Abstract
Purpose of review
In past decades, there has a been a paradigm shift concerning menopause, hormone therapy (HT), and cardiovascular disease (CVD). While initial observational studies suggested hormone replacement to provide a cardioprotective benefit for all menopausal women, subsequent large randomized trials have not confirmed these benefits and furthermore brought to light the risks of HT with regard to CVD, venous thrombosis, and stroke. The goal of this review is to summarize current recommendations regarding HT as it pertains to cardiovascular risk.
Recent Findings
Menopause HT remains the most effective treatment for vasomotor symptoms. Guidelines now suggest benefits outweigh the risk for in women with bothersome vasomotor symptoms, when initiated in early menopause (within 10 years of symptom onset or age < 60 years) without contraindications. Consideration of cardiovascular risk is necessary. For women with a moderate atherosclerotic CVD risk score, or with CVD risk modifiers, a transdermal formulation is preferred. For patients with known CVD, non-hormonal alternatives should be trialed. Current evidence does not support the use of HT for primary or secondary prevention of CVD.
Summary
Initiation of menopausal HT requires an individualized approach taking into account age of menopause, timing of initiation, vasomotor symptoms, and cardiovascular risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality for women in the USA with an increased prevalence of the disease after menopause [1••, 2]. Historically, estrogen had been postulated to be cardioprotective in women, due in part to its beneficial effects on cardiovascular hemodynamics, lipid metabolism, and the endothelium [3]. Estrogen had been shown to decrease the levels of total and LDL cholesterol and increase the release of nitric oxide resulting in vasodilation, possibly decreasing vascular injury and development of atherosclerosis in the long term [4]. Following this reasoning, it was believed that the increased prevalence of CVD in menopausal women was due to the loss of endogenous estrogen during menopause. This prevailing theory led many providers to conclude that women at menopause would derive cardioprotective benefits from hormone therapy (HT)–a recommendation that was backed by findings from animal and observational studies indicating that menopausal HT reduced the risk of CVD [5].
Over the past two decades, studies on HT have led to a better understanding of the role of HT use at and around the time of menopause and their role with CVD. In 1991, the Women’s Health Initiative (WHI) was the first randomized control trial (RCT) to determine if HT prevented CVD and other chronic diseases. Women with an intact uterus (n = 16,608) were randomized to daily oral conjugated equine estrogen (CEE) 0.625 mg plus medroxyprogesterone acetate (MPA) 2.5 mg or placebo while women with a hysterectomy (n = 10,739) were randomized to daily oral CEE (0.625 mg) alone or placebo. Women assigned to CEE + MPA had an increased incidence of CVD (HR 1.18; 95% CI 0.95–1.45), while women assigned to CEE saw nearly no difference (HR 0.94; 95% CI 0.78–1.14), as compared with placebo [6]. Women in both the CEE and CEE + MPA trials saw an increase in ischemic strokes by 35 and 37%, respectively. Given these findings, the investigators concluded that the risks outweighed the benefits and therefore did not support the use of HT in women [6].
Subsequently, the Heart and Estrogen/progestin Replacement Study (HERS) was a secondary prevention RCT in women and HT [7]. The trial randomized 2763 women with established CVD to receive either CEE 0.625 mg plus MPA 2.5 mg or placebo. The results from the trial showed a significant time trend, with HT being associated with an increased risk of recurrent CVD events in the first 2 years but a decreased risk in years 4 and 5. During an average follow-up of 4.1 years, CEE + MPA did not reduce the overall rate of recurrent CVD events compared with placebo (HR 0.99; 95% CI 0.80–1.22) and instead was associated with an increased risk of thromboembolic events [7]. As such, the researchers concluded against starting HT for the purpose of secondary prevention of CVD in menopausal women.
Findings from WHI and HERS that HT did not benefit primary or secondary CVD prevention and in some women, increased CVD risk, led to a considerable shift in clinical practices due to associated risk with CVD. Analyses of pharmacy databases found that in the year following publication of WHI, HT use declined drastically between 25 and 72% [8]. In the ensuing decade, nearly an 80% reduction in HT prescribing was observed, resulting in significant undertreating of women with bothersome vasomotor symptoms [9].
Impact of estrogen on the cardiovascular system
Estrogen affects serum lipid levels, coagulation, antioxidant systems, and the production of vasoactive substances such as nitric oxide and prostaglandins, all of which influence the cardiovascular system. Potential adverse physiological effects of estrogen contributing to an increased risk of CVD include an increase in serum triglyceride concentrations, a prothrombotic effect, particularly with oral estrogen, and an increase in vascular inflammatory markers [10]. Earlier studies supporting the cardioprotective effect of HT demonstrated a beneficial effect of oral estrogen on vasodilation, endothelial function, and lipids [4].
Estrogen influences serum lipid concentrations via estrogen-receptor-mediated effects on the hepatic expression of apoprotein genes [4]. In a randomized, double-blind crossover of healthy menopausal women with normal lipid levels at baseline, oral CEE 0.625 mg per day, and 1.25 mg per day were found to lower mean LDL cholesterol by 15 (95% CI 11–19%; p < 0.0001) and 19% (95% CI 15–23%; p < 0.0001), respectively; conversely high-density lipoprotein (HDL) cholesterol was found to increase by 16 (95% CI 12–20%; p < 0.001) and 18% (95% CI 14–22%; p < 0.0001), respectively. This decrease in LDL cholesterol has been thought to be a result of accelerated LDL catabolism. The study demonstrated an increase in very-low-density lipoprotein (VLDL) triglyceride levels by 24 (95% CI 8–40%; p < 0.003) and 42% (95% CI 26–58%; p < 0.0001) for the respective estrogen doses [11]. In this cohort of healthy patients without hyperlipidemia or CVD, transdermal estradiol (0.1 mg) did not significantly alter VLDL or LDL levels. However, in a study of 58 women with hyperlipidemia, randomized to receive simvastatin or CEE with MPA, HT produced smaller decreases in LDL and total cholesterol and an increase in triglycerides (29% versus 14% decrease) compared with simvastatin [12].
Other trials have suggested that oral estrogen therapy may have a prothrombotic effect leading to cardiovascular events, thrombosis and stroke via a reduction in serum fibrinogen, factor VII, and antithrombin [13]. In a trial of 28 menopausal women randomized to oral HT versus transdermal HT to placebo, it was found that oral HT also significantly increased levels of the inflammatory marker C-reactive protein (CRP) [14]. These pro-thrombotic and pro-inflammatory physiological effects are postulated to contribute to an increased prevalence of cardiovascular events in women with known disease. Indeed, both the WHI CEE + MPA (HR 1.87; 95% CI 1.37–2.54, p < 0.001) and CEE only (HR 1.48; 95% CI 1.06–2.07 p = 0.02) arms and HERS (HR 2.89; 95% CI, 1.50–5.58) trials demonstrated an increased risk of venous thromboembolism [6, 7].
Further studies have provided evidence that the biologic effects of estrogen on the vasculature is dependent on the extent of atherosclerosis present (Fig. 1) [4, 10]. A study of postmortem coronary artery specimens in premenopausal and postmenopausal women with and without coronary disease has shown that estrogen receptor expression is diminished in atherosclerotic arteries [15], potentially contributing to a decrease in the beneficial effects of estrogen. Additionally, estrogen has been shown to up-regulate members of the matrix metalloproteinase (MMP) family responsible for degrading the extracellular matrix of the arterial wall. In patients with established atherosclerosis, it is hypothesized that this increase in MMP expression in plaque could be associated with an increased risk of plaque rupture [16]. Other animal studies have suggested that the impaired cyclooxygenase-2 response in diseased atherosclerotic arteries may be implicated in the diminished or absent anti-atherosclerotic benefit of estrogen [17].
Estrogen’s role on healthy versus atherosclerotic vascular health. CAM = cell adhesion molecule; Cox-2 = cyclooxygenase 2; ER = estrogen receptor; HRT = hormone replacement therapy; LDL = low-density lipoprotein; MCP = monocyte chemoattractant protein; MMP = matrix metalloproteinase; TNF = tumor necrosis factor; VSMC = vascular smooth muscle cell. Permission for reprint requested from Ouyang, Michos & Karas [10]
Recent studies
Following WHI and HERS, there remained a substantial discrepancy between the results of these randomized trials and previous observational studies demonstrating cardioprotective benefit. Subsequently, a closer analysis of the WHI/HERS studies found a crucial difference between the two trials and the earlier observational studies—the average age of women enrolled. The WHI/HERS trials included women who were older (mean age of 67 years in HERS, mean age of WHI 62 years) given that the average age of menopause is 51 years [5]. Post hoc analyses of younger patients within the WHI revealed both lower absolute risks and attributable risks of adverse outcomes when menopausal HT was initiated in women aged 50 to 59. Notably, the use of HT within 10 years of menopause onset was associated with a lower risk of coronary heart disease compared with women who began therapy greater than 20 years since onset of menopause [6].
Out of these findings and observations, the “timing hypothesis” was proposed which posits there may be less risk when HT is initiated closer to the time of menopause onset before atherosclerosis develops. This hypothesis was first described based on basic science non-human primate studies that showed that initiation of estrogen at the time of oophorectomy reduced coronary atherosclerosis by 50–70%, but starting HT 2 years following oophorectomy (equivalent to 6 years in humans) in primates had no benefit [18]. In response to this hypothesis, more recent randomized trials have focused on the cardiovascular effects of HT in recently menopausal women.
In 2014, the KEEPS (Kronos Early Estrogen Prevention Study) found that the early use of HT does not affect atherosclerosis progression [19]. KEEPS randomized 727 healthy menopausal women between the ages 42–58 to daily oral CEE (0.45 mg/day) or transdermal estrogen (50 μg/day), both with cyclic progesterone treatment or placebo for 4 years and compared their markers of atherosclerosis progression. Intermediate markers for atherosclerosis were evaluated, including carotid artery intima-media (CIMT) thickness measured annually by ultrasonography, and coronary artery calcium (CAC) score at study entry and baseline. Blood pressure and other biochemical risk factors for CVD were also monitored.
Over the 4-year period, KEEPS found there was no difference in the rate of CIMT increase across all 3 groups with a mean rate increase of 0.0076 mm/year [20]. The rates of CAC score increase were also similar between the 3 groups: CAC score increased in 17.4% of the oral estrogen group, 18.9% of the transdermal estrogen group, and 21.0% of the placebo group with no significant differences. Despite no changes in intermediate markers of atherosclerosis, HT was found to have overall favorable effects on the biochemical risk factors for CVD: oral estrogens decreased LDL and non-HDL cholesterol while increasing HDL cholesterol and triglycerides, and transdermal estrogens decreased cholesterol and other insulin-related markers, such as fasting insulin and blood glucose [20]. No change in blood pressure in either of the groups were seen. These results of the KEEPS Trial showed that HT has no effect on atherosclerosis progression, however, may improve several CVD risk factors in recently menopausal women.
The Early versus Late Interventional Trial with Estradiol trial (ELITE) also sought to study intermediate markers of CVD on women close to the age of menopause onset. This study examined the effects of HT on CIMT and CAC in early (< 6 years from menopause) versus late menopausal women (>10 years from menopause) [21]. This single-center, randomized, controlled trial examined 643 healthy, menopausal women who were assigned to receive either oral 17β-estradiol (1 mg daily) plus progesterone vaginal gel or placebo and stratified them based on time since menopause. After a median of 5 years of intervention, the trial showed that early menopausal women taking HT had decreased rates of CIMT progression (0.0044 mm/year) compared with placebo (0.0078 mm/year). There were no differences in rates of CIMT progression between the late menopausal HT group and placebo. CAC scores showed no differences between the early or late menopausal HT group versus placebo (however, the late menopausal HT group did have higher CAC scores as expected for age).
The differences between ELITE and KEEPS may be attributed to several factors including estrogen dosing and time of follow-up. KEEPS used a lower dose of estrogen and thus may not have had the dose-response effect of estrogen needed for vascular benefit. In addition, KEEPS only followed patients for 4 years, whereas the ELITE trial began to see noticeable rates of change starting at year 3. Therefore, KEEPS may have been limited by the follow-up time necessary to evaluate CVD progression. Regardless of the small differences seen between the two studies, both trials demonstrated that HT does not cause atherosclerosis progression but perhaps may be beneficial to CVD in early, menopausal women.
In 2015, Cochrane published a systematic review of 19 trials of HT including a total of 40,410 menopausal women [22]. The study found that HT in both primary and secondary prevention conferred no protective effects for all-cause mortality, CVD death, non-fatal myocardial infarction, angina, or revascularization. There was an increased risk of stroke in the HT arm for combined primary and secondary prevention (RR 1.24, 95% CI 1.10 to 1.41), venous thromboembolic events (RR 1.92, 95% CI 1.36 to 2.69), and pulmonary emboli (RR 1.81, 95% CI 1.32 to 2.48). Additionally, this review found that when HT was started less than 10 years after menopause, HT caused lower rates of coronary heart disease (RR 0.52, 95% CI 0.26 to 0.96) and decreased all-cause mortality (RR 0.70, 95% CI 0.52 to 0.95) [22]. This effect was not found when HT was started 10 years after menopause.
Special consideration: Premature and early menopause
While the average age of natural menopause occurs at 51 years in the USA, women can enter natural menopause prematurely (<40 years) or early (<45 years) or otherwise undergo bilateral salpingo-oophorectomy (BSO) at an early age entering them into surgical menopause. Women that enter menopause prematurely or early and do not use HT through the natural age of menopause have been found to have a higher risk of CVD and CVD mortality. A 2016 pooled meta-analysis of over 300,000 women in 32 observational studies found that compared with women with onset of menopause before the age of 45, women with onset of menopause ≥45 years of age had a significantly higher risk of overall (RR 1.50, 95% CI 1.28–1.76) and fatal (RR 1.11, 95% CI (1.03–1.20) CVD, even after adjustment for established CVD risk factors [1, 23]. Other prospective studies have found that women with onset of menopause before the age of 45 have a significantly greater risk of heart failure (HR 1.33, 95% CI 1.150–1.53) [24]. The Framingham study demonstrated that higher total cholesterol, higher blood pressure, and other cardiovascular risk factors before menopause were associated with earlier menopause, independently of smoking status. Additionally, it has been shown that CVD risk is substantially higher in women undergoing BSO at a young age (40–45 years) [25].
Current recommendations
Along with the literature, evidence-based recommendations have evolved considerably over the past two decades. Currently, the most comprehensive position statement regarding hormone therapy is that of the North American Menopause Society (NAMS) in 2017 [26••]. This is an update from the NAMS 2012 position statement and will again be updated in 2022. This position statement affirms that hormone therapy remains the most effective treatment for both vasomotor symptoms and genitourinary syndrome of menopause and has also been shown to prevent bone loss and fracture. Hormone therapy is also indicated for premature hypoestrogenism due to hypogonadism, primary ovarian insufficiency, or premature surgical menopause [27]. The NAMS position statement did not recommend HT for the indication of cardiovascular prevention. Furthermore, the statement states the risks of HT differ depending on timing of initiation, type, dose, route of administration, and duration of use [26••].
Initiation of hormone therapy
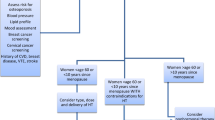
An approach to the initiation of HT should include an evaluation of clinical symptoms, exclusion of absolute contraindications, consideration of timing, and a thorough assessment of cardiovascular risk including using the 10-year American College of Cardiology/American Heart Association Atherosclerotic CVD (ASCVD) risk calculator (Table 1). For women younger than 60 years or those who are within 10 years of menopause onset (without contraindications including CVD, personal history of breast cancer, previous venous thromboembolic event or stroke, active liver disease or undiagnosed vaginal bleeding), the benefit-risk ratio is favorable for initiation of HT for bothersome vasomotor symptoms or patients at elevated risk of bone loss or fracture. The benefit-risk ratio is also favorable for women with 10-year ASCVD risk <5% or ≤ 1 cardiovascular risk factors. For women with premature (<40 years) or early menopause (<45 years), HT should be used at least until the age of 51 years, unless contraindicated [26••].
For women with 10-year ASCVD risk 5–10% or 10-year ASCVD risk <5% but ≥2 CVD risk factors, a transdermal estrogen formulation is preferable. For women who initiate HT more than 10 years from menopause onset or are 60 years of age or older, HT use is not recommended as the benefit-risk ratio appears less favorable due to greater absolute risks of CVD, stroke, venous thromboembolism, and dementia [26••]. For women with a 10-year ASCVD risk >10%, including those with risk enhancers such as elevated CAC > 100 and/or 75th percentile, uncontrolled hyperlipidemia or hypertriglyceridemia, history of pregnancy complications such as preeclampsia, and chronic inflammatory conditions (e.g., rheumatoid arthritis, systemic lupus erythematosus, psoriatic arthritis), the benefit-risk ratio also appears less favorable [26••, 28]. Periodic reevaluation should be undertaken to assess for ongoing symptoms and necessity of therapy.
Dosing
The therapeutic goal of estrogen therapy is to use the most appropriate (often, but not necessarily, lowest effective) dose to adequately control symptoms. Progestogens are indicated to counteract the effect of estrogen on the endometrial lining and prevent endometrial growth which may increase the risk of endometrial cancer. Oral progesterones (bioidentical) and oral or transdermal progestins (synthetic) have varying dosing that are dependent on the potency of the formulation and varies with estrogen use [26••].
Route of delivery
The route of administration is another consideration for the prescription of HT. Transdermal formulations bypass first-pass hepatic metabolism, thereby resulting in lower production of prothrombotic factors and inflammatory markers such as CRP when compared with oral regimens [13]. As such, the use of transdermal (rather than oral) estradiol is preferred for women at moderate risk for CVD with a 10-year ASCVD risk between 5 and 10% (Table 1).
Compounded hormones
The use of custom compounded bioidentical HT is not endorsed by several medical societies as these formulations present safety concerns and doses that are not standardized or regulated [26••, 29]. Additionally, these formulations may be administered in non-standard routes such as subdermal implants, pellets or troches [26••]. A recent report commissioned by the US Food and Drug Administration, the National Academies of Sciences, Engineering, and Medicine reviewed the available evidence on compounded HT and advised against the use of compounding HT with major concerns around inadequate labeling, lack of bioavailability data, and the lack of evidence on the safety and efficacy claims [30].
Government-approved and FDA regulated bioidentical hormone formulations including estradiol, estrone and micronized progesterone are available and are monitored for purity and efficacy. Of note, many of these formulations continue to carry black-box warnings for adverse events. Despite this, the continued use of compounded HT may be exacerbated by the lack of knowledge, by both providers and patients, that bioidentical formulations are available and generic on current prescription plans.
Non-hormonal options
In high-risk patients with CVD, history of venous thromboembolism, stroke, history of breast cancer, other estrogen sensitive cancers, or other contraindications, non-hormonal therapies should be considered. These recommendations are outlined in the NAMS 2015 position statement on nonhormonal management [31]. For vasomotor symptoms, the SSRI paroxetine is the only FDA-approved medication and is associated with a 33 to 67% reduction in hot flash frequency when compared with placebo. Other SNRIs and antidepressants have also shown to be effective, and it has been postulated that the decline in estrogen and progesterone levels in menopause trigger alterations in the neuroendocrine system, including changes in serotonin and norepinephrine levels, leading to thermoregulatory dysfunction in the hypothalamus [32]. Gabapentin and clonidine are other alternatives.
Non-pharmacologic approaches including cognitive-behavioral therapy and clinical hypnosis have been shown to have benefit [31]. Again, none of these above alternatives have shown to be as effective as estrogen therapy. For patients with debilitating menopausal symptoms and risk of CVD, it may be reasonable to initiate HT with close and stringent monitoring, after a thorough discussion of the risks and benefits.
Treatment of genitourinary syndrome of menopause
Genitourinary syndrome of menopause encompasses symptoms of vaginal dryness, burning, irritation, pain with intercourse, urinary urgency, dysuria, and recurrent urinary tract infection, for which low-dose topical vaginal estrogen preparations are effective. These may be used in women at risk for CVD as these preparations result in minimal systemic absorption. A 2018 prospective observational cohort study of participants who used vaginal estrogen in WHI demonstrated that the risks of cardiovascular disease and cancer were not elevated among menopausal women using vaginal estrogens compared with nonusers, providing reassurance for its safety [33]. Despite this, the FDA black box warning is included in this topical formulation and should be discussed with women.
Clinical vignette recommendations
Ms. A was started on HT for a “cardioprotective” benefit more than 10 years since onset of menopause. Furthermore, she has significant risk for CVD including an elevated coronary calcium score, hyperlipidemia, and family history of CAD and now recent onset of angina. Her HT should be tapered and discontinued as soon as feasible. If she experiences menopausal symptoms, non-hormonal options may be considered. Current evidence does not support the use of HT for primary or secondary CVD prevention. Furthermore, she is at high risk for CVD based on her risk factors and symptoms. Stress testing and/or coronary angiogram should be pursued for her anginal symptoms, as well as treatment for her hyperlipidemia. Tapering using a low-dose transdermal approach may be considered. For her genitourinary symptoms, a local vaginal estradiol may still be considered.
Ms. B has bothersome vasomotor symptoms and recent menopause (< 10 years since onset). Given this and lack of clinical contraindications, she is a candidate to continue HT. However, she should be switched from oral to transdermal estradiol due to her moderate 10-year ASCVD risk score of 7.5% and other CVD risk factors including hypertension, hyperlipidemia, and diabetes. Progesterone is not necessary given her history of hysterectomy. She should be started on statin with her history of diabetes and have close monitoring of her cholesterol and hemoglobin A1C levels. Risks and benefits should be discussed in depth with the patient, including the increased risk of breast cancer after 5 years of combined HT use.
Conclusion
There has been a considerable evolution of the understanding and recommendations regarding menopause, HT, and CVD since the WHI trials were published. Despite disparate findings from observational studies, these research trials have allowed for a better understanding of the role of HT and heart disease, leading away from its use for CVD prevention, to now treatment primarily targeted towards symptom management taking into account cardiovascular risk.
Currently, HT is considered an appropriate option when initiated in early menopause (within 10 years of symptom onset or age < 60 years) without contraindication and when vasomotor symptoms are bothersome. Current guidelines recommend an assessment of cardiovascular risk before initiation of therapy and can include evaluation of risk factors, lipid profile, and coronary calcium score as objective measurements. Given the variability of data regarding this topic historically, further studies and specifically randomized controlled trials are certainly warranted to validate recent findings of reduced CVD and all-cause mortality when hormone therapy is initiated in women near the onset of menopause. As cardiologists are often consulted on this topic, it is beneficial for practitioners in the specialty to understand the evolution of literature regarding hormone therapy and cardiovascular risk and the current recommendations for treatment.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
•• El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. 2020;142(25):e506–e32. https://doi.org/10.1161/CIR.0000000000000912 This 2020 scientific statement from the American Heart Association discusses menopause characteristics relevant to cardiovascular disease, with a summary of the evolving literature and particular focus on timing of hormone therapy initiation.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke Statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596. https://doi.org/10.1161/CIR.0000000000000757.
Subbiah MT. Mechanisms of cardioprotection by estrogens. Proc Soc Exp Biol Med. 1998;217(1):23–9. https://doi.org/10.3181/00379727-217-44201.
Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–11. https://doi.org/10.1056/NEJM199906103402306.
Johnson RR, Sweeney ME. Debate: the potential role of estrogen in the prevention of heart disease in women after menopause. Curr Control Trials Cardiovasc Med. 2000;1(3):139–42. https://doi.org/10.1186/cvm-1-3-139.
Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353–68. https://doi.org/10.1001/jama.2013.278040.
Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (HERS) research group. JAMA. 1998;280(7):605–13. https://doi.org/10.1001/jama.280.7.605.
Corbelli JA, Hess R. Hormone therapy prescribing trends in the decade after the Women's Health Initiative: how patients and providers have found a way to sleep better at night. Menopause. 2012;19(6):600–1. https://doi.org/10.1097/gme.0b013e318255b441.
Santen RJ, Stuenkel CA, Burger HG, Manson JE. Competency in menopause management: whither goest the internist? J Women's Health (Larchmt). 2014;23(4):281–5. https://doi.org/10.1089/jwh.2014.4746.
Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47(9):1741–53. https://doi.org/10.1016/j.jacc.2005.10.076.
Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17):1196–204. https://doi.org/10.1056/NEJM199110243251702.
Darling GM, Johns JA, McCloud PI, Davis SR. Estrogen and progestin compared with simvastatin for hypercholesterolemia in postmenopausal women. N Engl J Med. 1997;337(9):595–601. https://doi.org/10.1056/NEJM199708283370903.
Brosnan JF, Sheppard BL, Norris LA. Haemostatic activation in post-menopausal women taking low-dose hormone therapy: less effect with transdermal administration? Thromb Haemost. 2007;97(4):558–65.
Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, et al. Different effects of oral conjugated estrogen and transdermal estradiol on arterial stiffness and vascular inflammatory markers in postmenopausal women. Atherosclerosis. 2006;189(2):436–42. https://doi.org/10.1016/j.atherosclerosis.2005.12.030.
Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89(4):1501–10. https://doi.org/10.1161/01.cir.89.4.1501.
Karas R, Clarkson TB. Consideration in interpreting the cardiovascular effects of hormone replacement therapy observed in the WHI: timing is everything. Menopausal Med. 2003:8–12.
Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306(5703):1954–7. https://doi.org/10.1126/science.1103333.
Mehta JM, Chester RC, Kling JM. The timing hypothesis: hormone therapy for treating symptomatic women during menopause and its relationship to cardiovascular disease. J Women's Health (Larchmt). 2019;28(5):705–11. https://doi.org/10.1089/jwh.2018.7201.
Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161(4):249–60. https://doi.org/10.7326/M14-0353.
Miller VM, Naftolin F, Asthana S, Black DM, Brinton EA, Budoff MJ, et al. The Kronos early estrogen prevention study (KEEPS): what have we learned? Menopause. 2019;26(9):1071–84. https://doi.org/10.1097/GME.0000000000001326.
Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–31. https://doi.org/10.1056/NEJMoa1505241.
Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;3:CD002229. https://doi.org/10.1002/14651858.CD002229.pub4.
Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1(7):767–76. https://doi.org/10.1001/jamacardio.2016.2415.
Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR. Association of Age at Menopause With Incident Heart Failure: A Prospective Cohort Study and Meta-Analysis. J Am Heart Assoc. 2016;5(8). https://doi.org/10.1161/JAHA.116.003769.
Lobo RA. Surgical menopause and cardiovascular risks. Menopause. 2007;14(3 Pt 2):562–6. https://doi.org/10.1097/gme.0b013e318038d333.
•• The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2018;25(11):1362–87. https://doi.org/10.1097/GME.0000000000001241 This 2017 position statement from The North American Menopause Society provides the most current evidenced-based recommendations regarding hormone therapy with a discussion of cardiovascular disease.
Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–91. https://doi.org/10.3109/13697137.2015.1020484.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):e177–232. https://doi.org/10.1016/j.jacc.2019.03.010.
Cobin RH, Goodman NF, Committee ARES. American ASSOCIATION of clinical endocrinologists and AMERICAN COLLEGE of endocrinology position statement on MENOPAUSE-2017 update. Endocr Pract. 2017;23(7):869–80. https://doi.org/10.4158/EP171828.PS.
National Academies of Sciences Eg, and Medicine, Division HaM, Policy BoHS, Therapy CotCUoTPwCBHR. The Clinical Utility of Compounded Bioidentical Hormone Therapy: A Review of Safety, Effectiveness, and Use. 2020.
Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22(11):1155–72; quiz 73–4. https://doi.org/10.1097/GME.0000000000000546.
Carroll DG, Lisenby KM, Carter TL. Critical appraisal of paroxetine for the treatment of vasomotor symptoms. Int J Women's Health. 2015;7:615–24. https://doi.org/10.2147/IJWH.S50804.
Crandall CJ, Hovey KM, Andrews CA, Chlebowski RT, Stefanick ML, Lane DS, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative observational study. Menopause. 2018;25(1):11–20. https://doi.org/10.1097/GME.0000000000000956.
Funding
This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. K23 HL125941, K23 HL127262, and grants from the Society for Women’s Health Research (SWHR), Washington, D.C., the Women’s Guild of Cedars-Sinai Medical Center, Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, the Barbra Streisand Women’s Cardiovascular Research and Education Program, the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statements
Benita Tjoe, Breanna Fell, Alexis LeVee, Janet Wei, and Chrisandra Shufelt declare that they have no conflict of interest.
Human andAnimal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Reproductive Health and Cardiovascular Disease
Clinical Vignettes
• Ms. A is a 62-year-old female who presents to cardiology clinic for complaint of recent onset of angina. She reports to be on oral hormone therapy (HT) with combined estrogen and progesterone at the recommendation of another physician who states it has cardioprotective benefit. She started menopause at age 49 and was started on HT at age 60. She experienced hot flashes and night sweats closer to the age of menopause but currently reports no bothersome vasomotor symptoms. She does endorse vaginal dryness and frequent urinary tract infections. Both her father and grandfather died of heart disease in their late 60s. She recently had a coronary calcium scan showing a coronary artery calcium score > 300 and lipid panel with total cholesterol 226 mg/dL and low-density lipoprotein (LDL) cholesterol 142 mg/dL.
• Ms. B is a 53-year-old female with past medical history of hypertension, dyslipidemia and type-2 diabetes mellitus who presents to cardiology clinic, inquiring whether it is safe for her to be on HT. She endorses severe vasomotor symptoms of menopause. Her last menstrual period was 18 months ago, and she has been having 10–20 hot flashes daily, drenching night sweats disrupting her sleep and severe mood imbalance. For this, she was prescribed oral estradiol recently. She has a history of hysterectomy. Her mammogram and pap smear are both up-to-date and normal. Her lipid panel shows total cholesterol of 187 mg/dL and LDL 123 mg/dL. Her 10-year atherosclerotic cardiovascular disease (ASCVD) risk score is 7.5%.
Rights and permissions
About this article
Cite this article
Tjoe, B., Fell, B., LeVee, A. et al. Current Perspective on Menopause Hormone Therapy and Cardiovascular Risk. Curr Treat Options Cardio Med 23, 37 (2021). https://doi.org/10.1007/s11936-021-00917-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-021-00917-2