Opinion statement
Urgent reperfusion of the ischaemic brain is the aim of stroke treatment, and the last two decades have seen a rapid advancement in the medical and endovascular treatment of acute ischaemic stroke. Intravenous tissue plasminogen activator (tPA) was first introduced as a safe and effective thrombolytic agent followed by the introduction of newer thrombolytic agents as well as anticoagulant and antiplatelet agents, proposed as potentially safer drugs with more favourable interaction profiles. In addition to chemo-thrombolysis, other techniques including transcranial sonothrombolysis and microbubble cavitation have been introduced which are showing promising results, but await large-scale clinical trials. These developments in medical therapies which are undoubtedly of great importance due to their potential widespread and immediate availability are paralleled with gradual but steady improvements in endovascular recanalisation techniques which were initiated by the introduction of the MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Penumbra systems. The introduction of the Solitaire device was a significant achievement in reliable and safe endovascular recanalisation and was followed by further innovative stent retrievers. Initial trials failed to show a solid benefit in endovascular intervention compared with IV-tPA alone. These counterintuitive results did not last long, however, when a series of very well-designed randomised controlled trials, pioneered by MR-CLEAN, EXTEND-IA and ESCAPE, emerged, confirming the well-believed daily anecdotal evidence. There have now been seven positive trials of endovascular treatment for acute ischaemic stroke. Now that level I evidence regarding the superiority of endovascular recanalisation is abundantly available, the clinical challenge is how to select patients suitable for intervention and to familiarise and educate stroke care providers with this recent development in stroke care. It is important for the interventional services to be provided only in comprehensive stroke centres and endovascular interventions attempted by experienced well-trained operators, at this stage as an adjunct to the established medical treatment of IV-tPA, if there are no contraindications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is considered one of the major public health problems and the third most costly health condition in developed countries [1]. It is the most common cause of adult disability in these countries, requiring long-term costly rehabilitation, and is the principal cause of 1 out of every 16 deaths [2]. The majority of the strokes are ischaemic in nature with only about 15 % being due to haemorrhagic events [2].
In ischaemic stroke, a core area of tissue dies due to under-perfusion and an area of hypo-perfused tissue with patent collateral vessels remains salvageable. This has been referred to as the “penumbra” which, if revascularised in a timely manner, could be saved [3]. Therefore, urgent recanalisation of the occluded artery and restoration of blood flow are considered the most important therapeutic step to reperfuse the threatened brain parenchyma before an irreversible infarction is established, in order to reduce morbidity and mortality [4]. Studies have estimated 1.8 days of added healthy life benefit for each minute reduction in time to treatment [5], and a meta-analysis has demonstrated that successful recanalisation increases the chance of a good functional outcome with an odds ratio of 4.43 (95 % CI 3.32–5.91) and reduced mortality at 3 months (OR 0.24, 95 % CI 0.16–0.35) [6].
Almost 20 years ago, in 1996, intravenous tissue plasminogen activator (IV-tPA) was approved as the first thrombolysis medication and practically the only treatment option in ischaemic strokes, when administered within 3 h from onset. However, its success rate in recanalisation is reduced in large vessel occlusion and was never shown to be more than 50 % and is more likely to be around 30 % [1, 7]. This partial success in recanalisation, with critical time limitations, makes tPA less than ideal in managing a large proportion of the stroke patients with large vessel occlusion. In fact, it has been shown that approximately 70 % of patients with acute ischaemic stroke are not eligible for thrombolysis, mainly due to delayed presentation [8, 9].
Overall, the success of intravenous thrombolysis depends on numerous factors including thrombus type, location and extent, collateral circulation, underlying comorbidities, patient’s age, time to commencement of treatment and time to recanalisation [2]. Furthermore, studies have shown that large calibre proximal arteries are unlikely to be responsive to chemical thrombolysis alone [7, 10, 11]. Clinical trials have also shown that the likelihood of recanalisation negatively correlates with thrombus burden, with those having a clot size more than 8 mm in length having a significantly lower chance of achieving recanalisation by IV-tPA alone [2, 12].
The effectiveness of IV-tPA is also affected by the composition of the occlusive clot with emboli originated from large vessel atherosclerotic lesions shown to be less responsive, compared with the usually recanalisable fibrin-rich cardioembolic thrombi [13]. In addition, early reocclusion of the arteries has been shown in up to 20 % of cases, when recanalised initially by IV-tPA [14].
Therefore, there have been major initiatives over the last several years to examine and develop new techniques to improve recanalisation rates and clinical outcome. These newly proposed therapeutic techniques consist not only of other extra-arterial methods of thrombolysis but also a variety of endovascular techniques with the aim of improving revascularisation rates and clinical outcome.
Extra-arterial thrombolysis
Intravenous tPA is the standard first-line treatment in those who are eligible which normally follows a noncontrast CT brain excluding a haemorrhagic stroke. Provided no contraindications exist, it is administrated at a dose of 0.9 mg/kg (maximum of 90 mg), with 10 % of the medication given as a bolus and the remainder infused over 1 h [2, 15, 16]. The safety and efficacy of IV-tPA within the first 3 h of the onset of the symptoms was demonstrated in the National Institute of Neurological Disorders and Stroke (NINDS) study [17]; however, the envelope of the time limitation was stretched in subsequent research studies, like ECASS 3 (European Cooperative Acute Stroke Study 3) [18], with the proposed guidelines changed accordingly to include those patients who presented within 4.5 h [1, 15, 19]. Subgroup analyses of the data from the IST-3 trial and SITS-IST (Ischemic Stroke Recorded in the Safe Implementation) study furthermore showed no statistically significant detrimental effect for tPA treatment in those who presented up to 6 h after onset compared with 4.5 or 3 h, suggesting that delayed intravenous (IV) thrombolysis can still have beneficial effects [9, 20, 21].
Sonothrombolysis aims to increase the efficacy of IV-tPA by using the effect of energy delivered by sound waves on fibrin strands in the thrombus. Its feasibility and safety have already been proven by the CLOTBUST (Combined Lysis of Thrombus in Brain Ischemia With Transcranial Ultrasound and Systemic TPA) study [10, 22–24]. However, its efficacy is being further investigated in the phase III CLOTBUST-ER (Randomized, Placebo-Controlled, Double-Blind Study of the Combined Lysis of Thrombus With Ultrasound and Systemic Tissue Plasminogen Activator for Emergent Revascularization) study with recruitment completed but the results are yet to be released [25]. A similar idea has been proposed by using ultrasound microbubble contrast agents, which lead to cavitation within the occlusive thrombi, helping their degradation in the presence of IV-tPA, which is being examined in the TUSCAN (Transcranial Ultrasound in Clinical Sonothrombolysis) study [1, 8, 10, 26, 27, 28•].
On the other hand, there is a definite lack of evidence for the benefit of combination therapies of IV-tPA with anticoagulation or antiplatelet agents. The recent ARTIS (Antiplatelet Therapy in Combination with recombinant tPA Thrombolysis in Ischemic Stroke) study demonstrated an increased risk of symptomatic intracranial haemorrhage with concurrent administration of aspirin [10, 19, 29, 30].
Parallel to the worldwide ongoing use of tPA as the only clinically approved thrombolytic agent in ischaemic stroke (which works by converting plasminogen into active plasmin [2, 18, 31•, 32]), there are ongoing attempts to investigate other potential agents with the hope of finding substitutes with better risk-benefit profile, particularly given the emerging evidence of a potential detrimental effect of tPA on the blood-brain barrier and its resultant neurotoxicity [2, 10, 33–35].
Tenecteplase was one of the first agents introduced as a semi-synthetic and potential substitute for tPA, which has a longer half-life and an increased affinity to fibrin [10, 30, 36, 37], with at least one study so far demonstrating increased recanalisation success rate compared with IV-tPA with good final clinical outcome and no statistically significant difference in the risk of haemorrhagic complications [37]. However, NOR-TEST (Norwegian Tenecteplase Stroke Trial) is an ongoing RCT on tenecteplase with the results yet to be released [38].
Desmoteplase also demonstrates a higher affinity for fibrin with longer half-life compared with tPA. It is found in the bat saliva and has been suggested as an alternative thrombolytic agent. However, increased symptomatic haemorrhage was demonstrated in the initial feasibility and safety phase of the DIAS (Desmoteplase In Acute Ischemic Stroke) study, requiring dosage modification in the following phase II DEDAS (Dose Escalation of Desmoteplase for Acute Ischemic Stroke) and phase III DIAS-2 studies [10, 39]. Although there were unfavourable results released from the DIAS-2 study, however, given the subsequent criticisms regarding its sample size and patient selection, desmoteplase was again the subject of investigation in the phase III DIAS-4 study with preliminary results confirming its inferiority with the final results to be published in the near future [10, 40–42].
In addition to the above-mentioned novel thrombolytic agents, there are ongoing research studies into the potential benefit of the new anticoagulation and antiplatelet agents.
Ancrod is an anticoagulant agent extracted from the viper’s venom which reduces fibrinogen levels and blood viscosity. Although an initially better immediate outcome was demonstrated in STAT (Stroke Treatment with Ancrod Trial) in those patients with acute ischaemic stroke who received ancrod infusion, it was associated with a marginally significant increase in the risk of symptomatic haemorrhage with the long-term benefit being questionable [10, 43–45].
Argatroban, on the other hand, is a direct thrombin inhibitor which was shown to be of no significant benefit in the treatment of patients with acute ischaemic stroke who presented late, in the phase I ARGIS (Argatroban Anticoagulation in Patients with Acute Ischemic Stroke) study. However, it demonstrated a statistically significant benefit when combined with conventional tPA infusion in the ARTTS (Argatroban Tissue-Type-PA Stroke) study, increasing the recanalisation rate [10, 23, 46–51], and is now into the second phase of clinical investigation [52]. Other agents from this family include hirudin, bivalirudin, desirudin, lepirudin, dabigatran, melagatran and ximelagatran, and their roles in the management of acute ischaemic stroke are yet to be fully investigated [53–55].
Relatively novel GP-IIb/IIa antiplatelet agents, as direct platelet activation inhibitors [56], were also tried as adjuvant treatments in the setting of coronary artery occlusion, with immediate recanalisation improvement, but with questionable long-term benefit in that context [10, 57, 58], and now are the subject of multiple clinical trials to study their potential benefit in the management of ischaemic stroke.
Tirofiban was initially suggested for those patients with acute ischaemic stroke with delayed presentation and has been shown not only to be safe but also with a marginal benefit in decreasing mortality in the long term in the phase II SaTIS (Safety of Tirofiban in Acute Ischemic Stroke) trial [9, 10, 24].
Abciximab, however, demonstrated a significantly higher rate of symptomatic intracranial haemorrhage in the phase II AbESTT (Abciximab in Emergency Treatment of Stroke Trial) RCT compared with the control group with no significant benefit in outcome [9, 10, 59, 60].
Eptifibatide, on the other hand, decreased the risk of symptomatic intracranial haemorrhage when combined with IV-tPA compared with those treated with IV-tPA alone in the phase II CLEAR-ER (Combined Approach to Lysis Utilizing Eptifibatide and Recombinant Tissue Plasminogen Activator in Acute Ischemic Stroke) study, with a trend towards a better final clinical outcome [61].
Other members of this antiplatelet aggregation family, e.g. roxifiban and orbofiban, have also been the subject of different phases of clinical trials, with their advantage over conventional treatments yet to be shown [62, 63].
Endovascular treatment
PROACT II (Prolyse in Acute Cerebral Thromboembolism) was the first study to examine the efficacy of intra-arterial thrombolysis instead of intravenous thrombolysis. Despite the fact that some of the usual resilient patterns of occlusion such as ICA thrombosis were excluded from the study, there were still relatively poor recanalisation rates in patients treated with IA urokinase, with questionable overall clinical benefit, which could even be partially related to the heparin [32, 64]. Although the following IMS II (Interventional Management of Stroke II) trial demonstrated a slightly better outcome in those patients treated with intra-arterial tPA infusion via an EKOS (EkoSonic Endovascular System) microcatheter, there was also a higher rate of symptomatic intracranial haemorrhage demonstrated in this group with an overall poor recanalisation rate [65], demonstrating a failure in providing a tangible advantage in this technique [66].
Mechanical thrombectomy has always had the appealing potential of rapid recanalisation and accelerated reperfusion with a potential for reduced haemorrhagic risk [1]. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) device was a corkscrew-shaped device with helical nitinol loops, which was specifically designed for placement into the thrombus for enbloc removal. It was the first officially approved thrombectomy device with its safety and feasibility assessed in MERCI and multi-MERCI trials [28•, 67–69]. The device was practically only useful for proximal large arterial occlusions, predominantly M1, and although this was associated with an increased recanalisation rate in particular when combined with IV-tPA, approaching 70 %, there was no overall clinical benefit shown in the final outcome of patients with an increase in mortality rate noted in those treated with this device [2, 67, 70].
The earliest iteration of the PT-PAD (Penumbra Thrombus Perturbation and Aspiration Device) was a clot aspiration catheter with an inner mechanical clot separator, which was shown to be safe in the PPS (Penumbra Pivotal Stroke) trial [71]. Despite its relatively high recanalisation rate of ∼80 %, the trial failed to show any significant benefit in the final clinical outcome [68]. However, the newer Penumbra system is much simpler, but with more advanced catheter technology, comprised of Max/Neuron family distal intracranial catheters for aspiration thrombectomy using a controlled vacuum pump system, which are the subject of an ongoing trial, THERAPY (Table 1). THERAPY is a trial to assess the safety and effectiveness of the new Penumbra system as an adjunctive treatment to IV-tPA, over IV-tPA alone (an example of suction thrombectomy depicted in Fig. 1). The preliminary results of this study have been quite encouraging with a 7.6 % increase in good outcome as per modified Rankin Scale (mRS), 11.9 % decrease in mortality at 90 days and no difference in complication rate.
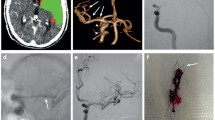
A case of difficult thrombectomy, utilising different techniques. a Hyperdense left MCA sign, with extension into the ipsilateral carotid terminus. b Occluded left carotid terminus and MCA on CTA with severe paucity of collaterals. c ICA occlusion from the bulb. d ICA recanalisation with carotid-T occlusion on angio with no MCA or ACA flow. e Partial flow restoration by Solitaire stent retriever thrombectomy. f Aspiration thrombectomy to improve MCA bifurcation flow. g ACA Catch-Mini stent retriever recanalisation. h TICI-2b final angio run.
The idea of using stents in the treatment of acute ischaemic stroke followed from the introduction of intracranial self-expanding stents for the treatment of intracranial atherosclerotic stenosis. The initial attempts made in the treatment of acute ischaemic stroke were by deployment of the stent across the thrombus at the occluded segment [2, 66, 72, 73]. One of the major conceivable complicating factors of this therapy was the need for concurrent antiplatelet-anticoagulation therapy and potential risk of haemorrhage [2, 74]. Despite the good technical success and significant improvement in the 90-day clinical outcome, the SARIS (Stent-Assisted Recanalization Acute Ischemic Stroke) study demonstrated significant subsequent in-stent stenosis requiring aggressive medical management with a resultant increased rate of haemorrhagic transformation [75, 76].
On the other hand, the introduction of detachable stents for stent-assisted coiling of cerebral aneurysms raised the possibility of using stents, which would retrieve a clot in mechanical intracranial thrombectomy, with the further advantage of rapid recanalisation without the above-mentioned risks and complications of a permanent stent. The first two devices to be approved for this purpose were Solitaire-FR™ from Covidien® and Trevo™ from Stryker®, which were followed by similar products including Trevo-ProVue™ (Stryker®), Revive™ (Johnson & Johnson®), EmboTrap™ (Neuravi®), Eric™ (MicroVention®), Catch™ and Catch-Mini™ (Balt®). Contrary to the suction thrombectomy or even stent insertion [77], these devices not only displace the thrombus to the periphery of the vessel and temporarily restore the flow, they also capture the thrombus by incorporating the clot through their interstices to make it possible to withdraw the trapped clot when the stent is removed [66, 78].
However, the initial promise demonstrated by the SWIFT (SOLITAIRE With the Intention For Thrombectomy) trial showing the superiority of the Solitaire stent over the MERCI retriever, with overall recanalisation rate of ∼90 % [79], further empowered by the phase II TREVO-2 (Thrombectomy Revascularisation of Large Vessel Occlusions in Acute Ischaemic Stroke) trial [80], suddenly diminished when three subsequent major RCTs failed to demonstrate any significant benefit in the final clinical outcome in endovascular intervention [66].
IMS III (Interventional Management of Stroke III) was a randomised control study, prematurely halted two thirds through its recruitment, when significant inferiority was noted in the endovascular intervention arm [81], which in retrospect may have been due to multiple inherent flaws in the study design, e.g. heterogeneity of the sample groups, and the interventional methods, and finally under-representation of the new stent retriever devices, which were only used in a small number of cases, despite their clear technical superiority [1, 7, 12, 64, 80, 82]. Despite the fact that SYNTHESIS (Synthesis Expansion: A Randomized Controlled Trial on Intra-arterial Versus Intravenous Thrombolysis in Acute Ischaemic Stroke), another unsuccessful study, benefited from a relatively better RCT design, it most certainly still suffered from multiple major flaws including the absence of a strict policy for vascular imaging to identify those with large arterial occlusion prior to intervention [64, 83]. Although the MR-RESCUE (Mechanical Retrieval and Recanalisation of Stroke Clots Using Embolectomy) trial tried to exploit advantages of advanced imaging techniques to stratify patients for the trial in multiple different arms, it still suffered from poor statistical power due to under-sampling in each group as well as a liberal intervention time window and poor overall technical success and recanalisation rates [3, 64, 84, 85].
The above-mentioned undoubtedly counterintuitive results certainly hindered activities regarding endovascular intervention in acute ischaemic stroke around the world until, in the second half of 2014, the Dutch MR-CLEAN (Multicenter Randomized CLinical trial of Endovascular treatment for Acute ischaemic stroke in the Netherlands) study demonstrated superiority in endovascular intervention, when the trial results were presented at the 9th World Stroke Congress (Table 1) [66].
MR-CLEAN was a randomised controlled trial which recruited 500 patients older than 18 years of age, with National Institutes of Health Stroke Scale (NIHSS) of at least 2 and anterior circulation large artery occlusion confirmed on CT angiography (CTA), presenting no later than 6 h from the onset of symptoms. All patients received full standard medical management, including IV-tPA where possible, and were randomised into an intra-arterial interventional group (233 patients) and control with ∼82 % of interventional patients treated by stent retrievers. The median NIHSS was 17 in the intervention and 18 in the control group, with the median Alberta Stroke Program Early CT Score (ASPECTS) of 9 for both, and 87.1 % and 90.6 % received IV-tPA in each group, respectively. Approximately 80 % of the patients who were intervened were completely recanalised in 24 h, with an odds ratio of 6.9 (95 % CI 4.3–10.9) compared to the control arm. This was associated with a significantly smaller infarct volume at 1 week in the interventional group compared with the control, 49 versus 80 ml, leading to a much better clinical outcome at 3 months (mRS less than 2, 33 versus 19 %) (Table 1) [86, 87••].
The positive and promising results from MR-CLEAN rejuvenated optimism regarding an endovascular treatment of stroke which was further confirmed by the EXTEND-IA (Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial) and ESCAPE (Endovascular Treatment for Small Core and Proximal Occlusion Ischaemic Stroke) results which were very notably positive. These two studies were halted by respective data safety and monitoring boards as equipoise was no longer in place after MR-CLEAN. The results of both were presented and published in February 2015.
EXTEND-IA was an Australian and New Zealand multicentre randomised controlled trial of intra-arterial reperfusion therapy after standard dose of IV-tPA versus IV-tPA alone. Patients were carefully selected cases with both large anterior circulation arterial occlusion and small core infarct using CT perfusion, analysed/processed by RAPID (Rapid Processing of Perfusion and Diffusion) [88], a technique that was developed at Stanford University with NINDS funding [89, 90]. Patients were included if they presented no later than 4.5 h after onset of symptoms, with the estimated infarct core less than 70 ml, or if there was a mismatch ratio greater than 1.2 between the estimated core and the under-perfused parenchyma. Patients were randomised into IV-tPA with subsequent Solitaire stent retrieval and IV-tPA alone [91••]. When the study was terminated, 70 patients had been enrolled with 35 in each arm. Median NIHSS was 17 and 13 in the interventional and control groups, respectively, with median age of 68.6 and 70.2 years. Recanalisation rate was more than 86 % in the interventional group with no significant difference in symptomatic intracranial haemorrhage. Significant improvement in the outcome was demonstrated in the interventional group with 31.4 % increase in those with mRS ≤2 and decreased mortality of 11.4 % compared to the control group (Table 1).
ESCAPE was a Canadian study with enrolment centres across Europe and North America, recruiting patients with NIHSS and ASPECTS of more than 5 with estimated moderate-to-good collaterals on multiphase CTA who did not present later than 12 h post onset of symptoms [92••]. At the time the study was terminated, 315 patients were enrolled with 165 and 150 allocated to the interventional and control arms, respectively, with median age and NIHSS of 71/70 and 16/17, respectively. The median ASPECTS was 9 in both arms with just below 80 % of the patients receiving IV-tPA. There was a significant increase in the overall favourable clinical outcome in those who underwent thrombectomy with a statistically significant decrease in mortality on a background recanalisation rate of approximately 70 % in the intervention arm (Table 1).
REVASCAT (Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours) [93] was a Spanish study which was again halted because of the broken equipoise. REVASCAT enrolled the first 160 patients aged between 18 and 80 who presented not later than 8 h post onset of symptoms with NIHSS equal to or more than 8 and ASPECTS of more than 6; however, the age criterion was subsequently modified to also include those aged between 81 and 85 if their ASPECTS was better than 9 [94]. In addition, a large anterior circulation artery occlusion was required to be demonstrated on CTA. Patients who received IV-tPA but failed to improve were included in the trial [94].
REVASCAT was terminated after 206 patients were randomised out of the initial target of 690 with 103 patients recruited in each arm. The average age of the subjects was 66.5 years with median ASPECTS of 7 and 8 for interventional and control groups, with 68 and 77 % of interventional and control arms receiving IV-tPA, respectively. The median NIHSS was 17 for both groups with all of the endovascular interventions performed using stent retrievers; however, the overall recanalisation rate was relatively lower (∼65 %), in particular, when compared to the other trials, but no increase in the complication rate compared to the control arm. Although there was a significant increase in the number of patients with good outcome with an adjusted odds ratio of 2.1 (95 % CI 1.1–4.0) and the number needed to treat to cause a favourable outcome of 6.4, there was a slight increase in total mortality in the interventional group compared with the control, which was not statistically significant. This was a well-organised trial with only a handful of the stroke patients presenting to the participating institutes not randomised. However, a post IV-tPA neurovascular imaging requirement as a part of the study protocol inevitably increased the delay to intervention and could together with increased times to enrol those who fail IV therapy be a contributing factor to the above-mentioned higher mortality [93].
SWIFT-PRIME (Solitaire™ FR as Primary Treatment for Acute Ischemic Stroke) trial also started with a design very similar to EXTEND-IA with a great emphasis on perfusion assessment of the infarcted core, which was again halted after demonstrating overwhelming efficacy for the endovascular group after the 196th patient was enrolled out of the total of 833 initially planned [95]. From the technical point of view, however, it is important to note that given the lack of standardisation of perfusion data, the current role of perfusion imaging is more to “rule-in” late presenters on the basis of penumbra rather than to “rule-out” early presenters, and attainment of perfusion data also should not significantly delay potential treatments [66].
Interestingly, the final study of this group of six, the French phase III THRACE (Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke), despite its relatively liberal approach to preprocedural imaging-based selection as well as endovascular techniques allowed into the study, was also terminated after positive intermediate analyses of the data from the first 395 enrolled patients. This may be significant as it may indicate overall endovascular superiority even in a less stringent patient selection routine, using a variety of techniques, although the final results are yet to be published [95, 96].
On the other hand, THRACE was the only trial in this wave of studies to include vertebrobasilar strokes. It is important to understand that although posterior circulation strokes only constitute less than 10 % of all large vessel ischaemic events, they appear to be a different entity all together with much poorer outcome with a few studies demonstrating significant mortality in the setting of unsuccessful recanalisation [2, 97, 98]. Prospective studies and in particular RCTs are hampered by relative low numbers and heterogeneity of acute vertebrobasilar occlusions. Nevertheless, BASICS (Basilar Artery International Cooperation Study), an observational registry of 592 patients, despite demonstrating a positive trend, failed to prove a statistically significant superiority of endovascular intervention over IV-tPA alone [99]. Further studies are emerging, claiming good recanalisation rates in vertebrobasilar occlusion with endovascular techniques, in particular using stent retrievers, with evidence increasing for good outcome and decrease in mortality, but at this stage, the rationale for aggressive treatment in vertebrobasilar occlusion is principally based on anecdotal evidence [100].
There are further ongoing thrombectomy trials (Table 2), e.g. the Brazilian study of RESILIENT, the transpacific trial of DAWN, the British and Norwegian trial of PISTE and the American trial of POSITIVE, as well as those yet to be concluded and published, e.g. Central European study of THRILL, which will provide further evidence on the management and appropriate intervention in acute ischaemic stroke [95].
Further meta-analysis of the pooled data from these studies will be performed in order to have enough statistical power, making detailed subgroup analyses possible. Such an approach will avoid the need for larger randomised controlled trials and will help provide advice on the generalisability and universality of this technique, as well as optimising patient selection and generating realistic outcome expectations. Some work has already been done on attempting to predict outcome [101•, 102], but these have not reached widespread applicability [95, 103].
In addition to all of the above-discussed medical and endovascular treatments for acute ischaemic stroke to re-establish parenchymal perfusion, there is a relatively new category of research focused on potential neuroprotective strategies to minimise a detrimental effect of ischemia, prolonging the window of opportunity to intervene [104]. This include newly emerging chemical agents with anti-oxidative or anti-excitotoxicity effect, as well as those protecting the blood-brain barrier (BBB) or preventing neuronal apoptosis and autophagy [104], to physical modifications including induced hypothermia [105].
In fact, some of these treatments, e.g. nitric oxide [106] and cannabinoids, have had promising results in early animal models and ad hoc clinical investigations whilst awaiting further evidence. Meanwhile, although NeuMAST (Neuroprotection With Minocycline Therapy for Acute Stroke Recovery Trial) was terminated due to futility of the interim results [107], there is an ongoing effort to establish a trial for a newly developed agent, PSD-95 inhibitor (NA-1), with encouraging results from laboratory tests [108•].
Conclusions
Allowing for the overwhelming results of the recent trials, the main question is no longer whether combined chemo-thrombolysis and endovascular intervention is superior, but to identify those who will benefit from this approach and transferring them urgently to the endovascular stroke centre, with the main goal being reperfusion of the brain in a rapid and safe fashion as possible.
Implementation of a comprehensive service will be a great challenge as the treatment for ischaemic stroke is expected to have a major effect on public health. It is interesting to consider that the number of patients needed to treat (NNT) by percutaneous coronary intervention (PCI) in the setting of acute coronary syndrome (ACS) to prevent one death is approximately 20, whilst this number for acute ischaemic stroke, not only to avoid death but also to be functioning independently after 90 days of treatment, is only ∼5 [109].
Now that the European Stroke Organisation has released an updated guideline recommending immediate availability of endovascular intervention to patients with AIS due to large vessel occlusion within 6 h of onset, the major logistical challenge is how to provide such a service in a uniform and equal manner, to avoid extra time wasted in the referral chain whilst, on the other hand, also avoiding potential deskilling and under-experience of the operators in centres with insufficient procedural volumes [109].
One of the tempting proposals to tackle this challenge is to have mobile clinical and imaging diagnostic units accompanying the ambulance services, not only to exclude a haemorrhagic stroke and commence IV-tPA on the spot [110] but also to diagnose a large vessel occlusion and transfer the patient directly to a centralised endovascular centre for thrombectomy [111–113], an ambitious but certainly attractive idea, which is yet to be tested in practice.
There are now a growing number of national and international organisations proposing guidelines for the endovascular management of stoke, including the consensus statement released by ESO, ESMINT and ESNR [114], the management guideline published by the American Stroke Association [115], or the Canadian best practice recommendations [116], and individual institutions around the world can adopt or modify these and establish their own protocols based on their particular need and availability.
Final recommendations
-
1.
Endovascular stroke intervention depends on the availability of skilled expertise in this technique; however, it should not preclude or delay initiation of the proven intravenous thrombolysis [2], if there are no contraindications.
-
2.
Each emergency and radiology department should be equipped with preassembled kits of ready-to-use IV-tPA if indicated immediately after haemorrhage has been excluded with noncontrast CT brain. CTA should be performed immediately following noncontrast CT brain.
-
3.
Endovascular intervention should only be performed in tertiary stroke centres of high volume with experienced multidisciplinary stroke teams and the required equipment and expertise available. The procedure should be conducted by interventional neuroradiologists with sufficient experience/training.
-
4.
The infrastructure for rapid assessment, diagnosis and stabilisation of patients by a multidisciplinary team is required, or alternative transportation to a comprehensive stroke centre should be considered, where the specialist care and technology is available. Such a model has been previously proposed under the comprehensive stroke systems of care, which also requires the implementation of a set of guidelines and the establishment of a system for quality control and assessment [2, 117].
-
5.
Stent retrievers should be the first-line treatment for endovascular treatment as they have shown the highest rates of recanalisation, although there is emerging evidence that fast and effective aspiration thrombectomy is also feasible and all available techniques should be utilised to achieve reperfusion as soon as possible.
-
6.
Although there is now undeniable evidence for the effectiveness of endovascular intervention, the need for appropriate selection of candidates with imaging guidelines, in particular to confirm the presence of a large vessel occlusion and if possible a salvageable penumbra, should not be forgotten.
-
7.
It is of paramount importance to minimise delays to treatment, by setting up a system of care consistent with existing regional structures to optimise the time of onset to the time of recanalisation, thus minimising brain tissue loss.
References and Recommended Reading
Papers of particular interest, published recently have been highlighted as: • Of importance •• Of major importance
Ferrell AS, Britz GW. Developments on the horizon in the treatment of neurovascular problems. Surg Neurol Int. 2013;4 Suppl 1:S31–7.
Blackham KA, Meyers PM, Abruzzo TA, et al. Endovascular therapy of acute ischemic stroke: report of the Standards of Practice Committee of the Society of NeuroInterventional Surgery. J Neuroint Surg. 2012;4(2):87–93.
Gonzalez RG, Copen WA, Schaefer PW, et al. The Massachusetts General Hospital acute stroke imaging algorithm: an experience and evidence based approach. J Neuroint Surg. 2013;5 Suppl 1:i7–12.
Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome—a meta-analysis. Stroke. 2007;38(967):973.
Meretoja A, Keshtkaran M, Saver JL, et al. Stroke thrombolysis: save a minute, save a day. Stroke J Cereb Circ. 2014;45(4):1053–8.
Rha J-H, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke J Cereb Circ. 2007;38(3):967–73.
Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke J Cereb Circ. 2007;38(3):948–54.
Eissa A, Krass I, Levi C, Sturm J, Ibrahim R, Bajorek B. Understanding the reasons behind the low utilisation of thrombolysis in stroke. Aust Med J. 2013;6(3):152.
Asadi H, Yan B, Dowling R, Wong S, Mitchell P. Advances in medical revascularisation treatments in acute ischemic stroke. Thrombosis. 2014; 2014
Barreto AD, Alexandrov AV. Adjunctive and alternative approaches to current reperfusion therapy. StrokeJ Cereb Circ. 2012;43(2):591–8.
del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32(1):78–86.
Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke J Cereb Circ. 2011;42(6):1775–7.
Molina CA, Montaner J, Arenillas JF, Ribo M, Rubiera M, Alvarez-Sabín J. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke J Cereb Circ. 2004;35(2):486–90.
Rubiera M, Alvarez-Sabín J, Ribo M, et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke J cereb Circ. 2005;36(7):1452–6.
Del Zoppo GJ, Saver JL, Jauch EC, Adams Jr HP. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke J Cereb Circ. 2009;40(8):2945–8.
Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):483S–512S.
Tissue plasminogen activator for acute ischemic stroke. New Engl J Med 1995; 333(24): 1581–7.
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29.
Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380(9843):731–7.
Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379(9834):2352–63.
Ahmed N, Kellert L, Lees KR, Mikulik R, Tatlisumak T, Toni D. Results of intravenous thrombolysis within 4.5 to 6 hours and updated results within 3 to 4.5 hours of onset of acute ischemic stroke recorded in the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR): an observational study. JAMA Neurol. 2013;70(7):837–44.
Alvarez-Fernandez JA. Ultrasound-enhanced systemic thrombolysis. An effective and underutilized treatment for acute ischemic stroke. Med intensiva Soc Esp Med Intensiva y Unidades Coronarias. 2011;35(2):134–5.
Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351(21):2170–8.
Siebler M, Hennerici MG, Schneider D, et al. Safety of Tirofiban in acute Ischemic Stroke: the SaTIS trial. Stroke J Cereb Circ. 2011;42(9):2388–92.
Cerevast. Phase 3, Randomized, Placebo-Controlled, Double-Blinded Trial of the Combined Lysis of Thrombus With Ultrasound and Systemic Tissue Plasminogen Activator (tPA) for Emergent Revascularization in Acute Ischemic Stroke (CLOTBUST-ER). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).2014 - [cited 13 December 2014]. Available from: http://clinicaltrials.gov/show/NCT01098981.
Molina CA, Barreto AD, Tsivgoulis G, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol. 2009;66(1):28–38.
Molina CA, Ribo M, Rubiera M, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke J Cereb Circ. 2006;37(2):425–9.
Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke J Cereb Circ. 2005;36(7):1432–8. One of the early mechanical thrombectomy trials.
Mikulik R, Dufek M, Goldemund D, Reif M. A pilot study on systemic thrombolysis followed by low molecular weight heparin in ischemic stroke. Eur J Neurol Off J Eur Fed Neurol Soc. 2006;13(10):1106–11.
Zinkstok SM, Vermeulen M, Stam J, de Haan RJ, Roos YB. A randomised controlled trial of antiplatelet therapy in combination with Rt-PA thrombolysis in ischemic stroke: rationale and design of the ARTIS-Trial. Trials. 2010;11:51.
del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke J Cereb Circ. 1998;29(1):4–11. One of the pioneer studies into intra-arterial thrombolysis in the setting of acute ischemic stroke.
Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA J Am Med Assoc. 1999;282(21):2003–11.
Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32(1):48–55.
Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke J Cereb Circ. 2005;36(10):2311–20.
Haley Jr EC, Thompson JL, Grotta JC, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke J Cereb Circ. 2010;41(4):707–11.
Haley Jr EC, Lyden PD, Johnston KC, Hemmen TM. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke J Cereb Circ. 2005;36(3):607–12.
Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366(12):1099–107.
Logallo N, Kvistad CE, Nacu A, et al. The Norwegian tenecteplase stroke trial (NOR-TEST): randomised controlled trial of tenecteplase vs. alteplase in acute ischaemic stroke. BMC neurology. 2014;14(1):106.
Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke J Cereb Circ. 2005;36(1):66–73.
Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke J Cereb Circ. 2006;37(5):1227–31.
Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8(2):141–50.
Kummer R, Albers GW, Mori E. The desmoteplase in acute ischemic stroke (DIAS) clinical trial program. Int J Stroke. 2012;7(7):589–96.
Sherman DG, Atkinson RP, Chippendale T, et al. Intravenous ancrod for treatment of acute ischemic stroke: the STAT study: a randomized controlled trial. Stroke Treatment with Ancrod Trial. JAMA J Am Med Assoc. 2000;283(18):2395–403.
Levy DE, del Zoppo GJ, Demaerschalk BM, et al. Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke J Cereb Circ. 2009;40(12):3796–803.
Hennerici MG, Kay R, Bogousslavsky J, Lenzi GL, Verstraete M, Orgogozo JM. Intravenous ancrod for acute ischaemic stroke in the European Stroke Treatment with Ancrod Trial: a randomised controlled trial. Lancet. 2006;368(9550):1871–8.
Jang IK, Brown DF, Giugliano RP, et al. A multicenter, randomized study of argatroban versus heparin as adjunct to tissue plasminogen activator (TPA) in acute myocardial infarction: myocardial infarction with novastan and TPA (MINT) study. J Am Coll Cardiol. 1999;33(7):1879–85.
Kawai H, Umemura K, Nakashima M. Effect of argatroban on microthrombi formation and brain damage in the rat middle cerebral artery thrombosis model. Jpn J Pharmacol. 1995;69(2):143–8.
Morris DC, Zhang L, Zhang ZG, et al. Extension of the therapeutic window for recombinant tissue plasminogen activator with argatroban in a rat model of embolic stroke. Stroke J Cereb Circ. 2001;32(11):2635–40.
LaMonte MP, Nash ML, Wang DZ, et al. Argatroban anticoagulation in patients with acute ischemic stroke (ARGIS-1): a randomized, placebo-controlled safety study. Stroke J Cereb Circ. 2004;35(7):1677–82.
Sugg RM, Pary JK, Uchino K, et al. Argatroban tPA stroke study: study design and results in the first treated cohort. Arch Neurol. 2006;63(8):1057–62.
Barreto AD, Alexandrov AV, Lyden P, et al. The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke J Cereb Circ. 2012;43(3):770–5.
Barreto AD. Randomized Controlled Trial of Argatroban With tPA for Acute Stroke (ARTSS-2). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2011 - [cited 13 December 2014]. Available from: https://clinicaltrials.gov/show/NCT01464788.
Javedani PP, Horowitz BZ, Clark WM, Lutsep HL. Dabigatran etexilate: management in acute ischemic stroke. Am J Crit Care. 2013;22(2):169–76.
Lee JY, Markland FS, Lucchesi BR. Hirudin and S18886 maintain luminal patency after thrombolysis with alfimeprase. J Cardiovasc Pharmacol. 2013;61(2):152–9.
Boudreau DM, Guzauskas GF, Chen E, et al. Cost-effectiveness of recombinant tissue-type plasminogen activator within 3 hours of acute ischemic stroke current evidence. Stroke. 2014;45(10):3032–9.
Eisenberg PR, Sobel BE, Jaffe AS. Activation of prothrombin accompanying thrombolysis with recombinant tissue-type plasminogen activator. J Am Coll Cardiol. 1992;19(5):1065–9.
Pereira H. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Revista portuguesa de cardiologia : orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese J Cardiol Off J Portuguese Soc Cardiol 2001;20(6):687–8.
Ohman EM, Kleiman NS, Gacioch G, et al. Combined accelerated tissue-plasminogen activator and platelet glycoprotein IIb/IIIa integrin receptor blockade with Integrilin in acute myocardial infarction. Results of a randomized, placebo-controlled, dose-ranging trial. IMPACT-AMI Investigators. Circulation. 1997;95(4):846–54.
Adams Jr HP, Effron MB, Torner J, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke J Cereb Circ. 2008;39(1):87–99.
Torgano G, Zecca B, Monzani V, et al. Effect of intravenous tirofiban and aspirin in reducing short-term and long-term neurologic deficit in patients with ischemic stroke: a double-blind randomized trial. Cerebrovasc Dis. 2010;29(3):275–81.
Pancioli AM, Adeoye O, Schmit PA, et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue plasminogen activator in acute ischemic stroke-enhanced regimen stroke trial. Stroke J Cereb Circ. 2013;44(9):2381–7.
Ciccone A, Motto C, Abraha I, Cozzolino F, Santilli I. Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke. Cochrane Database Syst Rev 2014;3.
Sandercock PA, Counsell C, Gubitz GJ, Tseng MC. Antiplatelet therapy for acute ischaemic stroke. The Cochrane Library 2008.
Mokin M, Khalessi AA, Mocco J, et al. Endovascular treatment of acute ischemic stroke: the end or just the beginning? Neurosurg Focus. 2014;36(1):E5.
The Interventional Management of Stroke (IMS) II Study. Stroke J Cereb Circ 2007; 38(7): 2127–35.
Asadi H, Dowling R, Yan B, Wong S, Mitchell P. Advances in endovascular treatment of acute ischemic stroke. Int Med J. 2014.
Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke J Cereb circ. 2008;39(4):1205–12.
The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke J Cereb Circ 2009; 40(8): 2761–8.
Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241–9.
Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and multi MERCI trials. Stroke J Cereb Circ. 2009;40(12):3777–83.
Investigators PPST. The penumbra pivotal stroke trial safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40(8):2761–8.
Levy EI, Mehta R, Gupta R, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007;28(5):816–22.
Nogueira RG, Schwamm LH, Buonanno FS, et al. Low-pressure balloon angioplasty with adjuvant pharmacological therapy in patients with acute ischemic stroke caused by intracranial arterial occlusions. Neuroradiology. 2008;50(4):331–40.
Fiorella DJ, Levy EI, Turk AS, et al. Target lesion revascularization after wingspan: assessment of safety and durability. Stroke J Cereb Circ. 2009;40(1):106–10.
Levy EI, Rahman M, Khalessi AA, et al. Midterm clinical and angiographic follow-up for the first Food and Drug Administration-approved prospective, single-arm trial of primary stenting for stroke: SARIS (Stent-Assisted Recanalization for Acute Ischemic Stroke). Neurosurgery. 2011;69(4):915–20. discussion 20.
Levy EI, Siddiqui AH, Crumlish A, et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (stent-assisted recanalization in acute ischemic stroke). Stroke J Cereb Circ. 2009;40(11):3552–6.
Fitzsimmons B-F, Becske T, Nelson P. Rapid stent-supported revascularization in acute ischemic stroke. Am J Neuroradiol. 2006;27(5):1132–4.
Fulkerson J, Ferrera DA, Cragg A. Acute stroke revascularization/recanalization systems processes and products thereby. Google Patents; 2009
Saver JL, Jahan R, Levy EI, et al. SOLITAIRE with the intention for thrombectomy (SWIFT) trial: design of a randomized, controlled, multicenter study comparing the SOLITAIRE Flow Restoration device and the MERCI Retriever in acute ischaemic stroke. Int J stroke Off J Int Stroke Soc. 2012.
Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380(9849):1231–40.
Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903.
Demchuk A, Investigators II. IMS III: comparison of outcomes between IV and IV/IA treatment in baseline CTA confirmed ICA, M1, M2 and basilar occlusions. Int Stroke Conference; 2013; 2013
Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(10):904–13.
Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368(10):914–23.
Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke J Cereb Circ. 2009;40(6):2046–54.
Keller DM. ‘Mr Clean’ Polishes Stroke Outcome With Endovascular Therapy. Medscape Medical News: 9th World Stroke Congress (WSC);2014.
Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl J Med. 2015;372(1):11–20. This is was the first randomised control trial into the efficacy of endovascular treatment for acute ischemic stroke, performed by a Dutch group as a large well-designed multicentric study, with the preliminary result announced during the international conference of stroke, breaking the equipoise and forcing several other trials to be on hold.
National Stroke Research Institute A. Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial (EXTEND-IA).
Campbell BCV, Yassi N, Ma H, et al. Imaging selection in ischemic stroke: feasibility of automated CT-perfusion analysis. Int J Stroke. 2015;10(1):51–4.
Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32(5):1024–37.
Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl J Med. 2015;372(11):1009––18. This is a randomised control trial into comparing endovascular thrombectomy combined with IV-tPA with IV-tPA alone with emphasis on patient selection based on CT perfusion and advanced penumbral imaging.
Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl J Med. 2015;372(11):1019–30. This is a randomised control trial into efficacy of endovascular thrombectomy compared with IV-tPA in patients presented not later than 12 h post onset with emphasis on ASPECT score at the time of presentation and the situations of the collaterals based on a three-phase CTA.
Molina CA, Chamorro A, Rovira À, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR® device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight‐hours of symptom onset. Int J Stroke 2013.
Smith WS, Yan B. REVASCAT trial: further advancement in endovascular stroke therapy. Stroke. 2015;46(10):3012–3.
Khatri P, Hacke W, Fiehler J, et al. State of acute endovascular therapy report from the 12th thrombolysis, thrombectomy, and acute stroke therapy conference. Stroke. 2015;46(6):1727–34.
Bracard S, Guillemin F, Ducrocq X. THRACE study: intermediate analysis results. Int J Stroke; 2015: Wiley-Blackwell, 111 River St, Hoboken 07030-5774, NJ, USA; 2015. p. 31
Baek J, Yoon W, Kim S, et al. Acute basilar artery occlusion: outcome of mechanical thrombectomy with solitaire stent within 8 hours of stroke onset. Am J Neuroradiol. 2014;35(5):989–93.
Levy EI, Firlik AD, Wisniewski S, et al. Factors affecting survival rates for acute vertebrobasilar artery occlusions treated with intra-arterial thrombolytic therapy: a meta-analytical approach. Neurosurgery. 1999;45(3):539–45. discussion 45–8.
Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. 2009;8(8):724–30.
Broussalis E, Hitzl W, McCoy M, Trinka E, Killer M. Comparison of endovascular treatment versus conservative medical treatment in patients with acute basilar artery occlusion. Vasc Endovasc Surg. 2013;1538574413488458.
Asadi H, Dowling R, Yan B, Mitchell P. Machine learning for outcome prediction of acute ischemic stroke post intra-arterial therapy. PLoS One. 2014;2:e88225. The first study published on the role of machine learning and artificial intelligence in prognostication and predication of the patients’ outcome in acute ischemic stroke.
Grech R, Galvin PL, Power S, et al. Outcome prediction in acute stroke patients considered for endovascular treatment: a novel tool. Interv Neuroradiol. 2014;20(3):312–24.
MacIsaac RL, Khatri P, Bendszus M, et al. A collaborative sequential meta‐analysis of individual patient data from randomized trials of endovascular therapy and tPA vs. tPA alone for acute ischemic stroke: ThRombEctomy And tPA (TREAT) analysis: statistical analysis plan for a sequential meta‐analysis performed within the VISTA‐Endovascular collaboration. Int J Stroke. 2015;10(A100):136–44.
Majid A. Neuroprotection in stroke: past, present, and future. ISRN Neurol. 2014;2014.
Choi K-E, Hall CL, Sun J-M, et al. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J. 2012;26(7):2799–810.
Li Y-S, Shemmer B, Stone E, Nardi MA, Jonas S, Quartermain D. Neuroprotection by inhaled nitric oxide in a murine stroke model is concentration and duration dependent. Brain Res. 2013;1507:134–45.
Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke. 2012;7(5):407–18.
Tymianski M. Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke. 2013;44(10):2942–50. Important breakthrough in the role of neuroprotective agents in acute ischemic stroke in animal models.
Campbell BC, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. 2015;14(8):846–54.
Walter S, Kostpopoulos P, Haass A, et al. Bringing the hospital to the patient: first treatment of stroke patients at the emergency site. PLoS ONE. 2010;5(10):e13758.
Perez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45(1):87–91.
Ebinger M, Fiebach JB, Audebert HJ. Mobile computed tomography: prehospital diagnosis and treatment of stroke. Curr Opin Neurol. 2015;28(1):4–9.
Cerejo R, John S, Buletko AB, et al. A mobile stroke treatment unit for field triage of patients for intraarterial revascularization therapy. J Neuroimaging. 2015;25(6):940–5.
Wahlgren N, Moreira T, Michel P, et al. Mechanical thrombectomy in acute ischemic stroke: consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT. ESNR and EAN Int J Stroke. 2016;11(1):134–47.
Powers WJ, Derdeyn CP, Biller J, et al. American Heart Association Stroke Council: 2015 AHA/ASA focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015.
Coutts SB, Wein TH, Lindsay MP, et al. Canadian Stroke Best Practice Recommendations: secondary prevention of stroke guidelines, update 2014. Int J Stroke. 2015;10(3):282–91.
Schwamm LH, Pancioli A, Acker 3rd JE, et al. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association’s Task Force on the Development of Stroke Systems. Circulation. 2005;111(8):1078–91.
Ding D. Endovascular mechanical thrombectomy for acute ischemic stroke: a new standard of care. J Stroke. 2015;17:123–6.
Acknowledgments
A special thank is due to Dr. Myrna Rosenfeld for taking the time to review this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Hamed Asadi declares no conflict of interest.
David Williams has received advisory board fees from Boehringer Ingelheim, Daiichi Sankyo, Bayer and Bristol-Myers Squibb, along with payment for manuscript preparation from Boehringer Ingelheim. Dr. Williams is also a local co-investigator of ARISE (Analysis of Revascularisation in Ischemic Stroke with EmboTrap®) sponsored by Neuravi Ltd.
John Thornton has received advisory board fees from Neuravi, Galway, Ireland.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cerebrovascular Disorders
Rights and permissions
About this article
Cite this article
Asadi, H., Williams, D. & Thornton, J. Changing Management of Acute Ischaemic Stroke: the New Treatments and Emerging Role of Endovascular Therapy. Curr Treat Options Neurol 18, 20 (2016). https://doi.org/10.1007/s11940-016-0403-8
Published:
DOI: https://doi.org/10.1007/s11940-016-0403-8