Opinion statement
Liver transplantation is the definitive therapy for cirrhosis, and malnutrition is the most frequent complication in these patients. Sarcopenia or loss of muscle mass is the major component of malnutrition in cirrhotics and adversely affects their outcome. In addition to the metabolic consequences, functional consequences of sarcopenia include reduced muscle strength and deconditioning. Despite nearly universal occurrence of sarcopenia and its attendant complications, there are no established therapies to prevent or reverse the same. Major reasons for this deficiency include the lack of established standardized definitions or measures to quantify muscle mass, as well as paucity of mechanistic studies or identified molecular targets to develop specific therapeutic interventions. Anthropometric evaluation, bioelectrical impedance analysis, and DEXA scans are relatively imprecise measures of muscle mass, and recent data on imaging measures to determine muscle mass accurately are likely to allow well-defined outcome responses to treatments. Resurgence of interest in the mechanisms of muscle loss in liver disease has been directly related to the rapid advances in the field of muscle biology. Metabolic tracer studies on whole body kinetics have been complemented by direct studies on the skeletal muscle of cirrhotics. Hypermetabolism and anabolic resistance contribute to sarcopenia. Reduced protein synthesis and increased autophagy have been reported in cirrhotic skeletal muscle, while the contribution of the ubiquitin-proteasome pathway is controversial. Increased plasma concentration and skeletal muscle expression of myostatin, a TGFβ superfamily member that causes reduction in muscle mass, have been reported in cirrhosis. Hyperammonemia and TNFα have been reported to increase myostatin expression and may be responsible for sarcopenia in cirrhosis. Nutriceutical interventions with leucine enriched amino acid mixtures, myostatin antagonists and physical activity hold promise as measures to reverse sarcopenia. There is even less data on muscle function and deconditioning in cirrhosis and studies in this area are urgently needed. Even though macronutrient replacement is a major therapeutic goal, micronutrient supplementation, specifically vitamin D, is expected to improve outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malnutrition is the most frequent complication in cirrhosis, affecting nearly 70 % of patients; it adversely affects survival, quality of life and the risk of developing other complications of cirrhosis [1•, 2, 3••, 4, 5]. These adverse consequences affect patients with cirrhosis during every stage of their life: those who have not been transplanted or waiting for liver transplantation, during the transplantation, and after liver transplantation [1•, 6•, 7, 8]. Despite widespread clinical recognition of the magnitude and clinical consequences of malnutrition, there are no effective therapeutic options [9•, 10]. The absence of effective treatments is principally due to the lack of mechanistic studies to help develop pathophysiology-based therapy. It is only recently that the term malnutrition in cirrhosis has been identified to consist of sarcopenia or loss of skeletal muscle mass and altered energy metabolism [2, 9•]. Most studies to date have focused on the consequences of sarcopenia, which is the major component of malnutrition in cirrhosis, and ongoing research has focused on improving our understanding of the mechanisms and reversal of sarcopenia of cirrhosis [1•, 3••, 7, 11, 12, 13•]. The clinical significance of sarcopenia of cirrhosis is due to the high prevalence in cirrhosis, and progressive worsening with increasing severity of liver disease combined with its adverse impact on outcomes [14]. Additionally, sarcopenia casts a long shadow by worsening the clinical outcome of cirrhotics before transplantation but unlike other complications of liver disease, sarcopenia worsens after transplantation [15•, 16, 17]. Contributions of micronutrients and vitamin deficiency are also of clinical significance in cirrhosis. Vitamin D deficiency and zinc deficiency are being increasingly recognized to contribute to worse clinical outcomes in these patients [18].
Clinical presentation
Patient symptoms include reduction in muscle mass and strength as well as reduced exercise capacity [12, 19]. Progression of cirrhosis is accompanied by worsening muscle mass and strength [20•]. Additionally, alcoholic cirrhosis and patients with cholestatic diseases have the most severe degree of muscle loss. A number of clinical factors are believed to contribute to the reduced nutritional status and muscle mass (Table 1). The major concern that patients express is the lack of clear guidelines for dietary modifications or exercise or communication with the hepatologist or transplant team to improve their nutritional status. Most patients define their nutritional status by the changes in muscle mass or strength. Lack of universal guidelines on nutritional interventions and clear outcome measures to monitor response to interventions, continued concern about protein intake and encephalopathy, and absence of guidelines regarding exercise with impaired exercise capacity contribute to reduced patient satisfaction with office visits [9•, 21, 22••].
Diagnosis of sarcopenia and functional impairment in cirrhosis
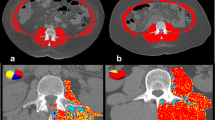
Establishing the diagnosis of sarcopenia has been challenging, but most previous studies have used relatively imprecise or indirect measures of muscle mass, including subjective global assessment (SGA), anthropometric measures, bioelectrical impedance analysis (BIA) and dual energy X-ray absorptiometry (DEXA) [7, 23, 24••]. Since skeletal muscle constitutes only about 60 % of lean or fat free mass, there is increasing interest in the use of computed tomogram at L3 or L4 to directly quantify muscle and visceral fat mass [25, 26]. Recently, two groups have reported normal values for skeletal muscle in control subjects [15•, 26]. The consensus seems to be that values below the 5th percentile of normal indicate severe sarcopenia and values between the 5th and 20th percentile are clinically significant but moderate sarcopenia. Using such direct measures of muscle mass, sarcopenia has been shown to directly correlate with survival in cirrhosis [13•]. Other imaging methods including ultrasound and magnetic resonance imaging (MRI) have also been used [24••]. However, most cirrhotics undergoing liver transplant evaluation have a computed tomography (CT) scan as part of their evaluation. Use of abdominal imaging is likely to become standard to diagnose sarcopenia. A limitation of this evaluation remains when the response to interventions needs to be determined. Protocol CT scans of the abdomen exclusively for quantifying muscle mass may be logistically difficult and cost prohibitive. Other options include the use of ultrasound of the thigh and single sections of mid thigh to quantify muscle and fat. Since muscle loss is a major component of the SGA scale and previous studies have suggested SGA to be a reliable measure of nutritional assessment [27, 28], given the cost benefit, it appears that SGA will be the assessment measure of choice, with modifications focusing on the muscle loss. However, given the potential for intra-observer, and to a greater extent, inter-observer variability, more objective measures like imaging quantification of muscle loss are likely to be the most acceptable method to define sarcopenia until less expensive measures have been validated across different populations.
The major contributors to deconditioning and functional impairment in cirrhotics are the reduced muscle mass and contractile strength. Consistently, a number of abnormalities have been reported in cirrhosis, including decreased gluconeogenesis, increased lactate production during exercise, and lower VO2 max compared to controls [22••, 29]. These are accompanied by reduced exercise capacity. Reduced VO2 max in cirrhosis has been correlated with reduced survival [30–32].
Clinical presentation and consequences of sarcopenia in cirrhosis
It is now universally recognized that survival is significantly shorter in cirrhotics with than in those without sarcopenia. Quality of life is impaired in nearly all cirrhotics and sarcopenia contributes significantly to reduced quality of life [2]. Complications of cirrhosis including infection, ascites, portal hypertension and hepatic encephalopathy are significantly greater in patients with than in those without muscle loss [1•, 3••, 7, 11]. After liver transplantation, muscle strength improves, but muscle mass continues to deteriorate and contributes to the post-transplant sarcopenia with obesity characterized by reduced muscle mass, increased fat mass, and the development of metabolic syndrome or its components [15•, 16, 33].
Mechanisms of sarcopenia in liver disease
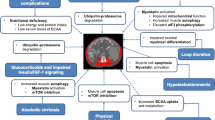
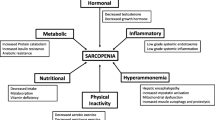
A number of potential clinical effects of cirrhosis have been reported to contribute to poor nutrient intake and increased energy expenditure contributing to malnutrition, specifically sarcopenia [34, 35]. However, other direct metabolic and molecular studies have contributed significantly to our understanding of mechanisms of muscle loss and more importantly, the reasons for the lack of effective interventions [36–42]. The major functional component of the skeletal muscle is protein with nearly 60 % of whole body protein located in the muscle. Thus, skeletal muscle mass is maintained by a balance between protein synthesis and breakdown. Metabolic tracer studies in a heterogeneous population of cirrhotics have reported conflicting results with increased, decreased or unaltered protein breakdown and decreased or unaltered protein synthesis [43, 44, 45•]. It is critical to reiterate that a reduction in protein synthesis alone is not adequate to explain a loss of muscle mass that is a progressive disorder in cirrhosis. Potential reasons for these conflicting data on protein synthesis and breakdown tracer studies are that whole body rather than muscle specific kinetics have been quantified, differences in tracers and methodology used, and heterogeneity in patient population in terms of severity, duration and etiology of cirrhosis. Overall, the most convincing data supports reduced whole body protein synthesis and conflicting data on protein breakdown in patients with cirrhosis. Cirrhosis is also believed to be a state of accelerated starvation based on whole body studies on substrate utilization [17, 29]. Early postprandial lowering of the respiratory quotient indicates a more rapid shift to fat as a primary fuel for energy [46]. This has been reported to be associated with reduced muscle mass, suggesting that the state of early starvation physiology contributes to reduced protein synthesis, as well as increased autophagy to provide essential nutrients to the surviving muscle cells.
More recently, the focus has shifted from whole body metabolic studies to detailed molecular characterization of skeletal muscle responses. Gene signatures for protein breakdown and synthesis have been identified [47]. The two major skeletal muscle proteolytic pathways include the ubiquitin-proteasome and the autophagy systems. It has been generally believed that the ubiquitin proteasome components are activated and contribute to the muscle loss in most chronic diseases like renal failure. However, direct studies in muscle biopsies from patients with cirrhosis suggest lower expression and activity of the proteasome components while skeletal muscle autophagy is increased [37, 38]. The portacaval anastamosis (PCA) rat has been shown to reproduce the metabolic, biochemical, hormonal and nutritional consequences of cirrhosis [39, 48, 49]. The major conclusions from these mechanistic studies were that the predominant mechanism of muscle loss depends on the time course of the disease, with increased ubiquitin-proteasome mediated proteolysis being a major mechanism of muscle loss early in the course of the disease. Once the disease is more advanced, then proteasome-mediated proteolysis is reduced, while autophagy and reduced protein synthesis become the dominant mechanisms responsible for the persistent and worsening sarcopenia. Studies on the molecular mechanisms of sarcopenia showed increased muscle expression of myostatin, a TGFβ superfamily member, in cirrhotics that results in reduced muscle mass [37]. Even though there is limited evidence that proteasome gene expression may be increased, there is convincing data that proteasome activity is not altered in the skeletal muscle of cirrhotics.
A number of mediators of the liver-skeletal muscle axis have been considered (Table 2), but hyperammonemia is a consistent abnormality in patients with cirrhosis [50, 51]. The skeletal muscle is believed to be a metabolic partner to the liver because increased muscle uptake of ammonia has been reported in cirrhosis [52•, 53]. The skeletal muscle responds to the increased ammonia uptake beyond being a metabolic sink, with alteration in molecular and biochemical responses [37]. Ammonia has been shown to induce autophagy by activating the early components, and may function via activation of AMPK in the muscle [38]. Additionally, hyperammonemia has been shown to transcriptionally upregulate myostatin by NFKB activation via the classical pathway. Other potential mechanisms of muscle loss in cirrhosis include the hypermetabolism and impaired energy rich phosphogens in the skeletal muscle that result in decreased energy for protein synthesis, and potentially accelerate autophagy to provide essential nutrients necessary for cellular function [54]. Reduced substrate availability, impaired protein synthesis, disordered energetics in the muscle, and potentially mitochondrial dysfunction contribute to the reduced muscle contractile strength and deconditioning of sarcopenia of cirrhosis.
Therapy of sarcopenia and functional impairment in cirrhosis
A number of interventions have been used to improve the “nutritional status” of cirrhosis (Table 3). A critical analysis of the data, however, shows that most studies to date focus on increased dietary intake, late evening snack and increased protein intake, and have been suggested to have limited improvement in nutritional outcomes in cirrhosis [10, 55••, 56]. As discussed above, these interventions have a very modest to no impact on improving the nutritional outcome of cirrhosis, because multiple molecular abnormalities contribute to a state of anabolic resistance. Reduced muscle protein synthesis despite nutrient ingestion and increased protein intake is termed anabolic resistance [57]. The diagnosis of anabolic resistance requires demonstration of reduced muscle protein synthesis, but has never been studied in cirrhosis. However, indirect measures in humans fed branched chain amino acid mixtures [9•] and preliminary studies by our group showed that the cirrhotics respond to leucine administration by increasing muscle protein synthesis [58].
Nutritional supplementation
Increase in caloric and protein intake
Lower whole body respiratory quotient in cirrhosis and activation of skeletal muscle AMPK by hyperammonemia suggest that cirrhosis is a state of accelerated starvation and increased nutritional intake will potentially reverse this process. Increased gluconeogenesis and decreased hepatic glycogen stores contribute to increased resting energy expenditure [59, 60]. Given the number of factors that contribute to reduced caloric and protein intake in cirrhosis, progressive sarcopenia ensues and worsens outcome. Estimated energy requirements are 35–40 kcal/kg body weight [55••, 61]. There is now widespread recognition that protein restriction is not necessary and may actually contribute to worse outcomes [55••]. The current European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend 1.2–1.5 g protein/kg/day in cirrhosis. However, there is controversy regarding the weight to be used for these calculations, and use of ideal body weight seems most reasonable even though there is no data to support this view. The pattern of dietary intake and the source of nitrogen have been shown to alter the response to nutritional interventions. In cirrhosis, the post-absorptive periods are characterized by increased protein breakdown and decreased protein synthesis, and the feeding pattern can be modified by altering the pattern of feeding (Fig. 1). Of the interventions, a late evening snack has been evaluated extensively and improves nutritional parameters, improved quality of life, nitrogen retention, and reverses the rapid switch to gluconeogenesis and reduction in respiratory quotient (RQ) [46]. Long-term benefits are confounded by low compliance in the outpatient setting, relatively imprecise measures of muscle mass in the studies performed, and the lack of consensus on the optimum snack. Based on the physiological studies that show increased gluconeogenesis and reduction in RQ in the post-absorptive phase, use of a high protein snack at night and breakfast are likely to provide the carbon skeleton for gluconeogenesis and can potentially prevent continued sarcopenia.
The importance and tolerance of the source of nitrogen is more contentious. Vegetable proteins that are rich in branched chain amino acids are believed to be better tolerated in terms of risk of hepatic encephalopathy, while animal proteins rich in aromatic amino acids are believed to confer a higher risk of encephalopathy [62, 63]. However, there are no high quality clinical studies to support the long-term preferential use of vegetable proteins [63]. Legumes, including soybeans, chickpeas and others of this class, have high protein content, but have a relative deficiency of methionine. Cereals including rice and wheat have low lysine content and have a relatively low total protein content. Combining cereal with legumes may thus have the advantage of providing a nearly ideal amino acid combination. Our recommendation has been to provide nearly one-third of the protein intake as animal protein, one-third from dairy products including egg white, and one-third from vegetable sources. Even though there is no definitive evidence to support this view based on the amino acid composition of different foods, this appears to be a reasonable approach. One of the major goals is reversal of sarcopenia, and there is a need for rapid assessment of the efficacy of interventions is critical. Based on our studies in cirrhotics in whom low RQ is associated with reduced muscle mass, it could be argued that the goal of interventions is to increase the RQ so that muscle protein synthesis is increased and breakdown is reduced [29].
Amino acid supplementation
Studies in animal models and in patients with cirrhosis have suggested a consistently low plasma branched chain amino acid (BCAA) and an elevated aromatic amino acid (AAA) concentration [64]. The mechanism of this pattern is related to the increased muscle utilization of the branched chain amino acids as a source of energy derived from muscle protein catabolism. Accelerated muscle protein breakdown contributes to the elevated plasma AAA concentration, since AAA can only be degraded in the liver. Hepatocellular dysfunction and portosystemic shunting in cirrhosis result in reduced AAA disposal, and consequent increased plasma concentrations. In contrast to the consistent data in the plasma, tissue concentrations of BCAA are not consistently reduced, and may be related to differential utilization of amino acids in the muscle. A number of studies in the past have used BCAA supplementation to reverse hepatic encephalopathy, but the few studies that examined nitrogen balance or retention as outcomes did not observe any benefit [9•]. Long-term BCAA supplementation has been shown to improve indices of malnutrition in cirrhosis [9•], but the major concern is that skeletal muscle mass or function were not evaluated as specific outcome measures. There is increasing interest in the use of leucine as a nutriceutical to reverse the anabolic resistance in cirrhosis based on data on sarcopenia of aging [65], even though the mechanisms of sarcopenia may be different in these clinical conditions.
Despite the heterogeneity of the data on nutritional supplementation and outcome measures in cirrhosis, there are no long-term high quality studies that demonstrate increased survival or reversal of sarcopenia with long-term nutritional interventions.
Oral vs. enteral feeding
Since cirrhosis is metabolically believed to be a state of accelerated starvation, one would anticipate that increased caloric intake would reverse or prevent progression of sarcopenia. However, despite extensive studies spanning decades, there is only very limited evidence that increased feeding via the oral or enteral route can potentially improve nutritional outcome [10, 56]. This can be explained by the molecular changes in the skeletal muscle that induce a state of anabolic resistance. Overcoming anabolic resistance has been a major focus in reversing and preventing sarcopenia of aging [57].
Micronutrient replacement
A number of micronutrient and vitamin deficiencies with consistently low plasma zinc and hypovitaminosis D have been reported in cirrhosis [18, 66, 67]. Evidence favoring zinc supplementation in improving metabolic function and outcomes in cirrhosis is compelling, but replacement to correct the deficiency is a reasonable approach. The data on vitamin D supplementation in improving outcomes is also not strong, but the data relating poor outcomes in cirrhosis to hypovitaminosis D is compelling. Hence, replacing vitamin D in cirrhotics should be standard of care in cirrhotic patients.
Physical activity
Increased physical activity specifically increases skeletal muscle mass and strength, in addition to reversing insulin resistance. However, there are no guidelines or recommendations for exercise in cirrhosis. Cirrhotics have decreased exercise capacity and ventilatory dysfunction that limits their physical activity [19, 22••, 31]. Even though physiological studies suggest that a combination of exercise and nutritional intake increases muscle mass, there are no data in patients with cirrhosis. One potential mechanism for an increase in muscle mass in response to exercise is an increased phosphatidic acid in the muscle that directly stimulates mTOR, a critical mediator of enhanced protein synthesis and suppressor of muscle autophagy [68, 69]. The response of skeletal muscle mass and function to exercise in cirrhotic patients is as yet not known.
Hormonal abnormalities and nutrition
Cirrhosis is characterized by reduced testosterone and androgenic stimulation due to elevated peripheral aromatase activity [41]. Testosterone supplements are therefore not likely to be effective in reversing sarcopenia, despite compelling evidence that androgens are potent stimuli to induce muscle growth and hypertrophy [70, 71]. Extrahepatic adverse effects, including those on the cardiovascular system, also limit interest in this therapy. Aromatase resistant androgens may have a therapeutic role, but this needs careful assessment, given the pleiotropic side effects of oxandrolone and androgens.
Molecular therapies
With the recognition that increased myostatin, mTOR suppressants and calcineurin inhibitors contribute to impaired protein synthesis, there is increasing interest in the use of specific antagonists. Myostatin antagonists, including monoclonal antibodies, antagomirs and small molecules, hold potential [72]. Given the pleiotropic metabolic effects of myostatin beyond muscle protein synthesis, including its effects on glucose metabolism, myostatin antagonists hold promise as future therapies for sarcopenia of liver disease [39]. Development of immune specific mTOR inhibitors or mTOR protection against the immunosuppressants is anticipated to reduce the incidence of post-OLT sarcopenia. Evidence that calcineurin inhibits myostatin and that calcineurin inhibitors can potentially increase the expression of myostatin and contribute to the development of post-orthotopic liver transplantation (OLT) metabolic syndrome are exciting opportunities in the field of sarcopenia in cirrhosis [73, 74].
It is critical to focus on mechanistic and target-based therapeutic studies to complement the enormous number of descriptive studies in the field of nutritional dysfunction in cirrhosis. A major advance in the field of nutritional support in cirrhosis has been the recognition that nutrition is a relatively broad term, and that clinical focus needs to be on the reduction in skeletal muscle mass known as sarcopenia. The increasing focus on specific triggers, including hyperammonemia, bile salts, alcohol, cytokines, hormonal abnormalities and amino acid and metabolic alterations in cirrhosis, contributing to varying extents to sarcopenia, will allow rapid progress to reverse this last frontier in the management of cirrhotic patients.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28(2):281–4. Clinical paper that shows that skeletal muscle uptake of ammonia has clinical consequences beyond the muscle. Evidence provided that the skeletal muscle is a novel therapeutic target in cirrhotics.
Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16(1):95–131.
Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23(11):982–9. Definitive demonstration that muscle loss contributes to aggravation of other complications of cirrhosis.
Thiele M, Askgaard G, Timm HB, Hamberg O, Gluud LL. Predictors of health-related quality of life in outpatients with cirrhosis: results from a prospective cohort. Hepat Res Treat. 2013;2013:479639.
Shiraki M, Nishiguchi S, Saito M, Fukuzawa Y, Mizuta T, Kaibori M, et al. Nutritional status and quality of life in current patients with liver cirrhosis as assessed in 2007-2011. Hepatol Res. 2013;43(2):106–12.
Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–8. A prospective study to show that pre transplant sarcopenia has persistent adverse effects even beyond transplantation, emphasizing the high clinical significance and the need to reverse this process to improve transplant outcomes.
Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, et al. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30(2):208–14.
Merli M, Nicolini G, Angeloni S, Riggio O. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002;18(11–12):978–86.
Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachex Sarcopenia Muscle. 2012;3(4):225–37. A comprehensive review of the current state of knowledge in muscle biology as applied to sarcopenia of cirrrhosis. Current studies, deficiencies and potential solutions are discussed.
Antar R, Wong P, Ghali P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can J Gastroenterol. 2012;26(7):463–7.
Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8(11):979–85.
Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? PINC (Policentrica Italiana Nutrizione Cirrosi). Hepatology. 1996;23(5):1041–6.
Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166–73. A carefully performed prospective study with precise measurment of muscle mass in cirrhosis that showed increased mortality with sarcopenia in cirrhosis.
Sam J, Nguyen GC. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int. 2009;29(9):1396–402.
Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eghtesad B, et al. Post- liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014. doi:10.1111/jgh.12524. First study to show that increased skeletal muscle myostatin persists even after transplantation, and combined with data that myostatin is increased in cirrhosis and contributes to muscle loss suggests that myostatin antagonists are an exciting therapeutic option in cirrhosis.
Dasarathy S. Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig Dis Sci. 2013;58(11):3103–11.
Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85(5):1257–66.
Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57(4):897–909.
Lemyze M, Dharancy S, Wallaert B. Response to exercise in patients with liver cirrhosis: implications for liver transplantation. Dig Liver Dis. 2013;45(5):362–6.
Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17(9):761–5. Data to show that worsening severity of liver disease is accompanied by more severe muscle loss.
Heyman JK, Whitfield CJ, Brock KE, McCaughan GW, Donaghy AJ. Dietary protein intakes in patients with hepatic encephalopathy and cirrhosis: current practice in NSW and ACT. Med J Aust. 2006;185(10):542–3.
Williams TJ, McKenna MJ. Exercise limitation following transplantation. Compr Physiol. 2012;2(3):1937–79. Despite the title, this is the definitive and comprehensive review of exercise in cirrhosis. An essential reading for all hepatologists and transplant teams interested in understanding the role of exercise as a therapeutic option.
Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int. 2011;31(9):1250–8.
Heymsfield SB, Adamek M, Gonzalez MC, Jia G, Thomas DM. Assessing skeletal muscle mass: historical overview and state of the art. J Cachexia Sarcopenia Muscle. 2014. doi:10.1007/s13539-014-0130-5. A comprehensive review of the literature, focusing on the methods to quantify muscle mass. Given the increasing interest in precise quantification of skeletal muscle in patients, the advantages and limitations of various available methods are discussed.
MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross-sectional body composition analysis. Curr Opin Support Palliat Care. 2011;5(4):342–9.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006.
Figueiredo FA, Perez RM, Freitas MM, Kondo M. Comparison of three methods of nutritional assessment in liver cirrhosis: subjective global assessment, traditional nutritional parameters, and body composition analysis. J Gastroenterol. 2006;41(5):476–82.
Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21(2):113–7.
Glass C, Hipskind P, Tsien C, Malin SK, Kasumov T, Shah SN, et al. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol (1985 ) 2013;114(5):559–65.
Galant LH, Forgiarini Junior LA, Dias AS, Marroni CA. Functional status, respiratory muscle strength, and quality of life in patients with cirrhosis. Rev Bras Fisioter. 2012;16(1):30–4.
Jones JC, Coombes JS, Macdonald GA. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012;18(2):146–51.
Dharancy S, Lemyze M, Boleslawski E, Neviere R, Declerck N, Canva V, et al. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation. 2008;86(8):1077–83.
Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15(12):1662–70.
Muller MJ. Malnutrition and hypermetabolism in patients with liver cirrhosis. Am J Clin Nutr. 2007;85(5):1167–8.
Guglielmi FW, Panella C, Buda A, Budillon G, Caregaro L, Clerici C, et al. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism. Multicentre prospective study by the 'Nutritional Problems in Gastroenterology' Section of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2005;37(9):681–8.
Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Prasad SV, et al. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10(4).
Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110(45):18162–7.
Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303(8):E983–93.
Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54(5):915–21.
Dasarathy S, Muc S, Hisamuddin K, Edmison JM, Dodig M, McCullough AJ, et al. Altered expression of genes regulating skeletal muscle mass in the portacaval anastomosis rat. Am J Physiol Gastrointest Liver Physiol. 2007;292(4):G1105–13.
Dasarathy S, Mullen KD, Dodig M, Donofrio B, McCullough AJ. Inhibition of aromatase improves nutritional status following portacaval anastomosis in male rats. J Hepatol. 2006;45(2):214–20.
Dasarathy S, Dodig M, Muc SM, Kalhan SC, McCullough AJ. Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. Am J Physiol Gastrointest Liver Physiol. 2004;287(6):G1124–30.
Morrison WL, Bouchier IA, Gibson JN, Rennie MJ. Skeletal muscle and whole-body protein turnover in cirrhosis. Clin Sci (Lond). 1990;78(6):613–9.
McCullough AJ, Mullen KD, Kalhan SC. Defective nonoxidative leucine degradation and endogenous leucine flux in cirrhosis during an amino acid infusion. Hepatology. 1998;28(5):1357–64.
Tessari P, Inchiostro S, Barazzoni R, Zanetti M, Orlando R, Biolo G, et al. Fasting and postprandial phenylalanine and leucine kinetics in liver cirrhosis. Am J Physiol. 1994;267(1 Pt 1):E140–9. A carefully performed, detailed study using tracers in cirrhotics to demonstrate whole body changes. Even though a number of papers using similar methods exist, the current shift is from whole body to muscle specific alterations in protein turnover.
Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27(3):430–41.
Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49(1):59–68.
Dasarathy S, Mullen KD, Conjeevaram HS, Kaminsky-Russ K, Wills LA, McCullough AJ. Preservation of portal pressure improves growth and metabolic profile in the male portacaval-shunted rat. Dig Dis Sci. 2002;47(9):1936–42.
Dasarathy S, Muc S, Runkana A, Mullen KD, Kaminsky-Russ K, McCullough AJ. Alteration in body composition in the portacaval anastamosis rat is mediated by increased expression of myostatin. Am J Physiol Gastrointest Liver Physiol. 2011;301(4):G731–8.
Noiret L, Baigent S, Jalan R. Arterial ammonia levels in cirrhosis are determined by systemic and hepatic hemodynamics, and by organ function: a quantitative modelling study. Liver Int. 2013. doi:10.1111/liv.12361.
Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver Int. 2011;31(2):163–75.
Ganda OP, Ruderman NB. Muscle nitrogen metabolism in chronic hepatic insufficiency. Metabolism. 1976;25(4):427–35. This work has perhaps not attracted the attention it deserves. Increased ammonia uptake by the skeletal muscle in cirrhosis as a mechanism to protect against hyperammonemia in combination with more recent work on ammonia-mediated muscle autophagy and myostatin transcription provide novel therapeutic targets.
Lockwood AH, McDonald JM, Reiman RE, Gelbard AS, Laughlin JS, Duffy TE, et al. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia. J Clin Invest. 1979;63(3):449–60.
Jacobsen EB, Hamberg O, Quistorff B, Ott P. Reduced mitochondrial adenosine triphosphate synthesis in skeletal muscle in patients with Child-Pugh class B and C cirrhosis. Hepatology. 2001;34(1):7–12.
Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58(1):325–36. Despite the title, this is a very careful evidence-based review of the nutritional management in cirrhosis. Provides a critical overview of current nutritional interventions in cirrhosis.
Ney M, Vandermeer B, van Zanten SJ, Ma MM, Gramlich L, Tandon P. Meta-analysis: oral or enteral nutritional supplementation in cirrhosis. Aliment Pharmacol Ther. 2013;37(7):672–9.
Dardevet D, Remond D, Peyron MA, Papet I, Savary-Auzeloux I, Mosoni L. Muscle wasting and resistance of muscle anabolism: the "anabolic threshold concept" for adapted nutritional strategies during sarcopenia. Sci World J. 2012;2012:269531.
Tsien C, Thapalaya S, Runkana A, Yoho S, Ten Have G, McCullough AJ, et al. Leucine reverses anabolic resistance of hyperammonemia in cirrhosis: a prospective study. Hepatology. 2013;58(S1):875A.
Bugianesi E, Kalhan S, Burkett E, Marchesini G, McCullough A. Quantification of gluconeogenesis in cirrhosis: response to glucagon. Gastroenterology. 1998;115(6):1530–40.
Petersen KF, Krssak M, Navarro V, Chandramouli V, Hundal R, Schumann WC, et al. Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. Am J Physiol. 1999;276(3 Pt 1):E529–35.
Plauth M, Cabre E, Campillo B, Kondrup J, Marchesini G, Schutz T, et al. ESPEN Guidelines on Parenteral Nutrition: hepatology. Clin Nutr. 2009;28(4):436–44.
Bianchi GP, Marchesini G, Fabbri A, Rondelli A, Bugianesi E, Zoli M, et al. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized cross-over comparison. J Intern Med. 1993;233(5):385–92.
Seymour CA, Whelan K. Dietary management of hepatic encephalopathy. BMJ. 1999;318(7195):1364–5.
Dejong CH, van de Poll MC, Soeters PB, Jalan R, Olde Damink SW. Aromatic amino acid metabolism during liver failure. J Nutr. 2007;137(6 Suppl 1):1579S–85S.
van Loon LJ. Leucine as a pharmaconutrient in health and disease. Curr Opin Clin Nutr Metab Care. 2012;15(1):71–7.
Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract. 2012;27(1):8–20.
Putz-Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, et al. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012;32(5):845–51.
Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A. 2006;103(12):4741–6.
You JS, Lincoln HC, Kim CR, Frey JW, Goodman CA, Zhong XP, et al. The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem. 2014;289(3):1551–63.
Gluud C. Testosterone and alcoholic cirrhosis. Epidemiologic, pathophysiologic and therapeutic studies in men. Dan Med Bull. 1988;35(6):564–75.
Yurci A, Yucesoy M, Unluhizarci K, Torun E, Gursoy S, Baskol M, et al. Effects of testosterone gel treatment in hypogonadal men with liver cirrhosis. Clin Res Hepatol Gastroenterol. 2011;35(12):845–54.
Argiles JM, Orpi M, Busquets S, Lopez-Soriano FJ. Myostatin: more than just a regulator of muscle mass. Drug Discov Today. 2012;17(13–14):702–9.
Sakuma K, Nakao R, Aoi W, Inashima S, Fujikawa T, Hirata M, et al. Cyclosporin A treatment upregulates Id1 and Smad3 expression and delays skeletal muscle regeneration. Acta Neuropathol. 2005;110(3):269–80.
Zhang P, Chen X, Fan M. Signaling mechanisms involved in disuse muscle atrophy. Med Hypotheses. 2007;69(2):310–21.
Compliance with Ethics Guidelines
Conflict of Interest
Srinivasan Dasarathy declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dasarathy, S. Treatment to Improve Nutrition and Functional Capacity Evaluation in Liver Transplant Candidates. Curr Treat Options Gastro 12, 242–255 (2014). https://doi.org/10.1007/s11938-014-0016-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-014-0016-9
Keywords
- Amino acids
- AMP activated kinase
- Anabolic resistance
- Anthropometrics
- Autophagy
- Cirrhosis
- Deconditioning
- Encephalopathy
- Enteral feeding
- Exercise
- Fat free mass
- Hyperammonemia
- Late evening snack
- Leucine
- Malnutrition
- Metabolic studies
- Molecular mechanisms
- Myostatin
- Nutriceutical
- Sarcopenia
- Protein breakdown
- Respiratory quotient
- Skeletal muscle
- Substrate oxidation
- Supplements