Abstract
Purpose of review
The goal of this review is to describe the benefits and limitations of robotic-assisted percutaneous coronary intervention (PCI), the most important and recent clinical data, and the future applications as robotic technology continues to develop.
Recent findings
Robotic-assisted PCI can reduce occupational hazards of ionizing radiation exposure and orthopedic injury to the interventional cardiologist while offering increased precision and fine control that may confer benefit to the patient. Recent studies have suggested the efficacy and safety of robotic-assisted PCI, yet widespread use of the technology has not been fully adopted due to limitations of the current technology and high costs.
Summary
Robotic-assisted PCI has potential to benefit both the operator and the patient. Despite some limitations of robotic-assisted PCI, it can safely and effectively be used in many patients with coronary artery disease requiring PCI. The value proposition for robotic-assisted PCI will depend on the evolution of robotic systems and its applicability to more complex coronary lesions, peripheral arterial interventions, and telemedicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Robotic systems have been established within healthcare procedural fields to enhance operator techniques and improve patient care. Robots can assist with fine control and positioning of devices, usually with less invasiveness to patients. Robots can perform either the entire or certain aspects of common procedures. One example in cardiology is the use of robotic systems for minimally invasive valve repairs and bypass graft surgery. Recently, robotic percutaneous coronary intervention (PCI) was developed to address drawbacks of traditional PCI, namely occupational hazards related to radiation exposure and orthopedic injury from wearing lead aprons. The technology offers a contemporary disruption to the traditional concept of operators physically standing tableside adjacent to the radiation source to manually manipulate coronary wires, balloons, and stents. This review focuses on the benefits and limitations of robotic-assisted PCI, the current clinical data available, and the future applications.
Robotic PCI system
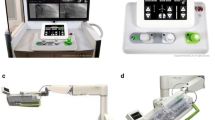
The robotic PCI system consists of two major components: a cockpit and a bedside robotic arm (Fig. 1a, b). The cockpit is radiation-shielded, mobile, and positioned away from the radiation source. This workspace houses the joysticks for manipulations of coronary devices and the guide catheter. The cockpit also features up-close angiographic and hemodynamic monitors and X-ray foot pedals. The interventional cardiologist can sit in the cockpit without wearing a lead apron. After obtaining vascular access and placing the guide catheter, the operator can perform PCI using the joysticks for fine rotational and longitudinal movements of the coronary guidewire and guide catheter, as well as for advancement and retraction of monorail coronary balloons and stents. Precision positioning of balloons and stents can occur with millimeter movements. Cables link the cockpit to the bedside, articulated robotic arm. A single-use, sterile cassette connects to the robotic arm, and the guide catheter is loaded within a guide track sleeve that pulls over the guide catheter and locks to the side port of the sheath. Coronary guidewires, balloons, and stents can subsequently be loaded into the cassette for robotic delivery into the coronary arteries. Support staff must remain tableside to initially load the interventional devices onto the cassette, inflate and deflate balloons and stents, and inject contrast [1, 2••, 3•]. The interventional cardiologist is still required to obtain arterial access and position the guide catheter into the coronary ostium prior to using robotic-assisted PCI. At any time, the procedure can be switched to manual control by disengaging the equipment from the cassette. The robotic CorPath 200 system (Corindus Vascular Robotics, Waltham, MA) has been evaluated in clinical studies and has two joysticks to control a guidewire and a balloon or stent device, while the newer generation CorPath GRX (Corindus Vascular Robotics) has three joysticks to also allow active control of the guide catheter [3•]. These robotic systems were designed to mitigate current occupational hazards of PCI for the primary operator and provide more precision to potentially improve outcomes for patients.
Occupational hazards of traditional PCI and benefits of robotic-assisted PCI
Radiation
Interventional cardiologists accumulate considerable lifetime ionizing radiation of 50 to 200 mSv, or the equivalent of 2500 to 10,000 chest X-rays [4,5,6]. Although some effects of radiation are cumulative and only occur once a threshold of exposure has been reached, other effects can occur with any level of radiation exposure. Therefore, there is truly no “safe” level of radiation exposure. The policy of keeping radiation “as low as reasonably achievable” (ALARA) alludes to the dangers of ionizing radiation but fails to provide a solution of truly avoiding radiation exposure.
Deterministic effects of ionizing radiation occur once a threshold of exposure has been exceeded and are caused by significant cell damage or death. Cataracts occur due to deterministic injury, and multiple studies have shown increased risk of cataract formation among interventional cardiologists [7,8,9]. One study performed ocular slit lamp examinations and surveyed 54 interventional cardiologists at an interventional cardiology meeting [7]. Fifty percent of the interventional cardiologists showed detectable posterior lens opacities compared with < 10% in a control group of age-matched non-medical professionals. The severity of the lens opacities correlated with cumulative occupational radiation dose. In a similar recent study, IC-CATARACT (CATaracts Attributed to Radiation in the CaTh lab), 99 catheterization laboratory staff—90% of whom were interventional cardiologists—had higher prevalence of cortical and posterior subcapsular lens changes on slit lamp examination compared with unexposed controls (47 vs 17%, p = 0.015) [8]. IC-CATARACT and other studies report lens changes with cumulative radiation exposures well below 1 Gy, suggesting that the threshold at which lens injury occurs is low [7, 8, 10, 11].
Stochastic effects have no threshold level of radiation dose at which injury occurs; however, the probability of incurring one of these effects increases as radiation exposure increases (“linear no-threshold model”) [12]. Stochastic effects of ionizing radiation occur due to symmetrical translocations of DNA injury. These effects include malignancies of the skin, gastrointestinal tract, brain, and thyroid gland [13, 14]. Indeed, interventional cardiologists develop DNA damage and chromosomal abnormalities at a much higher rate than non-interventional cardiologists [15, 16]. One study that evaluated a cohort of 31 interventionalists with brain cancer—23 of whom were interventional cardiologists—found that 85% of cancers were left-sided [17]. This finding, in the context of the operators’ left side being closest to the radiation source and exposed to double the radiation levels of the right side [17, 18], gives credence to the mechanism and increased cancer risk for interventional cardiologists in the catheterization laboratory.
Robotic-assisted PCI has been shown to decrease radiation exposure by over 95% to the primary operator compared to the levels found at the traditional table position [19••]. This decrease is due to a combination of the large radiation shield mounted on the cockpit and the increased distance from the radiation source. Tableside radiation exposure can be reduced with standard lead aprons and radiation protective pads, though these do not resolve the accompanying orthopedic strain. Zero gravity lead systems can also reduce tableside radiation exposure and spine stress, but lack the additional benefits of robotic-assisted device manipulation, including coronary wiring, lesion length measurements, and precise positioning of balloons and stents.
Orthopedic
Interventional cardiologists accumulate decades of cervical and lumbar stress from heavy lead apron use. Lead aprons can generate intervertebral disc pressures as high as 300 pounds per square inch [15, 20]. The result is often spinal disc disease, arthritis, chronic back pain, or hip, knee, and ankle pain. In a Society for Cardiac Angiography and Interventions survey, approximately half of the 424 responding interventional cardiologists reported spine problems. Of those with spine problems, one third missed work due to back pain. Approximately one quarter reported problems related to hips, knees, or ankles [12, 21]. Similarly, in a survey between interventional cardiologists (who stand and wear heavy lead), orthopedists (who stand and do not usually need to wear lead aprons), and rheumatologists (who stand only for short periods of time without lead), interventional cardiologists reported significantly higher rates of back and neck pain requiring treatment than the other physician groups. Interventional cardiologists also received more specific treatment such as non-steroidal anti-inflammatory drugs and mechanical support devices than either orthopedists or rheumatologists [22].
Due to the recent implementation of robotic-assisted PCI, no studies have directly assessed the long-term orthopedic benefits of this new technology. Intuitively, robotic-assisted PCI provides orthopedic benefits to the primary operator by providing the opportunity to complete a PCI without any lead or to sit while wearing the lead in the event emergent conversion to manual PCI is necessary. This requires that the secondary operator or support staff be proficient in loading and exchanging devices within the robotic cassette. There are also implications for robotic PCI reducing orthopedic stress when access is via the left radial artery. The robotic arm is closer to the patient’s left arm and allows for easy connection to a left-radial seated guide for PCI. This reduces the need for the operator to lean forward across the patient to perform manual device manipulations during PCI.
Potential benefits of robotic-assisted PCI to the patient
Patient benefits of robotic-assisted PCI include lesion length measurement for appropriate stent size selection and millimeter movements of balloons and stents for precise positioning of devices. In addition, stent positioning is enhanced as the operator is sitting closer to the fluoroscopy monitors while in the cockpit, potentially allowing improved attention to detail.
Traditionally, operators estimate lesion length visually, which is highly variable and often inaccurate even among experienced interventional cardiologists. In a recent study of 40 interventional cardiologists comparing visual estimation to qualitative coronary angiography, visually estimated lesion length was accurate in only 30% of cases [23]. Underestimation and overestimation of lesion length can both have deleterious effects. Underestimation of lesion length can cause (1) incomplete lesion coverage (longitudinal geographical miss [LGM]) or (2) placement of an additional, overlapping stent to avoid LGM. LGM has been shown to result in increased rates of target vessel revascularization (TVR) and myocardial infarction (MI) [24], and overlapping stents result in increased costs, risk of stent thrombosis, TVR, death, and MI [25]. Likewise, overestimation of lesion length results in excess stent lengths associated with increased restenosis risk [26••, 27, 28].
In a study of 60 consecutive patients who underwent robotic-assisted PCI using CorPath 200, operators provided visual estimates of lesion length and then used the robotic system to make measurements [26••]. Only 35% of visually estimated lesions resulted in accurate stent length selection, whereas 33% were overestimated and 32% were underestimated. In five patients, or 8.3% of cases, one less stent was placed based on the robotic lesion measurement, which both saved cost and potentially improved patient outcomes by decreasing the need for an extra, overlapping stent (Table 1) [26••, 27, 28]. This study was designed to assess changes in the immediate stent selection strategy of the operators. Therefore, limitations included a single treatment group and no assessment of LGM, long-term follow-up, or cost assessments. Future clinical studies and registry data will help shed light on these variables.
Robotic-assisted PCI clinical evidence
The PRECISE (Percutaneous Robotically Enhanced Coronary Intervention) study was the first large-scale clinical trial that evaluated the safety and efficacy of robotic-assisted PCI. One hundred sixty-four patients at nine sites were enrolled in this prospective, single-arm, multicenter trial. All patients underwent PCI using the first-generation CorPath 200 robotic system. The co-primary endpoints were (1) clinical procedure success defined as < 30% residual stenosis at the robotically treated lesions in the absence of cardiac death, MI, or TVR and (2) device technical success defined as the successful manipulation of PCI devices by the robot without conversion to manual operation. Clinical procedural success was achieved in 160 of the 164 patients (97.6%). In four patients, there were post-procedural elevations in creatine kinase myocardial band > 3 times the upper limit of normal in the absence of new pathological Q waves, and these patients ultimately experienced no clinical consequences. Importantly, no deaths, strokes, Q-wave MIs, or TVR occurred in the 30 days after the procedure. Device technical success was achieved in 162 of the 164 patients (98.8%). In two patients, the operators converted to manual operation due to severe resistance to delivery of the stent that ultimately required use of buddy wire and a guide extension catheter for stent delivery. The median radiation exposure to the operators at the interventional cockpit was 95.2% lower than at the procedure table [19••].
Based on the PRECISE study, the FDA approved CorPath 200 for PCI in 2012 [29]. However, the study evaluated relatively simple lesions, with 28.7% of treated lesions categorized as ACC/AHA class A and only 12.8% of treated lesions were ACC/AHA class C. The majority of these lesions were in the main epicardial vessels and not in branches such as the diagonal or obtuse marginal. All procedures were performed via femoral arterial access. Mean lesion length was short at 12.2 mm. Severe tortuosity or calcification, total occlusions, ostial lesions, intraluminal thrombus, involvement of bifurcations, and unprotected left main arteries were also excluded from the study [19••]. Therefore, while applicability to true real-world practice is limited, PRECISE demonstrated feasibility and safety of robotic-assisted PCI as a potential treatment strategy for uncomplicated lesions.
The subsequent CORA-PCI (Complex Robotically Assisted Percutaneous Coronary Intervention) study sought to evaluate robotic-assisted PCI in complex coronary artery disease more reflective of true clinical practice. All consecutive PCI procedures performed robotically (study group) or manually (control group) over 18 months at a single center were included. All robotic-assisted PCI were done using the CorPath 200 system. A total of 315 patients underwent 334 PCI procedures (108 robotic PCIs and 225 manual PCIs). Primary endpoints were (1) clinical success defined as < 30% residual stenosis with thrombolysis in myocardial infarction flow grade 3 without death, MI, stroke, or urgent repeat revascularization and (2) robotic technical success defined as clinical success and completion of the PCI procedure either robotically or with partial manual assistance. Clinical success was similar between patients receiving robotic-assisted PCI vs. manual PCI (98.8 vs 100%, p = 1.00). Robotic technical success was 91.7%. Stent use, contrast use, and fluoroscopy time were similar between the groups, although procedure time was longer in the robotic-assisted PCI group [2••].
The results of CORA-PCI suggest that robotic-assisted PCI can be an effective and safe initial strategy for an all-comer population with the caveat that manual assistance or conversion may be required. The lesions studied in CORA-PCI were certainly more complex than in PRECISE, with almost 70% of robotically treated PCIs categorized as ACC/AHA class C lesions. Furthermore, the mean lesion length was longer at 22.2 mm (compared with 12.2 mm in the PRECISE population). However, 20% of the robotic procedures required either partial manual assistance or manual conversion. For this reason, any conclusion that robotic-assisted PCI can currently replace traditional PCI is premature. In addition, the highest complexity lesions, such as those with severe calcification requiring atherectomy, planned bifurcation stenting, and hybrid chronic total occlusions, were excluded from analysis. These characteristics were represented in 24% of the manual PCI group. Almost 90% of the procedures were performed via femoral arterial access. Finally, since this was not a randomized controlled trial, bias could be a potential confounder since there were no selection criteria for allocating specific cases for robotic PCI, although baseline characteristics were similar between robotic and manual PCI groups [2••]. CORA-PCI shows that robotic-assisted PCI can be effective and safe for more complex coronary lesions via the femoral arterial approach. The results of the trial cannot be extrapolated to the highest complexity lesions that were excluded, and operators should be prepared to intervene manually if needed. The second-generation CorPath GRX system has an added feature of active guide control, which reduced the need for partial manual assistance or manual conversion. As robotic systems evolve to accommodate over-the-wire crossing and atherectomy devices, potential exists for utilization of this technology in the highest of complex lesions.
Limitations
Robotic-assisted PCI currently has limitations that have prevented it from being used widely. First, cost-effectiveness is still unclear, although the benefits of robotic PCI that cannot be easily quantitated such as reduction of operator radiation exposure and orthopedic injuries need to be considered. Second, current generation systems are incompatible with atherectomy devices, guide extension catheters, microcatheters, or 0.018 or 0.035-in guidewires. However, newer systems continue to make advancements as exemplified by the recent CorPath GRX release allowing for active control of the guide catheter. Third, robotic PCI has no tactile feedback and therefore requires an initial learning curve for proficiency of use. Fourth, cases still require the use of an assistant operator at the table to exchange equipment, to inject contrast, and to deploy balloons and stents. Thus, while the primary operator receives the benefit of reduced radiation behind the cockpit and reduced lead-wearing time, support staff will not yet receive these benefits.
Future directions
As robotic PCI technology continues to improve, a wider variety of applications for cardiac catheterization lab procedures is expected. Even with current technology, operators have demonstrated the potential of robotic-assisted PCI for more difficult lesion subsets. Successful robotic-assisted PCI for unprotected left mains, saphenous vein grafts, and acute ST elevation myocardial infarction (STEMI) lesions have been reported [1, 30]. The authors’ centers’ experience with the robotic CorPath GRX system has included successful use for intravascular ultrasound, embolic filter protection device deployment and retrieval during saphenous vein graft intervention, and diagnostic angiography via radial approach [31].
Future applications of robotic systems to peripheral arterial interventions are promising. The RAPID (Robotic-Assisted Peripheral Intervention for peripheral arterial Disease) trial was a single-arm, single-center, open-label, non-randomized study of robotic-assisted peripheral vascular intervention using the CorPath 200 system. This study enrolled 20 subjects with symptomatic femoropopliteal artery disease. Mean lesion length was 33.1 mm with the majority having either mild or moderate calcification. All provisional stenting, per protocol, was done manually and not considered a failure of the robotic system. Clinical procedural success, defined as < 50% residual stenosis without an unplanned switch to manual assistance or device-related serious adverse event in the periprocedural period, was achieved in all patients. The results of this study will need to be replicated with a larger sample size and more complex, calcified lesions [32•]. Results of the RAPID II trial, in which femoropopliteal artery lesions were treated with robotic-assisted drug-coated balloons for a complete robotic procedure, are currently pending release [33•, 34]. Operators have begun to successfully treat below-the-knee peripheral arterial disease robotically without manual assistance, and further improvements in robotic technology will continue to expand use in peripheral interventions [35].
Telestenting, in which robotic-assisted PCI is performed on a patient in an entirely separate location, is a viable future concept for the field of robotics. Telestenting can have numerous applications to overcome barriers in access to care for patients requiring coronary interventions in under-served regions. STEMI patients presenting to hospitals lacking immediate PCI capabilities can instead undergo telestenting after an invasive (non-interventional) cardiologist obtains the coronary angiography and remains at the tableside, thus decreasing delays in treatment or use of systemic thrombolytic therapy. One interventional cardiologist can also provide telestenting to multiple remote sites [3•]. Options for a back-up remote operator to assist with a challenging case or proctor remotely are additional benefits. To explore the feasibility of telestenting, the REMOTE-PCI study was conducted. This trial was a single-center, prospective, observational study in which the interventional cardiologist performed robotic-assisted PCI in a cockpit located in a separate room. Telecommunication devices via Wi-Fi and video cameras allowed both audio and visual contact with the patient and staff in catheterization laboratory. Twenty-two lesions in 20 patients were treated with a robotic technical success rate of 86% (19 of 22 lesions) [36••].
Conclusion
Robotic-assisted PCI is a current treatment option for patients with ischemic heart disease and provides benefits to both operators and patients. The current robotic system benefits the primary operator by nearly eliminating ionizing radiation exposure during critical aspects of a PCI procedure and reduces the orthopedic strain imposed by heavy lead aprons. Patient benefits include precise robotic measurements of lesion length for appropriate stent size selection and fine control movements of devices for optimal positioning. Clinical trial data in robotic PCI to date, albeit with limitations, supports the safety and feasibility of use. The ongoing PRECISION GRX Registry will gather longer term data on patient outcomes, economic benefits, and detailed procedural parameters [37]. As robotic technology evolves, adaptation to a broader range of complex procedures and reduced cost will enhance utilization of this novel technology.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kapur V, Smilowitz NR, Weisz G. Complex robotic-enhanced percutaneous coronary intervention. Catheter Cardiovasc Interv. 2014;83:915–21.
•• Mahmud E, Naghi J, Ang L, et al. Demonstration of the safety and feasibility of robotically assisted percutaneous coronary intervention in complex coronary lesions: results of the CORA-PCI study (Complex Robotically Assisted Percutaneous Coronary Intervention). J Am Coll Cardiol Intv. 2017;10:1320–7. Important study evaluating the safety, feasibility, clinical success, and technical success of CorPath 200 in an all-comer population at a single center.
• Maor E, Eleid MF, Gulati R, et al. Current and future use of robotic devices to perform percutaneous coronary interventions: a review. J Am Heart Assoc. 2017;6:e006239. A comprehensive review of robotic-assisted PCI.
Andreassi MG, Piccaluga E, Guagliumi G, et al. Occupational health risks in cardiac catheterization laboratory workers. Circ Cardiovasc Interv. 2016;9:e003273.
Picano E, Vano E. Radiation exposure as an occupational hazard. EuroIntervention. 2012;8:649–53.
Vano E, Gonzalez L, Fernandez JM, et al. Occupational radiation doses in interventional cardiology: a 15-year follow-up. Br J Radiol. 2006;79:383–8.
Vano E, Kleiman NJ, Duran A, et al. Radiation-associated lens opacities in catheterization personnel: results of a survey and direct assessments. J Vasc Interv Radiol. 2013;24:197–204.
Karatasakis A, Brilakis HS, Danek BA, et al. Radiation-associated lens changes in the cardiac catheterization laboratory: results from the IC-CATARACT (CATaracts Attributed to Radiation in the CaTh lab) study. Catheter Cardiovasc Interv. 2017; https://doi.org/10.1002/ccd.27173.
Vano E, Kleiman NJ, Duran A, et al. Radiation cataract risk in interventional cardiology personnel. Radiat Res. 2010;174:490–5.
Ciraj-Bjelac O, Rehani MM, Sim KH, et al. Risk for radiation-induced cataract for staff in interventional cardiology: is there reason for concern? Catheter Cardiovasc Interv. 2010;76:826–34.
Ciraj-Bjelac O, Rehnani MM, Minamoto A, et al. Radiation-induced eye lens changes and risk for cataract in interventional cardiology. Cardiology. 2012;123:168–71.
Klein LW, Miller DL, Balter S, et al. occupational health hazards in the interventional laboratory: time for a safer environment. J Vasc Inter Radiol. 2009;20:S278–83.
Smilowitz NR, Balter S, Weisz G. Occupational hazards of interventional cardiology. CRM. 2013;14:223–8.
Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64.
Hasan F, Bonatti J. Robotically assisted percutaneous coronary intervention: benefits to the patient and the cardiologist. Expert Rev Cardiovasc Ther. 2015;13(11):1165–8.
Andreassi MG, Cioppa A, Botto N, et al. Somatic DNA damage in interventional cardiologists: a case-control study. FASEB J. 2005;19:998–9.
Roguin A, Goldstein J, Bar O, et al. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111:1368–72.
Vano E, Gonzalez L, Guibelalde E, et al. Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol. 1998;71:954–60.
•• Weisz G, Metzger C, Caputo RP, et al. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) study. J Am Coll Cardiol. 2013;61:1596–600. First pivotal trial assessing the safety, technical success, and clinical success of CorPath 200 in simple coronary lesions.
Khalil TM, Rosomoff HL. Ergonomics. Back pain: a guide to prevention and rehabilitation. New York: Van Nostrand Reinhold; 1993.
Goldstein JA, Balter S, Cowley M, et al. Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 2004;63:407–11.
Ross AM, Segal J, Borenstein D, et al. Prevalence of spinal disc disease among interventional cardiologists. Am J Cardiol. 1997;79:68–70.
Campbell PT, Mahmud E, Marshall JJ. Interoperator and intraoperator (in)accuracy of stent selection based on visual estimation. Catheter Cardiovasc Interv. 2015;86(7):1777–83.
Costa MA, Angiolilo DJ, Tannenbaum M, et al. Impact of stent deployment procedural factors on long-term effectivenss and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol. 2008;101:1704–11.
Raber L, Juni P, Wandel S, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55(12):1178–88.
•• Campbell PT, Kruse KR, Kroll CR, et al. The impact of precise robotic lesion length measurement on stent length selection: ramifications for stent savings. CRM. 2015;16:348–50. Study that compared robotic-assisted PCI measurement of lesion length and stent length selection to visual estimation, and the implications on stent savings.
Mauri L, O’Malley AJ, Cutlip DE, et al. Effects of stent length and lesion length of coronary restenosis. Am J Cardiol. 2004;93:1340–6.
Mauri L, O’Malley AJ, Popma JJ, et al. Comparison of thrombosis and restenosis risk from stent length of sirolimus-eluting stents versus bare metal stents. Am J Cardiol. 2005;95:1140–5.
Pourdjabber A, Ang L, Reeves RR, et al. The development of robotic technology in cardiac and vascular interventions. Rambam Maimonides Med J. 2017;8(3):e0030.
Mahmud E, Dominguez A, Bahadorani J. First-in-human robotic percutaneous coronary intervention for unprotected left main stenosis. Catheter Cardiovasc Interv. 2016;88:565–70.
Swaminathan RV, Rao SV. Robotic-assisted transradial diagnostic coronary angiography. Catheter Cardiovasc Interv. 2018; https://doi.org/10.1002/ccd.27480.
• Mahmud E, Schmid F, Kalmer P, et al. Feasibility and safety of robotic peripheral vascular interventions: results of the RAPID trial. J Am Coll Cardiol Intv. 2016;9:2058–64. Small study of 20 patients demonstrating the safety and feasibility of CorPath 200 in femoropopliteal diesease.
• Mangels DR, Giri J, Hirshfeld J, et al. Robotic-assisted percutaneous coronary intervention. Catheter Cardiovasc Interv. 2017;90(6):948–55. Review of robotic-assisted PCI and limitations.
Brodmann M. Robotic-assisted peripheral intervention for peripheral arterial disease II (RAPID II). https://clinicaltrials.gov/ct2/show/NCT02742077. Last updated: December 16, 2016.
Behnamfar O, Pourdjabbar A, Yalvac E, et al. First case of robotic percutaneous vascular intervention for below-the-knee peripheral arterial disease. J Invasive Cardiol. 2016;28(11):E128–31.
•• Madder RD, VanOosterhout SM, Jacoby ME, et al. Percutaneous coronary intervention using a combination of robotics and telecommunications by an operator in a separate physical location from the patient: an early exploration into the feasibility of telestenting (the REMOTE-PCI study). Eurointervention. 2017;12:1569–76. Thought-provoking trial introducing the concept of robotic-assisted PCI for telestenting.
Mahmud, E. Precision GRX Registry. https://clinicaltrials.gov/ct2/show/record/NCT03278301. Last updated: September 11, 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Coronary Artery Disease
Rights and permissions
About this article
Cite this article
Lo, N., Gutierrez, J.A. & Swaminathan, R.V. Robotic-Assisted Percutaneous Coronary Intervention. Curr Treat Options Cardio Med 20, 14 (2018). https://doi.org/10.1007/s11936-018-0608-0
Published:
DOI: https://doi.org/10.1007/s11936-018-0608-0