Opinion statement
Thoracic radiation remains an effective treatment for many types of neoplasms. The clinical benefit of radiation therapy on cancer mortality is counterbalanced by an increased risk of cardiovascular events in survivors. The long-term cardiovascular sequelae of thoracic radiation include premature coronary artery disease, valvular disease, pericardial disease, myocardial disease with systolic and especially diastolic dysfunction, and conduction system abnormalities. Radiation heart disease progresses over time and may manifest decades after the initial exposure. Since the risk of cardiac complications is significantly increased following chest irradiation, appropriate screening and long-term cardiac follow-up of these patients is essential. This article will summarize the pathophysiological features, clinical presentations, and current recommendations for screening and prevention of the wide spectrum of radiation induced cardiovascular disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mediastinal radiation therapy (RT) is commonly a part of treatment protocols for many types of neoplasms, including breast cancer, Hodgkin lymphoma and other mediastinal tumors. RT can increase the risk of complications in the decades following initial treatment with secondary malignant neoplasms and cardiovascular disease being the most common [1].
Radiation-induced heart disease (RIHD) is a serious side effect of thoracic RT and is associated with significant morbidity and mortality.
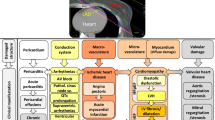
The possibility of cardiac radio-sensitivity was initially described by Pearson in 1957 when he postulated that intimal proliferation after chest wall radiation might have caused a myocardial infarction in two patients with breast cancer [2]. There is a wide spectrum of cardiac manifestations, which can range from acute to chronic and can involve the pericardium, myocardium, coronary arteries, heart valves, and conduction system (Table 1). The most important factors affecting the risk of subsequent cardiovascular damage after radiotherapy include [3]:
-
Total radiation dose and dose per fraction, especially doses >35 Gray (Gy)
-
Adjuvant cardiotoxic chemotherapeutic regimens, such as anthracyclines and cisplatin
-
Irradiated tissue volume and fraction size
-
Presence of other cardiac risk factors, especially hypertension and smoking.
These risks primarily involve patients treated at a younger age with older approaches using two-dimensional (2D) treatment planning and high-dose and wide-field RT. Contemporary RT using low-dose radiation with improved targeting and 3D treatment planning have led to a reduction in normal tissue radiation and a significant decline in the rates of RIHD [4]. Long-term follow-up studies are needed to confirm this observation. Nevertheless, it is important to note that there is no minimum radiation dose that is considered safe.
Pathophysiology of radiation-induced cardiovascular disease
Vascular injury and endothelial dysfunction due to increased expression of adhesion molecules and cytokines appear to be the primary mechanism in the pathogenesis of normal tissue radiation injury [5, 6]. Endothelial dysfunction contributes to pro-fibrotic and pro-inflammatory environments with secondary changes that lead to fibrosis [5, 7].
These changes can lead to the following cardiovascular manifestations:
Coronary artery disease (CAD)
In irradiated arteries, the expression of various inflammatory cytokines and adhesion molecules leads to adhesion and migration of monocytes into the sub-endothelial space. These monocytes may transform into activated macrophages and with ingestion of lipids subsequently form fatty streaks in the intima, thereby initiating or accelerating atherosclerosis [8].
CAD may be the most significant cause of mortality from RIHD. In an analysis of 7,033 Hodgkin lymphoma survivors, the risk of death from myocardial infarction was increased significantly compared to the general population (standardized mortality ratio [SMR] = 2.5, 95 % CI 2.1–2.9), and was limited to those patients who had received thoracic radiation with or without anthracycline therapy [9]. Similarly, in a large meta-analysis of 20,000 women enrolled in 40 randomized trials of patients with breast cancer, RT increased non-breast cancer related mortality, primarily due to an excess number of deaths from vascular causes (annual event rate ratio: 1.27, p = 0.0001) [10]. Another randomized trial comparing pre- or post-operative RT to surgery alone in 960 patients with breast cancer showed an increase in mortality from CAD in women treated with radiation (hazard ratio [HR]: 3.2, p < 0.05) [11].
The risk of radiation-induced CAD is directly related to the mean dose received by the heart. In a case control study of 2,168 women who were treated for breast cancer with surgery and RT the risk of a major coronary event increased linearly by 7.4 % per Gy of radiation to the heart, with no apparent threshold [12•]. In patients with early stage breast cancer treated with breast conservation surgery, left-sided radiation was associated with a 4.6-fold higher risk of developing CAD compared to right-sided RT [13]. Furthermore, involvement of the internal mammary nodes in the radiation field was associated with a higher risk of CAD (18 % vs. 7 %, p < 0.001), regardless of laterality [13].
The risk of RIHD increases with time from treatment. In a retrospective study of 415 patients treated with RT for Hodgkin lymphoma, the median time for development of CAD was 9 years [14]. Early studies in survivors of breast cancer showed that the incidence of CAD only increases 10 years after RT, with a higher rate of cardiac deaths in patients receiving left- compared to right-sided RT (6.4 % vs. 3.6 % at 20 years) [13]. More recently, however, a case control study of 2,168 women who were treated for breast cancer with surgery and RT showed that the increased risk of major coronary events occurs much earlier than previously shown and extends from 5 to 20 years after RT [12•].
The clinical presentation and diagnosis of radiation-induced CAD is similar to that of non-radiation atherosclerotic disease. Necropsy studies have shown however that radiation induced coronary obstruction more frequently involves the coronary ostia and proximal segments of the coronary arteries that are in the radiation field rather than distal segments [15, 16]. Given the increased risk of RIHD, the Children’s Oncology Group Survivorship Guidelines recommend an annual visit with attention to cardiac symptoms, evaluation of cardiac risk factors including lipids and fasting glucose every 2 years, and consideration of a baseline stress test 5 to 10 years from RT in all patients who received ≥40 Gy of chest RT alone or ≥30 Gy of chest RT in combination with anthracyclines [17•].
There are no trials that have addressed treatment options for primary or secondary prevention in this select patient group. Treatment remains the same as for non-radiation atherosclerotic disease and includes medical therapy, percutaneous coronary angioplasty, and coronary artery bypass graft surgery depending on the nature and extent of disease. However, surgical revascularization can be challenging in these patients.
In a cohort study of 230 patients undergoing cardiac surgery after mediastinal radiation, patients receiving extensive radiation exposure (such as for Hodgkin disease, thymoma, or testicular cancer) had a significantly decreased 1-year survival compared to those receiving tangential radiation (such as for breast cancer)(78 % vs. 88 %), in whom survival approximated that for the general population [18]. Involvement of the lungs in the radiation field leads to respiratory dysfunction manifesting as pneumonia, intractable pleural effusions, and re-intubation secondary to poor ventilatory mechanics, thus leading to increased post-operative morbidity and mortality [18]. Furthermore, biventricular dysfunction secondary to radiation induced diastolic heart failure may also contribute to adverse outcomes in these patients [18]. Thus, careful pre-operative assessment of pulmonary function should be performed in order to risk stratify RT patients appropriately.
For coronary artery bypass grafting, using left internal mammary artery (LIMA) grafting, while preferable, can be limited by radiation induced damage to the artery itself. In a cohort study of 138 patients with coronary artery bypass grafting after mediastinal radiation, the internal thoracic artery graft patency was less than expected and similar to that for venous or radial grafts (2-year stenosis rate of 32 % vs. 27 %, p = 0.72) [19]. Despite this, late survival was superior among patients receiving a LIMA graft to the left anterior descending artery [19]. Therefore, the LIMA should be assessed angiographically prior to surgery, and used when acceptable, in these patients.
Carotid artery disease
Cranial, neck, and mantle radiation affect the intracerebral vasculature, carotid arteries and cardiac valves, and accordingly have been associated with higher risk of stroke.
Irradiation of the cerebral and neck arteries elicits an acute phase of endothelial damage with nuclear disruption, platelet aggregation, and fibrin deposition. This is followed by medial degeneration and adventitial thickening and fibrosis leading to narrowing of the vessel lumen and occlusive vasculopathy [20]. RT for intracranial tumors can also lead to other radiation-induced vascular abnormalities such as venous-based cavernous malformations, aneurysms, telangectasias and a moyamoya-like vasculopathy in addition to occlusive disease [21].
A study of ≥5-year survivors of childhood leukemia and brain tumors showed that the relative risk (RR) of stroke was 6.4 (95 % CI 3.0–13.8) for leukemia survivors and 29.0 (95 % CI 13.8–60.6) for brain tumor survivors, relative to healthy siblings [22]. Similarly, in 331 adults with pituitary tumors, cranial RT increased the risk of stroke (RR 4.1 compared to a matched population) in a dose-dependent manner, with the greatest risk occurring at doses >50 Gy [23]. In patients with head and neck squamous cell carcinomas, the RR of stroke with neck RT was 1.8 (95 % CI 1.22–2.56) in patients <55 years of age, but was not increased in older individuals [24] This is important, since the rate of head and neck cancers, especially in younger men, has grown substantially over the last three decades due to its association with oral human papilloma virus. A cohort study comparing 1,387 childhood cancer survivors treated with mantle radiation for Hodgkin disease to healthy siblings showed that the RR of stroke was 5.6 (95 % CI, 2.59–12.25) with a median time to presentation of 17.5 years [22].
Radiation-induced carotid lesions are more extensive and often involve longer segments of the artery than traditional atherosclerotic carotid disease which usually involves bifurcation sites [25]. Like radiation-induced coronary artery disease, the risk of cerebrovascular disease increases with dose and time from treatment. In a retrospective study of 224 asymptomatic patients treated with RT for head and neck cancer who underwent screening carotid ultrasound, the HR for significant carotid artery stenosis was 1.4 for every 10-Gy increase in mean RT dose to the carotid bulb + 2 cm [26]. The median time to presentation varies with the type of tumor with estimates of up to 14 % of patients developing significant carotid artery stenosis 4 years from RT for head and neck cancer [26].
The diagnosis and presentation of radiation-induced carotid disease is similar to the general population. The survivorship guidelines recommend an annual history and physical examination focused on carotid disease and a screening carotid ultrasound 10 years after RT in all cancer survivors who have received head and neck radiation ≥40 Gy [17•].
Once again, there are no specific recommendations for the treatment of these patients. Surgical carotid endarterectomy and carotid artery stenting are both reasonable alternatives when intervention is deemed necessary for significant post-radiation carotid stenosis. In a meta-analysis of 21 studies involving 661 patients with post-radiotherapy carotid stenosis, there was no statistically significant difference in 30-day stroke or death rates between surgical revascularization and carotid artery stenting (odds ratio [OR], 0.54; 95 % CI 0.17–1.70) [27]. However, endarterectomy is confounded by the need to operate through scarred tissue planes and carries a higher risk of wound complications and cranial nerve injury while stenting carries an increased risk of restenosis [27]. Therefore, treatment decisions have to be individualized for each patient.
Pericardial disease
Historically pericardial disease has been considered one of the most common manifestations of RIHD. In a necropsy study of RIHD, 70 % of cases had radiation-related pericardial disease [14].
Varying degrees of pericardial fibrosis can be present secondary to RT. Fibrotic changes more severely involve the parietal pericardium with fibrosis replacing the adipose tissue [28, 29].
The pathological pericardial changes can present as acute pericarditis, pericardial effusion and delayed pericarditis in the form of constriction. Acute pericarditis is a rare adverse effect and is seen primarily in patients treated with high-dose RT for Hodgkin lymphoma [30]. Because of the benign course of acute pericarditis and fear of recurrence of the primary neoplasm, treatment of the malignancy is usually not withheld. Delayed pericarditis can present anywhere from months to years after RT. Most commonly, it presents within 4–26 months in the form of pericardial effusion usually manifesting as an enlarged cardiac silhouette. Small, hemodynamically insignificant pericardial effusions seen on routine echocardiogram do not need active intervention and can be followed up routinely.
Constrictive pericarditis is a delayed form of RIHD, which may present years after radiation exposure in the form of congestive heart failure. In rare cases, when patients are not responsive to medical therapy, pericardial stripping may be considered. However, the overall impact of radiation on cardiac structures, including premature coronary artery disease, valvular abnormalities, and myocardial fibrosis, are likely to have an adverse effect on perioperative mortality and overall survival, irrespective of constriction. In addition, mediastinal fibrosis from prior radiation exposure may also complicate the surgery. In a single center study of 163 patients who had undergone pericardial stripping for constrictive pericarditis of different etiologies, post-radiation constrictive pericarditis had the worst prognosis (7-year Kaplan–Meier survival: 27 %, 95 % CI 9–58 %) [31]. Therefore, careful attention should be given to other cardiopulmonary abnormalities and surgical stripping should be reserved for severely symptomatic patients who are not responsive to medical therapy.
Cardiomyopathy
Thoracic radiation can cause both systolic and diastolic dysfunction. Radiation-induced cardiomyopathy can present as a dilated or restrictive phenotype. Overall, RT induced cardiomyopathy presents more commonly as a restrictive cardiomyopathy and left ventricular systolic dysfunction is usually seen when RT is co-administered with chemotherapeutic agents such as anthracyclines [32].
Pathologically, most patients with myocardial involvement have non-specific, diffuse interstitial fibrosis. In an autopsy study of 27 hearts, the most common pattern of fibrosis was pericellular and perivascular fibrosis [15]. There is an increase in type I collagen compared to type III resulting in altered elastic properties of the myocardium and decreased diastolic relaxation [29].
For children treated with mediastinal RT before the age of 5 years, the survivorship guidelines recommend a screening echocardiogram every 2 years in the absence of, and every year in the presence of, anthracycline-based chemotherapy [17•]. For survivors treated with RT but no anthracyclines after the age of 5 years, a screening echocardiogram is recommended every 5 years for exposure <30 Gy and every 2 years if ≥30 Gy [17•]. For survivors treated with any RT dose after the age of 5 years, an echocardiogram is recommended every 2 years for those receiving <300 mg/m2 and every year for those receiving ≥300 mg/m2 of anthracyclines [17•].
Treatment of radiotherapy induced cardiomyopathy is similar to other forms of cardiomyopathy with a focus on symptomatic management. Heart transplantation may represent the only treatment option for patients with end-stage RIHD, but outcomes tend to be poor [33]. In a retrospective, single-center study of nine patients with RIHD who underwent cardiac transplantation, three died in the peri-operative period, and while five patients were still alive at a mean follow-up of 10 ± 8 years, three had developed secondary malignancies [33].
Valvular disease
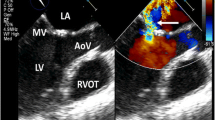
Mediastinal radiation frequently causes valvular heart disease. In a postmortem study, radiation-related valvular disease was identified in 71 % of patients in whom valves were available [15]. In this study, the aortic valve was most commonly affected, followed by the mitral and tricuspid valves, respectively. More than one valve can be involved and valvular disease often occurs in conjunction with other cardiac complications [34]. The spectrum of radiation-induced valvular disease can range from endocardial thickening without functional abnormality, to asymptomatic valvular dysfunction, and eventually to symptomatic valvular stenosis or regurgitation. Asymptomatic thickening is the most common manifestation of radiation-induced valvular disease. When significant, combined aortic regurgitation and stenosis are the most common findings, with mitral regurgitation following in incidence [35].
Surgical management can be challenging due to similar concerns as those for coronary artery bypass surgery (as mentioned above) [18]. With the advent of new technologies such as transcatheter aortic valve replacement and Mitraclip, non-surgical approaches in patients with isolated valve disease may be considered in selected patients.
Conduction system abnormalities
Electrocardiographic changes after irradiation range from nonspecific ST-T changes to QT interval prolongation, low voltage, right bundle branch block (RBBB) and sinus tachycardia [36]. The true incidence of radiation-induced rhythm abnormalities is unknown. Inappropriate and persistent sinus tachycardia similar to that seen in denervated transplanted hearts can occur [37]. Conduction system abnormalities, albeit rare, can be serious sequelae of RT. Injury to the conduction system can occur either directly from radiation or indirectly from associated myocardial fibrosis and ischemia. RBBB is the most common conduction abnormality seen with mediastinal irradiation because of the close proximity of the right bundle to the endocardium on the right side [38, 39]. Complete heart block is a serious manifestation that can be a delayed presentation of RIHD, necessitating pacemaker implantation [40].
Prevention and screening
While there is a growing appreciation of the long-term cardiovascular sequelae of RT, little is known about how to prevent and treat these complications. Certain measures can be taken to reduce incidental cardiac irradiation and protect patients from RIHD. These include:
-
Avoiding radiation at a young age, if possible
-
Reducing overall radiation dosage
-
Decreasing the heart volume being irradiated
-
Using proper shielding of the heart
-
Avoidance of concurrent cardiotoxic agents, such as anthracyclines
Newer techniques such as involved field RT are associated with decreased cardiac irradiation and thus presumably with reduced toxicity [41]. Longitudinal studies of patients treated with these newer modalities will be needed to identify those techniques that maximize cancer survival while minimizing toxicity.
Radiation-induced cardiovascular risk increases with time from treatment and requires long-term follow-up. The Children’s Oncology Group has published general guidelines for the long-term evaluation of cardiovascular complications in survivors of childhood cancers (survivorshipguidelines.org) [17•]. However, these guidelines have not been validated in a prospective manner.
In general, traditional cardiac risk factors, especially smoking, increase the risk of RIHD [13]. It has therefore been proposed that routine screening and modification of traditional risk factors such as smoking, hyperlipidemia, diabetes, and hypertension can help reduce the risk of RIHD (Table 2).
The guidelines recommend screening patients for ischemic heart disease 5–10 years from radiation, independent of symptoms (Table 2). However, the most appropriate imaging tool to screen for radiation-induced coronary artery disease is unknown. Exercise-treadmill testing, stress echocardiography, nuclear myocardial perfusion imaging, CT calcium scores, and coronary CT angiography (CCTA) have all been used as screening modalities. In a study comparing several screening tests including exercise treadmill testing, stress echocardiography, and myocardial perfusion imaging, stress-induced perfusion defects were the least specific for significant coronary disease: 55 % of patients with positive studies had no coronary stenosis exceeding 50 % on angiography [42]. Each imaging modality has its own inherent limitations. Hence, patient-related factors such as contrast allergy and renal function as well as center availability and local expertise should determine the choice of modality.
Cardiac auscultation for detection of murmurs is a simple first line screening method to detect asymptomatic valvular dysfunction. However, the diagnostic yield of cardiac auscultation is poor. Transthoracic echocardiography is a safe and cost effective imaging tool for the detection of radiation-induced valvular heart disease. It also permits evaluation for asymptomatic Stage B heart disease and therefore it is recommended that screening echocardiography be performed periodically, based on the radiation exposure and/or anthracycline therapy. The exact interval at which these surveillance echocardiograms should be performed remains a matter of significant debate and controversy.
As previously discussed, history and physical examination of cancer survivors who have received head and neck radiation should include assessment for possible symptoms and signs of carotid artery disease.
Carotid ultrasound with Doppler is the imaging modality of choice for screening of patients who have carotid bruits or signs or symptoms suggestive of stroke or transient ischemic attack (TIA).
Conclusion
There is a clear link between thoracic radiation and incidence of cardiovascular disease. Newer and improved radiation techniques and targeted therapy along with cardiovascular risk factor modification have substantially reduced the risk of cardiovascular complications. However, the exact magnitude of this reduction is unknown. Long-term data on the cardiac toxicity associated with treatment approaches in the modern era is needed. Since cardiac complications may appear years following incidental irradiation of the heart, screening and long-term cardiac follow-up of these patients is essential. However, studies evaluating the appropriate testing intervals and the sensitivity and specificity of different imaging modalities need to be conducted in order to monitor these patients in an effective manner.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Andratschke N, Maurer J, Molls M, et al. Late radiation-induced heart disease after radiotherapy. Clinical importance, radiobiological mechanisms and strategies of prevention. Radiother Oncol. 2011;100:160–6.
Pearson HES. Coronary occlusion following thoracic radiotherapy; 2 cases. Proc Soc Med. 1957;60:516.
Haybittle JL, Brinkley D, Houghton J, et al. Postoperative radiotherapy and late mortality: evidence from the Cancer Research Campaign trial for early breast cancer. BMJ. 1989;298:1611–4.
Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65.
Wang J, Boerma M, Fu Q, et al. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13(22):3047–55.
Hopewell JW, Calvo W, Jaenke R, et al. Microvasculature and radiation damage. Recent Results Cancer Res. 1993;130:1–16.
Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound’. Radiother Oncol. 2002;63(2):129–45.
Tribble DL, Barcellos-Hoff MH, Chu BM, et al. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol. 1999;19:1387–92.
Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99(3):206–14.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106.
Rutqvist LE, Lax I, Fornander T, et al. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys. 1992;22:887–96.
Darby S, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. A population based case‐control study of major coronary events in 2168 women treated with radiotherapy for breast cancer between 1958 and 2001 in Denmark and Sweden. The study shows that the risk of major coronary events increases linearly with the mean dose of cardiac radiation and occurs 5 to 20 years after radiotherapy.
Harris E, Correa C, Hwang WT. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–6.
Hull M, Morris C, Pepine C, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290(21):2831–7.
Veinot J, Edwards W. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27(8):766–73.
Brosius FC, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70(3):519–30.
Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, version 3.0. Children's Oncology Group, Arcadia, CA (October 2008) www.survivorshipguidelines.org. Consensus based guidelines that provide recommendations for screening and management of potential late toxicities of cancer treatment in survivors of pediatric malignancies.
Chang AS, Smedira NG, Chang CL, et al. Cardiac surgery after mediastinal radiation: extent of exposure influences outcome. J Thorac Cardiovasc Surg. 2007;133(2):404–13.
Brown ML, Schaff HV, Sundt TM, et al. Conduit choice for coronary artery bypass grafting after mediastinal radiation. J Thorac Cardiovasc Surg. 2008;136(5):1167–71.
Fonkalsrud EW, Sanchez M, Zerubavel R, et al. Serial changes in arterial structure following radiation therapy. Surg Gynecol Obstet. 1977;145(3):395–400.
Partap S. Stroke and cerebrovascular complications in childhood cancer survivors. Semin Pediatr Neurol. 2012;19(1):18–24.
Bowers DC, McNeil DE, Liu Y, et al. Stroke as a late treatment effect of Hodgkin's disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(27):6508–15.
Brada M, Burchell L, Ashley S, et al. The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys. 1999;45(3):693–8.
Huang YS, Lee CC, Chang TS, et al. Increased risk of stroke in young head and neck cancer patients treated with radiotherapy or chemotherapy. Oral Oncol. 2011;47(11):1092–7.
Cheng SW, Ting AC, Lam LK, et al. Carotid stenosis after radio therapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126(4):517–21.
Dorth JA, Patel PR, Broadwater G, et al. Incidence and risk factors of significant carotid artery stenosis in asymptomatic survivors of head and neck cancer after radiotherapy. Head Neck. 2013 Apr 2. doi:10.1002/hed.23280. [Epub ahead of print].
Kasivisvanathan V, Thapar A, Davies KJ, et al. Periprocedural outcomes after surgical revascularization and stenting for postradiotherapy carotid stenosis. J Vasc Surg. 2012;56(4):1143–52.
Rodemann P, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995;35(2):83–90.
Chello M, Mastroroberto P, Romano R, et al. Changes in the proportion of types I and III collagen in the left ventricular wall of patients with post-irradiative pericarditis. Cardiovasc Surg. 1996;4(2):222–6.
Morton DL, Glancy DL, Joseph WL, et al. Management of patients with radiation induced pericarditis with effusion: a note on the development of aortic regurgitation in two of them. Chest. 1973;64(3):291–7.
Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43(8):1445–52.
Kinsella TJ, Ahmann DL, Giuliani ER, et al. Adriamycin toxicity in stage IV breast cancer: possible enhancement with prior left chest radiation therapy. Int J Radiat Oncol Biol Phys. 1979;5:1979.
Uriel N, Vainrib A, Jorde UP, et al. Mediastinal radiation and adverse outcomes after heart transplantation. J Heart Lung Transplant. 2010;29(3):378–81.
Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55(6):1145–52.
Warda M, Khan A, Massumi A, et al. Radiation-induced valvular dysfunction. J Am Coll Cardiol. 1983;2:180–5.
Wehr M, Rosskopf BG, Schwenk D, Prignitz R. An electrocardiographic study of cardiac damage following post-operative radiation in patients with mammary carcinoma on the left (author's transl). Z Kardiol. 1982;71(2):112–8. German.
Hurst's the Heart. Valentine fuster. 13th ed; 2010.
Adams MJ, Lipshultz SE, Schwartz C, et al. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol. 2003;13(3):346–56.
Totterman KJ, Pesonen E, Siltanen P, et al. Radiation related chronic heart disease. Chest. 1983;83(6):875–8.
Nakao T, Kanaya H, Namura M, et al. Complete atrioventricular block following radiation therapy for malignant thymoma. Japan J Med. 1990;29(1):104–10.
Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012;83(4):1232–7.
Lind PA, Pagnanelli R, Marks LB, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys. 2003;55:914–20.
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Negareh Mousavi and Dr. Anju Nohria reported no potential conflicts of interest relevant to this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousavi, N., Nohria, A. Radiation-Induced Cardiovascular Disease. Curr Treat Options Cardio Med 15, 507–517 (2013). https://doi.org/10.1007/s11936-013-0259-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-013-0259-0