ERT should be continued in pregnancy, as no damage on mother or on the child has been observed [82].
Main drug interactions
An interaction between ERT and amiodarone has been reported [83].
Main side effects
The most frequent adverse events are infusion reactions, including headaches, paraesthesias, redness, hot flushes, fever, chills, cold sensation, nausea, vomiting, and fatigue. They usually are mild–moderate, occurring, as a rule, in the first 3 months after the start of ERT and reducing in frequency with time.
After resolution of the symptoms with non-steroidal anti-inflammatory drugs, antihistamines and/or glucocorticoids, the infusion may be continued after a few weeks/months.
Reducing the infusion rate may be also useful to avoid adverse effects.
A possible reason for infusion reactions or failure/decline in the efficacy of ERT is seroconversion. Unfortunately, up to 55–80 % of patients develop antibodies against human proteins, which are mainly generated in classically affected male patients.
In clinical studies, more than 80 % of patients treated with agalsidase beta developed IgG antibodies within 3 months, whereas in patients treated with agalsidase alpha this rate was about 24 % in the group of male, whereas no antibodies were detectable in female patients.
The clinical effect of these antibodies is unclear but inhibitory properties have been seen in vitro and may result in recurrence of storage material in urine, plasma, and skin and potentially impacts clinical effectiveness. If this effect is clinically significant, a change in dosing might be needed to achieve optimum treatment effect [84].
Recommendation for ERT therapy
Recommendations for initiation and cessation of ERT in patients with FD have been recently published, underlying the concept of starting ERT as soon as there are early clinical signs of kidney, heart, or brain involvement consistent with FD both in classical and non-classical FD and also in affected females [81••].
In male patients with classical FD, ERT may even be considered before clinical signs develop; consensus criteria recommend (class IIB recommendation) to start ERT at 16 years or older even in the absence of any signs or symptoms to prevent Gb3 deposits and irreversible tissue damage [81••].
In non-classical FD, signs of tissue involvement consistent with FD should be ascertained, performing extensive biochemical investigations and biopsy of damaged organ before starting ERT. Evaluation of sole SNC involvement implicates great difficulty in diagnostic work-up of non-classical FD patients, being obvious that tissue sample examination is not feasible.
In particular, concerning to nervous system involvement, neither algasidase alfa nor beta have been observed to significantly reduce the frequency of cerebrovascular events or the progression of white matter disease [85–87]. Possible explanations for ERT treatment failure include the incapability of the two compounds to cross the blood brain barrier and that irreversible endothelial damage occurs before the initiation of treatment.
Symptomatic or preventive treatments
Nephrological add-on therapy
Proteinuria and/or hypertension should be treated with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB) according to the treatment guidelines for chronic kidney disease (KDIGO).
Cardiological add-on therapy
Progressive cardiomyopathy should be treated with (ACE) inhibitors; if rhythm disorder is in present beta-blocker or anti-arrhythmic therapy after performing, a 24-hours ECG monitoring is recommended.
Neurological add-on therapy
Antiplatelet therapy and statins [88] are indicated in patient with prior stroke or transient ischemic attack according to the AHA/ASA guidelines on secondary prevention. For neuropathic pain, if pain is not controlled by the usual analgesics, gabapentin and cabamazepine may be administered.
Emerging therapies
Alternative strategies to deliver modified enzymes from the circulation across the blood–brain barrier are being investigated, including genetically engineered enzymes such as alfa N-actetyl-galactosaminidase (a-NAGAL), which may represent a non-immunogenic alternative, and the use of active site specific chaperones that will favor enzyme trafficking to an appropriate location increase retention of the enzyme in the endoplasmic reticulum and reduce the misfolding of the mutant protein [89–93, 94•]. 1-Deoxygalactonojirimycin (DGJ) (Migalastat) was shown to be safe and well tolerated in a trial of 27 patients, treated for 2 years. It was associated with increased α-Gal-A activity in 24 patients. Chaperone therapy offers the advantage of oral administration. Lastly, the injection into a Fabry mouse model of a recombinant adeno-associated viral vector encoding human α-Gal-A showed increased enzyme and decreased plasma Gb3 levels [95, 96].
Androgen receptor pathway has been recently implicated in pathogenesis of FD: blockade of AR signaling has been showed to prevent and to reverse cardiac and kidney hypertrophic phenotype in a mouse model of Fabry disease. These findings suggest blocking AR signaling as a novel therapeutic approach [97].
Discussion
Although FD is considered a rare disorder, it is probably underdiagnosed given the wide phenotypic spectrum, in which neurological features may be only a minor manifestation. Nephrologists and geneticists are the medical specialists who more often make the diagnosis of FD. However, given the frequency of neurological manifestations neurologists must be aware of the disease clinical manifestations to allow for prompt diagnosis. In fact, cerebrovascular diseases occur in 24–48 % of FD subjects and are a major cause of morbidity and early mortality in both male and female patients with FD [21, 22]. Cerebrovascular events may represent the first serious clinical manifestation of the disease and may be the symptoms leading to the diagnosis of FD. Similarly, neuropathy and particularly SFN are detected in almost 80 % of FD patients, and neuropathic pain is considered, in association with anhidrosis, a predictor of a decreased quality of life (QoL) [98].
The rarity of disease, as well as incomplete knowledge regarding the natural history and pathogenic mechanisms, makes FD a challenge for many clinicians who are not able to properly diagnose, treat, or follow-up affected patients. Although diagnostic and management guidelines have been published, clear diagnostic and care criteria are lacking [99••, 100]. This is mostly due to the heterogeneity of clinical decisions, treatment protocols, and supportive care that are at the discretion of the treating physician. Published datasets are incomplete and sometimes do not include untreated, mildly affected patients hampering the possibility of developing standardized care guidelines but also tailored management approach to patient. Also, the management of subjects carrying molecular variants of the GLA gene of unknown significance is unclear. Figure 2 represents a proposal for a diagnostic algorithm based on the literature and our experience. The acquisition of detailed data on large series of well-phenotyped FD patients and on disease natural history are mandatory given the development of new treatments such as ERT that may contribute in reducing the disease burden and morbidity and mortality. The introduction of ERT which changed the care of FD patients is still not supported by controlled trials [34] that clarify the efficacy of this therapy, particularly on neurological manifestations, and whether early ERT administration really prevents the development of irreversible secondary cellular and tissue damage.
The uncertainties of the efficacy of ERT treatment highlights the need for (1) clinical trial really assessing the efficacy of ERT and (2) the development of new treatment approaches that are better able to access the central nervous system aimed at bypassing neutralizing antibodies and improving heart and kidney functions with an impact on survival, progression, and quality of life.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sweeley C, Klionsky B. Fabry’s disease: classification as a sphingolipidosis and partial characterization of a novel glycolipid. J Biol Chem. 1963;238:3148–50.
Hers H. Inborn lysosomal diseases. Gastroenterology. 1965;48:625–33.
Brady RO. Enzymatic abnormalities in diseases of sphingolipid metabolism. Clin Chem. 1967;13:565–77.
Schiffmann R. Fabry disease. Pharmacol Ther. 2009;122(1):65–77.
Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. 2008;372(9647):1427–35. A detailed review about pathophysiology, neurological and extraneurological manifestations in Fabry disease.
Ishii S, Kase R, Sakuraba H, Suzuki Y. Characterization of a mutant alphagalactosidase gene product for the late-onset cardiac form of Fabry disease. Biochem Biophys Res Commun. 1993;197(3):1585–9.
Sakuraba H, Oshima A, Fukuhara Y, Shimmoto M, Nagao Y, Bishop DF, et al. Identification of point mutations in the alpha-galactosidase A gene in classical and atypical hemizygotes with Fabry disease. Am J Hum Genet. 1990;47(5):784–9.
Bernstein HS, Bishop DF, Astrin KH, Kornreich R, Eng CM, Sakuraba H, et al. Fabry disease: six gene rearrangements and an exonic point mutation in the alphagalactosidase gene. J Clin Invest. 1989;83(4):1390–9.
Shimotori M, Maruyama H, Nakamura G, Suyama T, Sakamoto F, Itoh M, et al. Novel mutations of the GLA gene in Japanese patients with Fabry disease and their functional characterization by active site specific chaperone. Hum Mutat. 2008;29(2):331.
Kornreich R, Bishop DF, Desnick RJ. Alpha-galactosidase A gene rearrangements causing Fabry disease. Identification of short direct repeats at breakpoints in an Alu-rich gene. J Biol Chem. 1990;265(16):9319–26.
Ishii S, Chang HH, Kawasaki K, Yasuda K, Wu HL, Garman SC, et al. Mutant alphagalactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem J. 2007;406(2):285–95.
Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249–54.
Shin SH, Kluepfel-Stahl S, Cooney AM, Kaneski CR, Quirk JM, Schiffmann R, et al. Prediction of response of mutated alpha-galactosidase A to a pharmacological chaperone. Pharmacogenet Genomics. 2008;18:773–80.
Desnick RJ, Ioannou YA. α-Galactosidase a deficiency. Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 6th ed. New York: McGraw-Hill; 1996. p. 2741–84.
Desnick RJ, Ioannou YA. α-Galactosidase a deficiency. Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2001. p. 3733–74.
Rombach SM, Twickler TB, Aerts JM, Linthorst GE, Wijburg FA, Hollak CE. Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab. 2010;99(2):99–108.
Shu L, Park JL, Byun J, Pennathur S, Kollmeyer J, Shayman JA. Decreased nitric oxide bioavailability in a mouse model of Fabry disease. J Am Soc Nephrol. 2009;20(9):1975–85.
Shen JS, Meng XL, Moore DF, Quirk JM, Shayman JA, Schiffmann R, et al. Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol Genet Metab. 2008;95(3):163–8.
Moore DF, Kaneski CR, Askari H, Schiffmann R. The cerebral vasculopathy of Fabry disease. J Neurol Sci. 2007;257(1–2):258–63.
Buechner S, Moretti M, Burlina AP, Cei G, Manara R, Ricci R, et al. Central nervous system involvement in Anderson-Fabry disease: a clinical and MRI retrospective study. J Neurol Neurosurg Psychiatry. 2008;79(11):1249–54.
Sims K, Politei J, Banikazemi M, Lee P. Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events: natural history data from the Fabry registry. Stroke. 2009;40:788–94.
Buechner S, Moretti M, Burlina AP, Cei G, Manara R, Ricci R, et al. Central nervous system involvement in Anderson-Fabry disease: a clinical and MRI retrospective study. J Neurol Neurosurg Psychiatry. 2008;79:1249–54.
Rolfs A, Böttcher T, Zschiesche M, Morris P, Winchester B, Bauer P, et al. Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005;366(9499):1794–6.
Brouns R, Sheorajpanday R, Braxel E, Eyskens F, Baker R, Hughes D, et al. Middelheim Fabry Study (MiFaS): a retrospective Belgian study on the prevalence of Fabry disease in young patients with cryptogenic stroke. Clin Neurol Neurosurg. 2007;109(6):479–84.
Baptista MV, Ferreira S, Pinho-E-Melo T, Carvalho M, Cruz VT, Carmona C, et al. Mutations of the GLA gene in young patients with stroke: the PORTYSTROKE study—screening genetic conditions in Portuguese young stroke patients. Stroke. 2010;41(3):431–6.
Brouns R, Thijs V, Eyskens F, Van den Broeck M, Belachew S, Van Broeckhoven C, et al. Belgian Fabry study: prevalence of Fabry disease in a cohort of 1000 young patients with cerebrovascular disease. Stroke. 2010;41(5):863–8.
Rolfs A, Fazekas F, Grittner U, Dichgans M, Martus P, Holzhausen M, et al. Acute cerebrovascular disease in the young: the Stroke in Young Fabry Patients study. Stroke. 2013;44(2):340–9. A large observational study on the prevalence of Fabry disease in young patients with stroke.
Shi Q, Chen J, Pongmoragot J, Lanthier S, Saposnik G. Prevalence of Fabry disease in stroke patients—a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2014;23(5):985–92.
Steinicke R, Gaertner B, Grittner U, Schmidt W, Dichgans M, Heuschmann PU, et al. Kidney function and white matter disease in young stroke patients: analysis of the stroke in young fabry patients study population. Stroke. 2012;43(9):2382–8.
Kolodny E, Fellgiebel A, Hilz MJ, Sims K, Caruso P, Phan TG, et al. Cerebrovascular involvement in Fabry disease: current status of knowledge. Stroke. 2015;46(1):302–13. An update of cerebrovascular involvement in FD, including epidemiology, clinical features and typical neuroadiological findings.
Uçeyler N, Homola GA, Guerrero González H, Kramer D, Wanner C, Weidemann F, Solymosi L, Sommer C. Increased arterial diameters in the posterior cerebral circulation in men with Fabry disease. PLoS One. 2014;9(1):e87054.
Crutchfield KE, Patronas NJ, Dambrosia JM, Frei KP, Banerjee TK, Barton NW, et al. Quantitative analysis of cerebral vasculopathy in patients with Fabry disease. Neurology. 1998;50:1746–9.
Böttcher T, Rolfs A, Tanislav C, Bitsch A, Köhler W, Gaedeke J, et al. Fabry disease—underestimated in the differential diagnosis of multiple sclerosis? PLoS One. 2013;8:e71894.
Bersano A, Lanfranconi S, Valcarenghi C, Bresolin N, Micieli G, Baron P. Neurological features of Fabry disease: clinical, pathophysiological aspects and therapy. Acta Neurol Scand. 2012;126(2):77–97.
Valeriani M, Mariotti P, Le Pera D, Restuccia D, De Armas L, Maiese T, et al. Functional assessment of A delta and C fibers in patients with Fabry’s disease. Muscle Nerve. 2004;30:708–13.
Kocen RS, Thomas PK. Peripheral nerve involvement in Fabry’s disease. Arch Neurol. 1970;22(1):81–8.
Charrow J. A 14-year-old boy with pain in hands and feet. Pediatr Ann. 2009;38(4):190.
Kolodny EH, Pastores GM. Anderson-Fabry disease: extrarenal, neurologic manifestations. J Am Soc Nephrol. 2002;13:S3–150.
Üçeyler N, Magg B, Thomas P, Wiedmann S, Heuschmann P, Sommer C. A comprehensive Fabry-related pain questionnaire for adult patients. Pain. 2014;155(11):2301–5.
Schiffmann R, Scott LJC. Pathophysiology and assessment of neuropathic pain in Fabry disease. Acta Paediatr. 2002;439:S48–52.
Hilz MJ, Stemper B, Kolodny EH. Lower limb cold exposure induces pain and prolonged small fiber dysfunction in Fabry patients. Pain. 2000;84:361–5.
Üçeyler N, Ganendiran S, Kramer D, Sommer C. Characterization of pain in Fabry disease. Clin J Pain. 2014;30:915–20.
Luciano CA, Russell JW, Banerjee TK, Quirk JM, Scott LJ, Dambrosia JM, et al. Physiological characterization of neuropathy in Fabry disease. Muscle Nerve. 2002;26:622–9.
Toyooka K, Said G. Nerve biopsy findings in hemizygous and heterozygous patients with Fabry’s disease. J Neurol. 1997;244:464–8.
Gemignani F, Marbini A, Bragaglia MM, Govoni E. Pathological study of the sural nerve in Fabry’s disease. Eur Neurol. 1984;23:173–81.
Kennedy WR. Unmyelinated nerves, challenges, and opportunities: skin biopsy and beyond. Suppl Clin Neurophysiol. 2004;57:8–14.
Üçeyler N, He L, Schönfeld D, Kahn A-K, Reiners K, Hilz MJ, et al. Small fibers in Fabry disease: baseline and follow-up data under enzyme replacement therapy. J Peripher Nerv Syst. 2011;16:304–14.
De Greef BT, Hoeijmakers JG, Wolters EE, Smeets HJ, van den Wijngaard A, Merkies IS, et al. No Fabry disease in patients presenting with isolated small fiber neuropathy. PLoS One. 2016;11(2):e0148316.
Cable WJ, Kolodny EH, Adams RD. Fabry disease: impaired autonomic function. Neurology. 1982;32:498–502.
Schiffmann R, Floeter MK, Dambrosia JM, Gupta S, Moore DF, Sharabi Y, et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 2003;28(6):703–10.
Moore DF, Ye F, Schiffmann R, Butman JA. Increased signal intensity in the pulvinar on T1-weighted images: a pathognomonic MR imaging sign of Fabry disease. AJNR Am J Neuroradiol. 2003;24(6):1096–101.
Uçeyler N, Homola GA, Guerrero González H, Kramer D, Wanner C, Weidemann F, et al. Increased arterial diameters in the posterior cerebral circulation in men with Fabry disease. PLoS One. 2014;9(1). e87054.
Fazekas F, Enzinger C, Schmidt R, Grittner U, Giese AK, Hennerici MG, et al. Brain magnetic resonance imaging findings fail to suspect Fabry disease in young patients with an acute cerebrovascular event. Stroke. 2015;46(6):1548–53. This study does not support the utility of brain MRI for diagnosis of FD diagnosis; pulvinar sign, verterbobasilar vessels ectasia, CWMH are not considered typical of FD.
Linthorst GE, De Rie MA, Tjiam KH, Aerts JM, Dingemans KP, Hollak CE. Misdiagnosis of Fabry disease: importance of biochemical confirmation of clinical or pathological suspicion. Br J Dermatol. 2004;150:575–7.
Desnick RJ, Allen KY, Desnick SJ, Raman MK, Bernlohr RW, Krivit W. Fabry’s disease: enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J Lab Clin Med. 1973;81(2):157–71.
Martins AM, D’Almeida V, Kyosen SO, Takata ET, Delgado AG, Gonçalves AM, et al. Guidelines to diagnosis and monitoring of Fabry disease and review of treatment experiences. J Pediatr. 2009;155(4 Suppl):S19–31.
Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001;308(1–2):195–6.
Caudron E, Germain DP, Prognon P. Fabry disease: enzymatic screening using dried blood spots on filter paper. Rev Med Interne. 2010;31 Suppl 2:S263–9.
Mehta A, Beck M, Eyskens F, Feliciani C, Kantola I, Ramaswami U, et al. Fabry disease: a review of current management strategies. QJM. 2010;103(9):641–59.
Yang CC, Lai LW, Whitehair O, Hwu WL, Chiang SC, Lien YH. Two novel mutations in the alpha-galactosidase A gene in Chinese patients with Fabry disease. Clin Genet. 2003;63:205–9.
Desnick RJ, Bernstein HS, Astrin KH, Bishop DF. Fabry disease: molecular diagnosis of hemizygotes and heterozygotes. Enzyme. 1987;38:54–64.
Schirinzi A, Centra M, Prattichizzo C, Gigante M, De Fabritiis M, Giancaspro V, et al. Identification of GLA gene deletions in Fabry patients by Multiplex Ligation-dependent Probe Amplification (MLPA). Mol Genet Metab. 2008;94:382–5.
Aerts JM, Kallemeijn WW, Wegdam W, Joao Ferraz M, van Breemen MJ, Dekker N, et al. Biomarkers in the diagnosis of lysosomal storage disorders: proteins, lipids, and inhibodies. J Inherit Metab Dis. 2011;34(3):605–19.
DeGraba T, Azhar S, Dignat-George F, Brown E, Boutière B, Altarescu G, et al. Profile of endothelial and leukocyte activation in Fabry patients. Ann Neurol. 2000;47(2):229–33.
Vedder AC, Biró E, Aerts JM, Nieuwland R, Sturk G, Hollak CE. Plasma markers of coagulation and endothelial activation in Fabry disease: impact of renal impairment. Nephrol Dial Transplant. 2009;24(10):3074–81.
Moore DF, Krokhin OV, Beavis RC, Ries M, Robinson C, Goldin E, et al. Proteomics of specific treatment-related alterations in Fabry disease: a strategy to identify biological abnormalities. Proc Natl Acad Sci U S A. 2007;104(8):2873–8.
Rombach SM, van den Bogaard B, de Groot E, Groener JE, Poorthuis BJ, Linthorst GE, et al. Vascular aspects of Fabry disease in relation to clinical manifestations and elevations in plasma globotriaosylsphingosine. Hypertension. 2012;60(4):998–1005.
Auray-Blais C, Blais CM, Ramaswami U, Boutin M, Germain DP, Dyack S, et al. Urinary biomarker investigation in children with Fabry disease using tandem mass spectrometry. Clin Chim Acta. 2015;438:195–204.
Vedder AC, Linthorst GE, van Breemen MJ, Groener JE, Bemelman FJ, Strijland A, et al. The Dutch Fabry cohort: diversity of clinical manifestations and Gb3 levels. J Inherit Metab Dis. 2007;30(1):68–78.
Sueoka H, Ichihara J, Tsukimura T, Togawa T, Sakuraba H. Nano-LC-MS/MS for quantification of Lyso-Gb3 and its analogues reveals a useful biomarker for Fabry disease. PLoS One. 2015;10(5):e0127048.
Togawa T, Kodama T, Suzuki T, Sugawara K, Tsukimura T, Ohashi T, et al. Plasma globotriaosylsphingosine as a biomarker of Fabry disease. Mol Genet Metab. 2010;100(3):257–61.
Rombach SM, Dekker N, Bouwman MG, Linthorst GE, Zwinderman AH, Wijburg FA, et al. Plasma globotriaosylsphingosine: diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta. 2010;1802(9):741–8.
Kampmann C, Linhart A, Devereux RB, Schiffmann R. Effect of agalsidase alfa replacement therapy on Fabry disease-related hypertrophic cardiomyopathy: a 12- to 36-month, retrospective, blinded echocardiographic pooled analysis. Clin Ther. 2009;31(9):1966–76.
West M, Nicholls K, Mehta A, Clarke JT, Steiner R, Beck M, et al. Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol. 2009;20(5):1132–9.
Linthorst GE, Germain DP, Hollak CE, Hughes D, Rolfs A, Wanner C, et al. European Medicines Agency Expert opinion on temporary treatment recommendations for Fabry disease during the shortage of enzyme replacement therapy (ERT). Mol Genet Metab. 2011;102(1):99–102.
Schiffmann R, Hauer P, Freeman B, Ries M, Scott LJ, Polydefkis M, et al. Enzyme replacement therapy and intraepidermal innervation density in Fabry disease. Muscle Nerve. 2006;34(1):53–6.
Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al. Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry’s disease. N Engl J Med. 2001;345(1):9–16.
Schaefer RM, Tylki-Szymańska A, Hilz MJ. Enzyme replacement therapy for Fabry disease: a systematic review of available evidence. Drugs. 2009;69(16):2179–205.
Sakurab H, Murata-Ohsawa M, Kawashima I, Tajima Y, Kotani M, Ohshima T, et al. Comparison of the effects of agalsidase alfa and agalsidase beta on cultured human Fabry fibroblasts and Fabry mice. J Hum Genet. 2006;51:180–8.
Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79(1):31–40.
Biegstraaten M, Arngrímsson R, Barbey F, Boks L, Cecchi F, Deegan PB, Feldt-Rasmussen U, Geberhiwot T, Germain DP, Hendriksz C, Hughes DA, Kantola I, Karabul N, Lavery C, Linthorst GE, Mehta A, van de Mheen E, Oliveira JP, Parini R, Ramaswami U, Rudnicki M, Serra A, Sommer C, Sunder-Plassmann G, Svarstad E, Sweeb A, Terryn W, Tylki-Szymanska A, Tøndel C, Vujkovac B, Weidemann F, Wijburg FA, Woolfson P, Hollak CE. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015;10:36. Recommendations for initiation and cessation of ERT in FD patients. The recommendations can be used as a benchmark for initiation and cessation of ERT, although final decisions should be made on an individual basis.
Politei JM. Treatment with agalsidase beta during pregnancy in Fabry disease. J Obstet Gynaecol Res. 2010;36(2):428–9.
Whitley CB, Tsai MY, Heger JJ, Prystowsky EN, Zipes DP. Amiodarone phenocopy of Fabry’s keratopathy. JAMA. 1983;249(16):2177–8.
Linthorst GE, Hollak CE, Donker-Koopman WE, Strijland A, Aerts JM. Enzyme therapy for Fabry disease: neutralizing antibodies toward agalsidase alpha and beta. Kidney Int. 2004;66(4):1589–95.
Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, et al. Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet. 2004;75(1):65–74.
Eng CM, Germain DP, Banikazemi M, Warnock DG, Wanner C, Hopkin RJ, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8(9):539–48.
Rombach SM, Smid BE, Bouwman MG, Linthorst GE, Dijkgraaf MG, Hollak CE. Long term enzyme replacement therapy for Fabry disease: effectiveness on kidney, heart and brain. Orphanet J Rare Dis. 2013;8:47.
Politei JM. Can we use statins to prevent stroke in Fabry disease? J Inherit Metab Dis. 2009;32(4):481–7.
Tajima Y, Kawashima I, Tsukimura T, Sugawara K, Kuroda M, Suzuki T, et al. Use of a modified alpha-N-acetylgalactosaminidase in the development of enzyme replacement therapy for Fabry disease. Am J Hum Genet. 2009;85(5):569–80.
Yam GH, Bosshard N, Zuber C, Steinmann B, Roth J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol Cell Physiol. 2006;290(4):C1076–82.
Yam GH, Zuber C, Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005;19(1):12–8.
Fan JQ, Ishii S. Active-site-specific chaperone therapy for Fabry disease. Yin and Yang of enzyme inhibitors. FEBS J. 2007;274(19):4962–71.
Germain DP, Fan JQ. Pharmacological chaperone therapy by active-site-specific chaperones in Fabry disease: in vitro and preclinical studies. Int J Clin Pharmacol Ther. 2009;47 Suppl 1:S111–7.
El-Abassi R, Singhal D, England JD. Fabry’s disease. J Neurol Sci. 2014;344(1–2):5–19. Complete review on clinical, pathogenetic and therapeutic aspects of FD.
Jung SC, Han IP, Limaye A, Xu R, Gelderman MP, Zerfas P, et al. Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice. Proc Natl Acad Sci U S A. 2001;98(5):2676–81.
Takahashi H, Hirai Y, Migita M, Seino Y, Fukuda Y, Sakuraba H, et al. Long-term systemic therapy of Fabry disease in a knockout mouse by adeno-associated virus-mediated muscle-directed gene transfer. Proc Natl Acad Sci U S A. 2002;99(21):13777–82.
Shen JS, Meng XL, Wight-Carter M, Day TS, Goetsch SC, Forni S, et al. Blocking hyperactive androgen receptor signaling ameliorates cardiac and renal hypertrophy in Fabry mice. Hum Mol Genet. 2015;24:3181–91.
Gold KF, Pastores GM, Botteman MF, Yeh JM, Sweeney S, Aliski W, et al. Quality of life of patients with Fabry disease. Qual Life Res. 2002;11:317–27.
Rombach SM, Smid BE, Linthorst GE, Dijkgraaf MG, Hollak CE. Natural course of Fabry disease and the effectiveness of enzyme replacement therapy: a systematic review and meta-analysis: effectiveness of ERT in different disease stages. J Inherit Metab Dis. 2014;37(3):341–52. Effectiveness of ERT in different disease stages. ERT is effective in reducing left ventricular mass, but has a limited effect on renal function. Improved treatment options are needed for Fabry disease.
Salvati A, Burlina AP, Borsini W. Nervous system and Fabry disease, from symptoms to diagnosis: damage evaluation and follow-up in adult patients, enzyme replacement, and support therapy. Neurol Sci. 2010;31:299–306.
Acknowledgments
A special thank you to Dr. Myrna Rosenfeld for taking the time to review this manuscript and to Dr. Marzia Rivello, from Shire Pharmaceuticals Limited, for the support in the organization of multidisciplinary symposia on the disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cerebrovascular Disorders
Rights and permissions
About this article
Cite this article
Ranieri, M., Bedini, G., Parati, E.A. et al. Fabry Disease: Recognition, Diagnosis, and Treatment of Neurological Features. Curr Treat Options Neurol 18, 33 (2016). https://doi.org/10.1007/s11940-016-0414-5
Published:
DOI: https://doi.org/10.1007/s11940-016-0414-5