Opinion statement
Coronary artery disease (CAD) is a major cause of heart failure with preserved ejection fraction (HFpEF). In studies of HFpEF, the reported prevalence of CAD varies widely, which may be the result of inconsistent definitions of CAD, geographic and ethnic differences in CAD burden, varying definitions of HFpEF (including different cutoffs for “preserved ejection fraction”), and differences in study design. Despite these limitations, pooled analysis of prospective HFpEF studies demonstrates that CAD is common in HFpEF, with an estimated prevalence of approximately 50%. Based on available data, patients with signs and symptoms of heart failure who have preserved left ventricular ejection fraction and evidence of CAD (HFpEF-CAD) most likely comprise a distinct etiologic and pathophysiologic subset of HFpEF. Therefore, future clinical trials in HFpEF should a priori stratify by CAD or specifically target patients with CAD, strategies that may improve the disappointing track record of therapies tested in HFpEF. The combination of systematic evaluation and management of CAD in HFpEF, along with promising future therapies for HFpEF-CAD, may lead to improved outcomes for this challenging clinical syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a challenging clinical entity [1•,2]. Previously underappreciated, it now is well known that HFpEF is common, costly, and deadly [3–6]. Development of successful therapies for patients with HFpEF has suffered from the heterogeneity of the HFpEF syndrome [1•,7•], controversy regarding its underlying pathophysiology [8–11], disagreement over whether it is truly a distinct clinical entity or simply part of a continuum with heart failure (HF) and reduced ejection fraction (EF; systolic heart failure) [8,12,13•,14–16,17•,18–23], and the presence of several HFpEF comorbidities that represent competing risks for morbidity and mortality [24•].
Our understanding of the relationship between coronary artery disease (CAD) and HFpEF also is incomplete, owing to the heterogeneous definitions of CAD used in prior pathophysiologic, epidemiologic, observational registry, and clinical trial studies of HFpEF [25], as well as differences in CAD prevalence based on geographic location and ethnic composition of the study sample. Emerging data suggest that HFpEF associated with CAD may be a distinct clinical subset and one that may be the future target of novel therapies. This review highlights these data and presents a framework for evaluating and managing CAD in patients with HFpEF.
Scope of the HFpEF problem
HFpEF, already a major public health problem and one of the most common causes of hospitalization for those older than 65 years, will continue to increase in prevalence as the population ages [5,26,27]. Treatment for HFpEF therefore is a high-priority unmet need. Epidemiologic studies have demonstrated a high mortality for patients after HF hospitalization, regardless of underlying EF and despite differences in comorbidities, age, gender, and cardiac structure and function [3,6]. More recent meta-analyses suggest that when data from several large studies, including clinical trials, are combined (whether using aggregate study data or individual patient-level data), patients with HFpEF have a better outcome than those with HF and reduced EF [28••,29]. However, these data should not be interpreted to mean that HFpEF patients are at low risk. Although these meta-analyses found that HFpEF carries a lower risk of death than HF with reduced EF, patients with HFpEF nonetheless still have a high mortality rate. Furthermore, other studies have shown that patients with HF, regardless of EF, have severely reduced exercise capacity and peak oxygen consumption on cardiopulmonary exercise testing [30], underscoring the significantly decreased quality of life that accompanies HFpEF.
In HFpEF, variation in outcomes among studies may be a result of differences in study design (eg, inpatient vs outpatient, clinical trials vs epidemiologic studies), underlying comorbidities, or geographic/ethnic differences. For example, clinical characteristics and outcomes may differ significantly depending on the proportion of patients in the study sample who are African American, in large part because there are known ethnic differences in the prevalence of obstructive CAD. Compared with Caucasians, African Americans have less obstructive epicardial coronary disease but more frequently have severe systemic hypertension and left ventricular (LV) hypertrophy [31–35]. Therefore, in geographic regions where the proportion of African Americans is higher, HFpEF-CAD may be less common than in other areas.
Where are patients with HFpEF? Who are patients with HFpEF?
Although HFpEF is common and quite prevalent, especially in the elderly population [3,6,36,37], identification of patients with HFpEF is far from straightforward. Nowhere is this more apparent than in clinical trials of HFpEF. Identifying patients with systolic HF is straightforward because a reduced EF is quite apparent on imaging modalities such as echocardiography and left ventriculography. HFpEF, however, suffers from lack of a “number,” such as reduced EF, to make the diagnosis readily apparent. Recent studies suggest that the product of left atrial volume and LV mass [38•] and the presence of elevated pulmonary artery systolic pressure on echocardiography [39•] may be important clues to the presence of HFpEF. Nonetheless, there is no one easy-to-use test to reliably diagnose HFpEF.
Besides the challenge of diagnosis, a variety of health care providers currently care for most patients with HFpEF. Although these patients comprise up to half the general HF population, they make up a much smaller percentage of those visiting HF specialty clinics, making enrollment of patients in HFpEF clinical trials quite challenging. Large HFpEF clinical trials, such as Irbesartan in Heart Failure With Preserved Systolic Function (I-PRESERVE) [40], have had difficulty identifying and enrolling patients, and have required large numbers of sites across the world to meet enrollment goals.
Beyond difficulties in identifying, diagnosing, and recognizing HFpEF, there also are significant differences between underlying etiology and pathophysiology among various study types [41]. HFpEF clinical trials often enroll younger, more commonly male, less ethnically diverse patients with relatively preserved renal function [42,43]. Epidemiologic studies and observational registries, on the other hand, tend to encompass a broader HFpEF population that includes more women and elderly patients, is more ethnically diverse, and includes those with worse renal function [3,6,36,37]. Small pathophysiologic studies, although key to understanding the HFpEF syndrome, have notoriously studied homogenous subsets of patients with HFpEF and commonly have excluded patients with CAD [8,30,44,45]. Omission of patients with CAD from some pathophysiologic studies may be one of the critical factors underlying the controversy regarding mechanisms of HFpEF. For example, some investigators have argued that patients with HFpEF, despite a normal EF, have reductions in systolic function, noting that HFpEF patients have lower EF than matched controls and have reductions in longitudinal systolic tissue Doppler velocities [12,14,19,20,46,47]. In these studies, patients with CAD were not excluded and comprised significant proportions of the study groups (Fig. 1a). Meanwhile, other investigators have argued that overall systolic function (especially chamber function) is similar when patients with HFpEF are compared with matched hypertensive controls [8,45]; however, these studies have excluded patients with significant CAD. Therefore, it is possible that in HFpEF, underlying systolic function differs based on the presence or absence of CAD, and that the presence of CAD has a major effect on the pathophysiologic substrate predisposing these patients to the HF syndrome.
Evidence for systolic dysfunction in patients with heart failure and preserved ejection fraction (HFpEF) associated with coronary artery disease (CAD). a Mitral annular systolic tissue Doppler velocities (S’): patients with HFpEF compared with control subjects. b Reduced preload recruitable stroke work (PRSW): a predictor of heart failure hospitalization in 885 patients with stable coronary artery disease and preserved ejection fraction (>50%). For panel B, univariate P < 0.0001 and multivariate P = 0.035 after adjustment for age, comorbidities, pulse pressure, renal function, and cardiac structure and function (including ejection fraction, left ventricular mass, and diastolic dysfunction).
How common is CAD in HFpEF?
Pooled analysis of prospective HFpEF studies suggests that CAD is present in approximately 50% of patients with HFpEF, but definitions of CAD are quite variable and often poorly documented (Table 1). Several studies have found that the prevalence of CAD in HFpEF is lower than that in systolic HF; for example, among 52,187 patients hospitalized for acute decompensated HF, 26,322 had preserved EF and were less likely to have CAD compared with those with reduced EF (50% vs 59%, P < 0.0001) [37], and recent data from the Framingham Heart Study showed that prior myocardial infarction (MI) decreased the odds of HFpEF compared with systolic HF (odds ratio, 0.32; 95% CI, 0.19–0.53). However, the prevalence and severity of angiographically documented CAD in patients with HFpEF has not been well studied [25]; thus, the true prevalence of CAD in HFpEF remains unknown.
The presence of CAD is associated with increased risk of developing HFpEF, and when documented angiographically, CAD is associated with increased mortality in HFpEF. In the Cardiovascular Health Study (CHS), prevalent CAD was associated with a threefold higher rate (41% vs 15%) of incident HF during follow-up in community-dwelling subjects older than 65 years [48,49]. In CHS, the same investigators also found that the population attributable risk of prevalent CAD was equivalent to that of hypertension [48,49]. In the Coronary Artery Surgery Study (CASS), which documented CAD by coronary angiography, the presence and extent of CAD were major determinants of prognosis in patients with HFpEF [50]. Similarly, in the Duke Cardiovascular Databank of patients with angiographically documented CAD, the presence of severe multivessel CAD was associated with increased mortality in patients with HFpEF [51]. Based on the aforementioned data, it is clear that the contribution of CAD as a comorbid condition in HFpEF cannot be ignored.
Mechanisms of HFpEF in patients with CAD
The syndrome of HFpEF, including the pathophysiology underlying it, is quite heterogeneous [7•,52]. One or more (typically multiple) different etiologic and pathophysiologic factors lead to the development of the HFpEF syndrome, complicating the elusive search for a rational classification system for these patients. The various pathophysiologic mechanisms for HFpEF that have been identified include diastolic dysfunction [53], systolic dysfunction (despite a preserved EF) [12,14,18–21,46,54,55], LV enlargement [56,57], abnormal ventricular-arterial coupling [58], noncardiac mechanisms of fluid overload [7•,59], and chronotropic incompetence [60,61•]. Of these mechanisms, CAD has most often been associated with diastolic dysfunction. However, there are compelling data associating CAD with systolic dysfunction and LV enlargement in patients with HFpEF.
There are several determinants of diastolic function, but the two major ones are 1) active relaxation, an energy-dependent process vulnerable to ischemia, and 2) passive LV chamber compliance, which decreases (the left ventricle becomes stiffer) with ischemia and infarction as fibrosis ensues. Several studies have demonstrated the interplay between myocardial ischemia and diastolic dysfunction, including demonstration that acute myocardial ischemia impairs both regional and global diastolic function, inducing impaired relaxation and slowing diastolic filling [25]. Acute ischemia also results in an upward and leftward shift of the LV end-diastolic pressure-volume relationship, resulting in decreased LV chamber compliance and LV diastolic pressure elevation [62]. If longstanding, chronic ischemia can cause cardiac hypertrophy and changes in the extracellular matrix, which eventually will alter LV compliance permanently [63]. Therefore, in the setting of both acute and chronic ischemia, any additional insult, such as acute hypertension or increased central blood volume, will quickly raise LV filling pressures even further and result in pulmonary edema. Diastolic dysfunction itself also may lead to ischemia. Calcium-overloaded myocytes cannot relax effectively; therefore, myocytes (and the myocardium as a whole) exist in a state of prolonged tension that interferes with diastolic coronary filling, thereby exacerbating ischemia [25]. LV hypertrophy acts synergistically with CAD to worsen diastolic function. In addition to increased LV wall thickness leading to subendocardial ischemia, studies have shown that patients with LV hypertrophy and CAD have higher pulmonary capillary wedge pressure (PCWP) at rest and strikingly high PCWP with exercise [64].
Emerging data suggest that systolic dysfunction (despite a preserved EF) and LV enlargement also may underlie the pathophysiology of HFpEF-CAD. Several studies have shown that patients with HFpEF have reduced longitudinal systolic mitral annular velocities compared with matched controls (Fig. 1a) [12,14,18–21,46] and that these decreases in systolic tissue velocities lie on a continuum between controls and overt systolic HF. Many of these studies included a large proportion of patients with CAD, highlighting the possibility that CAD and myocardial ischemia are causes of reductions in longitudinal cardiac systolic function. However, none of these studies specifically investigated this possibility. Other, more global systolic function parameters, such as increased LV end-systolic volume, reduced EF (within the range of normal), reduced end-systolic pressure-volume ratio, and reduced preload recruitable stroke work (Fig. 1b) [55,57], have been shown to be risk factors for HF hospitalization in patients with CAD and preserved EF.
CAD and HFpEF: diagnostic considerations
Evaluation of CAD in patients with HFpEF: should all patients with HFpEF undergo coronary angiography?
CAD is important in the pathogenesis of HFpEF, symptoms of ischemia can mimic symptoms of HF, and CAD is a treatable comorbidity in HFpEF. Therefore, identification of CAD is extremely important in HFpEF. The lower frequency of CAD observed in HFpEF compared with overt systolic HF may partly be the result of less systematic evaluation of CAD in HFpEF. The frequency with which CAD evaluation occurs in HFpEF is unknown but is likely lower than that of systolic HF, in which evaluation for the presence of CAD is nearly universal. Yet studies have shown that even in patients with preserved EF and no suspicion or evidence of CAD who are hospitalized with acute HF, CAD prevalence is as high as 35% [65,66•]. Therefore, systematic evaluation of CAD in HFpEF is essential.
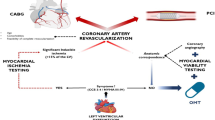
As stated earlier, CAD prevalence in HFpEF varies widely among studies but is most likely in the 50% range. If the 50% prevalence of CAD is taken as the pretest probability of CAD in the HFpEF patient population, the utility of noninvasive stress testing seems questionable if there are no contraindications for coronary angiography. Consider the following: best estimates of stress test sensitivity and specificity in the literature are 90% and 75%, respectively, for adenosine myocardial perfusion studies and 80% and 84%, respectively, for dobutamine stress echocardiography [67]. Using a pretest probability of 50%, the posttest probability for a normal stress test in HFpEF therefore is 12% to 19%. If “real-world” estimates of stress test sensitivity and specificity are used, the posttest probability of CAD despite a normal stress result is even higher (eg, if sensitivity and specificity are both 80%, the posttest probability is 20%, and if sensitivity and specificity are both 70%, the posttest probability is 30%). These high posttest probabilities in the setting of a normal stress test result suggest that coronary angiography should be performed in HFpEF as a first-line test. If there are contraindications to coronary angiography (eg, high risk for contrast nephropathy due to significant chronic kidney disease) or if performing stress testing will help localize and evaluate the burden of ischemia, stress testing may be performed, but only with the knowledge that a normal stress test result does not push the posttest probability of CAD below the threshold at which most clinicians would feel comfortable completely excluding CAD. Figure 2 outlines a practical approach to evaluating CAD in patients with HFpEF.
Diagnosis and treatment algorithm for coronary artery disease (CAD) in patients with heart failure and preserved ejection fraction (HFpEF). ACE—angiotensin-converting enzyme; ACS—acute coronary syndrome; ARB—angiotensin receptor blocker; BP—blood pressure; CABG—coronary artery bypass grafting; CI—chronotropic incompetence; CKD—chronic kidney disease; HF—heart failure; LV—left ventricular; LVEF—left ventricular ejection fraction; MI—myocardial infarction; PCI—percutaneous coronary intervention.
Risk factors for HFpEF development in patients with stable CAD
Recent guidelines advocate a staging system for HF that highlights the importance of preventing HF as opposed to simply treating it after it becomes clinically evident [27]. Indeed, over the next several years, the biggest improvement in HF morbidity and mortality likely will come from HF prevention. The presence of CAD is one of the criteria for stage A HF (high risk for developing HF). Patients with a history of MI, regardless of EF, meet the criteria for stage B HF (asymptomatic HF). In patients with CAD and preserved EF, clinical and echocardiographic risk factors for HF development have been identified. Recognizing these risk factors should help determine which patients with stage A or B HF have the highest likelihood of progression to stages C and D. In the Prevention of Events With Angiotensin Converting Enzyme Inhibition (PEACE) study, which randomly assigned 8290 patients aged ≥50 years with documented CAD and documented EF ≥40% to receive the angiotensin-converting enzyme (ACE) inhibitor trandolapril or placebo, a risk score was developed to predict incident HF (patients with prevalent HF were excluded from the study) [68••].
During a median follow-up of 4.8 years, the PEACE study identified the following risk factors for incident HF: increased age, elevated body mass index, history of MI, coronary artery bypass grafting (CABG) surgery, diabetes, hypertension, angina, cerebrovascular disease, current smoking, glomerular filtration rate less than 60 mL/min/m2, EF of 41% to 50% (compared with EF ≥50%), and use of calcium channel blockers, diuretics, digitalis, or antiarrhythmic medications. Non-use of lipid-lowering medication also was a risk factor for HF development [68••].
In the Heart and Soul Study of stable outpatients with CAD, the great majority of whom had preserved EF >50% at baseline, several echocardiographic variables predicted future risk of HF hospitalization. Increased LV mass index, increased left atrial volume index, decreased LV outflow tract velocity time integral (a surrogate for stroke volume), greater severity of mitral regurgitation, and higher grade of diastolic dysfunction all were independent predictors of HF hospitalization during follow-up. A risk score using these five echocardiographic parameters was a powerful predictor of subsequent HF hospitalization over 4.4 years of follow-up [69••].
Treatment
-
Compared with the number of large-scale randomized trials for patients with systolic HF, relatively few have been designed specifically for those with HFpEF. The latter trials have shown little benefit, leading some to conclude that there are no evidence-based treatments for this patient population. However, comorbidities drive adverse outcomes as often as or more frequently than the syndrome of HF in patients with HFpEF [24•]. Furthermore, the pathophysiology of HF in patients with HFpEF is directly related to underlying comorbidities such as CAD. Therefore, treatment of comorbidities such as CAD is vital to the management of HFpEF and currently is the main treatment option for these patients [24•].
Coronary revascularization
-
At first glance, revascularization of the epicardial coronary arteries with CABG or percutaneous coronary intervention (PCI) would seem to be a good option for patients with HFpEF and CAD. However, based on available data (which are limited), there is no known benefit (or harm) from revascularizing patients with CAD in the setting of HFpEF. In CASS, coronary revascularization with CABG in patients with HFpEF did not improve mortality (although it should be noted that morbidity and mortality rates for CABG have improved since CASS was published; thus, the contemporary utility of CABG in HFpEF is unknown). PCI data from 1997 to 2001 suggest that after undergoing PCI, patients with HFpEF have a higher adverse event rate compared with those without HF, although more recent data on the outcomes of PCI in HFpEF are lacking [70]. Finally, although some studies have demonstrated improvements in LV diastolic function after coronary revascularization, others have shown that pulmonary edema recurs in patients with CAD and HFpEF despite coronary revascularization [25,50,71–74].
-
Despite these challenges, patients may have alternative indications for revascularization based on other HFpEF-related comorbidities or in the setting of an acute coronary syndrome. In addition, revascularization is an important diagnostic maneuver in some patients, such as those with predominant dyspnea or exertional intolerance but little in the way of volume overload. Resolution of symptoms after revascularization in these patients can exclude the diagnosis of HFpEF.
Standard medical therapy for coronary artery disease
-
Many of the standard medical therapies for patients with CAD, such as β-blockers, ACE inhibitors, and angiotensin receptor blockers, have been tested in HFpEF clinical trials, with overall disappointing results [43,75,76•]. However, very few of these trials have specifically undertaken subgroup analysis in patients with CAD, and none has done so in an a priori fashion. Nonetheless, given the large evidence base for these therapies in CAD, there is no reason to withhold them from patients with HFpEF, as long as the caveats listed in the following text are followed.
-
β-Blockers are a cornerstone of CAD treatment. However, clinicians who treat HFpEF should take advantage of the beneficial effects of vasodilating β-blockers for treating both CAD and hypertension (which is almost universal in the HFpEF syndrome). Support for the use of vasodilating β-blockers also comes from the Coreg Heart Failure Registry (COHERE) and the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure (SENIORS), a randomized controlled trial [75,77•]. In COHERE, patients with HF treated with carvedilol had better outcomes than those treated with other β-blockers, regardless of underlying EF [77•]. Carvedilol, which now is available in generic form, therefore is an ideal choice, especially because it is a powerful antihypertensive and does not raise hemoglobin A1c [78]. The SENIORS study showed that in elderly patients with HF, nebivolol reduced mortality regardless of EF [75]. The “preserved EF” arm of the SENIORS study, however, was defined as EF greater than 35%, and few patients had an EF greater than 50%, which is not characteristic of the typical HFpEF patient population. Although β-blockers are first-line therapy for patients with CAD, and studies such as COHERE and SENIORS support the use of vasodilating β-blockers in HFpEF, these drugs can exacerbate chronotropic incompetence, an important cause of exercise intolerance in some patients with HFpEF [60,61•], as noted earlier. Therefore, whenever possible, patients with HFpEF should undergo exercise testing to determine their heart rate response to exercise. If a patient is found to have chronotropic incompetence, insertion of a dual-chamber, rate-adaptive pacemaker should improve heart rate response to exercise and will allow use of a β-blocker. Some elderly patients may refuse pacemaker therapy; in these cases, treatment must be individualized, and the benefits of β-blocker therapy for CAD must be weighed against the adverse consequences of worsening chronotropic incompetence. Of note, the use of rate-adaptive pacing for chronotropic incompetence in patients with HFpEF is being tested in the Restoration of Chronotropic Competence in Heart Failure Patients With Normal Ejection Fraction (RESET) study.
-
Inhibition of the renin-angiotensin-aldosterone axis also is an important therapy for both CAD and HFpEF. ACE inhibitor use in CAD is most well-established in patients with symptomatic systolic HF and in those with asymptomatic LV dysfunction. However, high-risk CAD patients, such as those with HFpEF, likely benefit from ACE inhibitor therapy. For example, in the Perindopril for Elderly Patients With Chronic Heart Failure (PEP-CHF) trial, which had difficulty enrolling patients, the use of perindopril in patients with HFpEF was not associated with a mortality benefit. However, in this trial, there was symptomatic improvement with perindopril and a mortality benefit in the subgroup of patients who had prior MI, suggesting a benefit for ACE inhibitors in patients with HFpEF and CAD [76•]. The Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM)-Preserved and I-PRESERVE trials of candesartan and irbesartan, respectively, for the treatment of HFpEF were both disappointing, and it is not clear whether the subgroup of patients with CAD in these trials benefited from these drugs [43]. In addition, the Hong Kong Diastolic Heart Failure study failed to show that angiotensin receptor blockade was beneficial above and beyond its effects on blood pressure [79]. Aldosterone blockade (with eplerenone) has been shown to improve post-MI outcomes in patients with reduced EF. Whether aldosterone antagonists can improve outcomes in the HFpEF population as a whole currently is being tested in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) study. Subgroup analysis of patients in TOPCAT who have CAD should be undertaken to determine whether aldosterone blockade helps in this patient population.
-
Potent statins, such as atorvastatin and simvastatin, are essentially required drugs for treating CAD and should not be withheld in patients with HFpEF-CAD. Observational data suggest beneficial effects of statins in HFpEF [80,81], but in reality, most HFpEF patients have one or more comorbidities (eg, CAD or diabetes) or a high Framingham risk score, so they should receive statins anyway.
-
Treating angina in patients with both CAD and HFpEF may be challenging. Ranolazine may be the best option in these patients because it is a potent antianginal agent and there is experimental data showing improvement in diastolic dysfunction with ranolazine [82]. Nitrates, commonly used to treat angina, can improve diastolic relaxation directly and reduce central blood volumes through venodilation, leading to rapid resolution of pulmonary edema and congestion. Thus, nitrates should be especially useful in patients with HFpEF-CAD. In reality, however, patients with HFpEF-CAD often develop tolerance to nitrates after a few months, and in some HFpEF patients with abnormal ventricular-arterial coupling, the addition of nitrates may lead to lightheadedness and dizziness. Calcium channel blockers (CCBs) also are used commonly in the setting of angina; however, these drugs may be detrimental in patients with HFpEF-CAD. CCBs, especially dihydropyridines, may exacerbate lower-extremity edema. In addition, CCBs were associated with increased risk of incident HF in the PEACE trial. Therefore, CCBs should be used only if the aforementioned options have been exhausted and angina or blood pressure control remains a problem.
-
Digoxin should not be used in patients with HFpEF-CAD, based on the results of the Digitalis Investigation Group (DIG) ancillary trial, a study of patients with HFpEF and EF greater than 45% treated with digoxin versus placebo. In the digoxin group, there was a trend toward decreased HF hospitalizations but a trend toward increased hospitalizations for unstable angina [42]. Therefore, use of digoxin in HFpEF-CAD may exacerbate myocardial ischemia and lead to adverse outcomes.
-
Although data are limited, cardiac rehabilitation and exercise training may be important adjunctive treatments for patients with HFpEF-CAD. The utility of cardiac rehabilitation has been demonstrated for secondary prevention in patients with CAD [83]; therefore, it should be used in HFpEF-CAD, given the possible beneficial increases in exercise capacity.
Evolving approaches
Establishment of a dedicated multidisciplinary HFpEF clinical program
-
Despite HFpEF’s high prevalence, morbidity, and mortality rates, there have been few clinical programs dedicated specifically to patients with this condition. Although HF specialty clinics are widespread and have been associated with clinical benefit, patients with HFpEF make up only a small proportion of these larger HF clinics. Given the paucity of proven treatments and ongoing clinical trials for HFpEF compared with systolic HF, patients with HFpEF may easily become marginalized in standard HF clinics, and enrollment in clinical trials may be challenging.
-
For these reasons, we have established a multidisciplinary HFpEF clinical program incorporating the benefits of a standard HF clinic but specializing in the subset of HF patients with a preserved EF. Patients are identified for enrollment in the clinic based on a query on the inpatient electronic medical record. Each day, the inpatient record is scanned for patients who meet at least one of the following criteria: 1) key words or a diagnosis of heart failure in the patient notes, 2) a B-type natriuretic peptide (BNP) level greater than 100 pg/mL, or 3) two or more doses of intravenous diuretics. EF (available for most patients) and BNP levels are documented on all identified patients, and the medical records of the patients on the list with an EF greater than 50% are reviewed on a daily basis, starting with patients with the highest BNP levels. If patients meet Framingham criteria for HF, their physicians are contacted to arrange for follow-up in the HFpEF clinic. Once patients arrive in the HFpEF clinic, a major aspect of their evaluation is determining the presence and severity of CAD, along with treatment of CAD if it exists, as outlined in Fig. 2. Although anecdotally this program has been quite successful in diagnosing and treating patients with HFpEF and enrolling them in clinical trials, further research must be conducted to determine whether specialized HFpEF clinical programs truly are beneficial.
Ranolazine
-
Ranolazine, an inhibitor of the late inward sodium channel (INa), is an effective agent for treating chronic stable angina [82]. In both LV hypertrophy and myocardial ischemia, late INa is augmented, resulting in increased cytosolic calcium, increased myocyte diastolic tension, impaired relaxation, and worsening of ischemia. Through inhibition of late INa, ranolazine can reduce diastolic calcium concentration and has been shown to decrease diastolic tension [84•,85]. Interestingly, in patients with congenital long QT syndrome due to a genetic defect in the late INa channel, treatment with intravenous ranolazine shortened the prolonged QTc and significantly improved diastolic function [86•]. We recently showed that in patients with suspected HF, including those with HFpEF, increased QTc interval is independently associated with decreased early mitral annular tissue Doppler velocity (E’) [87]. Therefore, it is possible that ranolazine, by inhibiting late INa and shortening the QTc interval, may improve diastolic function in these patients. Further study is required to determine whether ranolazine can truly improve diastolic function in HFpEF and whether this improvement will translate into meaningful clinical benefit.
Lusitropic agents
-
In a preclinical model of ischemic HF, istaroxime, a novel sodium-potassium ATPase and SERCA2a stimulator, improved systolic and diastolic function without increasing myocardial oxygen demand [88]. Istaroxime causes cytosolic calcium accumulation during systole and rapid sequestration of calcium during diastole. The beneficial clinical effects of istaroxime on both systolic and diastolic function were demonstrated in a phase 2 human study in patients with acute decompensated systolic HF [89,90•]. Although the capability of improving lusitropy without increasing oxygen demand would be desirable in HFpEF-CAD, it remains to be seen whether istaroxime, or other SERCA2a stimulators, will be beneficial in this setting.
Nonpharmacologic treatment of left ventricular hypertrophy and HFpEF
-
As discussed earlier, the combination of hypertensive LV hypertrophy and CAD compounds the problem of myocardial ischemia and leads to more severe elevations in LV filling pressures at rest and with exercise. Therefore, optimal treatment of systemic hypertension is critical in patients with HFpEF-CAD. Indeed, treatment of hypertension has been shown in multiple studies to be associated with reductions in future risk of HF, especially when thiazide diuretics are used [91,92]. Unfortunately, compliance with multiple antihypertensive agents may be difficult because of medication-medication interactions, medication intolerance, and unwanted side effects. Therefore, nonpharmacologic interventions for hypertension in patients with HFpEF would be an exciting development. Studies of two such therapies, radiofrequency ablation for denervation of renal sympathetic nerves [93••,94] and carotid sinus stimulation [95], were published recently, and if the effect of blood pressure lowering using these nonpharmacologic measures emulates the effects of blood pressure lowering in pharmacologic trials, both will be promising new potential therapies for patients with HFpEF-CAD.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Maeder MT, Kaye DM: Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 2009, 53(11):905–918.
Yip GW, Frenneaux M, Sanderson JE: Heart failure with a normal ejection fraction: new developments. Heart 2009, 95(19):1549–1552.
Bhatia RS, Tu JV, Lee DS, et al.: Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006, 355(3):260–269.
Dauterman KW, Massie BM, Gheorghiade M: Heart failure associated with preserved systolic function: a common and costly clinical entity. Am Heart J 1998, 135(6 Pt 2 Su):S310–319.
Lloyd-Jones D, Adams R, Carnethon M, et al: Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009, 119(3):480–486.
Owan TE, Hodge DO, Herges RM, et al.: Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006, 355(3):251–259.
Bench T, Burkhoff D, O’Connell JB, et al.: Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep 2009, 6(1):57–64.
Aurigemma GP, Zile MR, Gaasch WH: Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation 2006, 113(2):296–304.
Burkhoff D, Maurer MS, Packer M: Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation 2003, 107(5):656–658.
Maurer MS, Spevack D, Burkhoff D, Kronzon I: Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J Am Coll Cardiol 2004, 44(8):1543–1549.
Oh JK, Hatle L, Tajik AJ, Little WC: Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol 2006, 47(3):500–506.
Bruch C, Gradaus R, Gunia S, et al.: Doppler tissue analysis of mitral annular velocities: evidence for systolic abnormalities in patients with diastolic heart failure. J Am Soc Echocardiogr 2003, 16(10):1031–1036.
Gaasch WH, Delorey DE, Kueffer FJ, Zile MR: Distribution of left ventricular ejection fraction in patients with ischemic and hypertensive heart disease and chronic heart failure. Am J Cardiol 2009, 104(10):1413–1415.
Garcia EH, Perna ER, Farias EF, et al: Reduced systolic performance by tissue Doppler in patients with preserved and abnormal ejection fraction: new insights in chronic heart failure. Int J Cardiol 2006, 108(2):181–188.
Nikitin NP, de Silva R, Cleland JG: The utility of a comprehensive cardiac magnetic resonance examination for the evaluation of patients with heart failure. Heart 2004, 90(10):1166.
Paulus WJ, Tschope C, Sanderson JE, et al: How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007, 28(20):2539–2550.
Tan YT, Wenzelburger F, Lee E, et al.: The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 2009, 54(1):36–46.
Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG: “Pure” diastolic dysfunction is associated with long-axis systolic dysfunction. Implications for the diagnosis and classification of heart failure. Eur J Heart Fail 2005, 7(5):820–828.
Yip G, Wang M, Zhang Y, et al.: Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart 2002, 87(2):121–125.
Yu CM, Lin H, Yang H, et al.: Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 2002, 105(10):1195–1201.
Brucks S, Little WC, Chao T, et al.: Contribution of left ventricular diastolic dysfunction to heart failure regardless of ejection fraction. Am J Cardiol 2005, 95(5):603–606.
Heusch G: Diastolic heart failure: a misNOmer. Basic Res Cardiol 2009, 104(5):465–467.
De Keulenaer GW, Brutsaert DL: The heart failure spectrum: time for a phenotype-oriented approach. Circulation 2009, 119(24):3044–3046.
Shah SJ, Gheorghiade M: Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA 2008, 300(4):431–433.
Choudhury L, Gheorghiade M, Bonow RO: Coronary artery disease in patients with heart failure and preserved systolic function. Am J Cardiol 2002, 89(6):719–722.
Heart Failure Society of America: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail 2006, 12(1):e1–e2.
Hunt SA, Abraham WT, Chin MH, et al: 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009, 119(14):e391–e479.
Somaratne JB, Berry C, McMurray JJ, et al.: The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail 2009, 11(9):855–862.
Doughty RN, Kaye DM: MAGGIC: Survival in patients with heart failure and preserved versus impaired left ventricular ejection fraction: an individual patient data meta-analysis. Available at http://www.escardio.org/congresses/esc-2009/congress-reports/Pages/711011-711012-doughty-kaye.aspx. Accessed December 2009. This recent patient-level meta-analysis found that HFpEF has an improved but still high mortality compared with systolic HF.
Kitzman DW, Little WC, Brubaker PH, et al.: Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002, 288(17):2144–2150.
Doherty TM, Tang W, Detrano RC: Racial differences in the significance of coronary calcium in asymptomatic black and white subjects with coronary risk factors. J Am Coll Cardiol 1999, 34(3):787–794.
Kawakubo M, LaBree L, Xiang M, et al.: Race-ethnic differences in the extent, prevalence, and progression of coronary calcium. Ethn Dis 2005, 15(2):198–204.
Newman AB, Naydeck BL, Whittle J, et al.: Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol 2002, 22(3):424–430.
Tang W, Detrano RC, Brezden OS, et al.: Racial differences in coronary calcium prevalence among high-risk adults. Am J Cardiol 1995, 75(16):1088–1091.
Whittle J, Kressin NR, Peterson ED, et al.: Racial differences in prevalence of coronary obstructions among men with positive nuclear imaging studies. J Am Coll Cardiol 2006, 47(10):2034–2041.
Fonarow GC, Stough WG, Abraham WT, et al.: Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007, 50(8):768–777.
Yancy CW, Lopatin M, Stevenson LW, et al.: Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 2006, 47(1):76–84.
Melenovsky V, Borlaug BA, Rosen B, et al.: Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 2007, 49(2):198–207.
Lam CS, Roger VL, Rodeheffer RJ, et al.: Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009, 53(13):1119–1126.
Massie BM, Carson PE, McMurray JJ, et al: Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008, 359(23):2456–2467.
McMurray JJ, Carson PE, Komajda M, et al.: Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail 2008, 10(2):149–156.
Ahmed A, Rich MW, Fleg JL, et al.: Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation 2006, 114(5):397–403.
Yusuf S, Pfeffer MA, Swedberg K, et al.: Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003, 362(9386):777–781.
Baicu CF, Zile MR, Aurigemma GP, Gaasch WH: Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation 2005, 111(18):2306–2312.
Kasner M, Westermann D, Steendijk P, et al: Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 2007, 116(6):637–647.
Nikitin NP, Witte KK, Clark AL, Cleland JG: Color tissue Doppler-derived long-axis left ventricular function in heart failure with preserved global systolic function. Am J Cardiol 2002, 90(10):1174–1177.
Sanderson JE, Fraser AG: Systolic dysfunction in heart failure with a normal ejection fraction: echo-Doppler measurements. Prog Cardiovasc Dis 2006, 49(3):196–206.
Gottdiener JS, Arnold AM, Aurigemma GP, et al.: Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 2000, 35(6):1628–1637.
Gottdiener JS, McClelland RL, Marshall R, et al: Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002, 137(8):631–639.
Judge KW, Pawitan Y, Caldwell J, et al.: Congestive heart failure symptoms in patients with preserved left ventricular systolic function: analysis of the CASS registry. J Am Coll Cardiol 1991, 18(2):377–382.
O’Connor CM, Gattis WA, Shaw L, et al.: Clinical characteristics and long-term outcomes of patients with heart failure and preserved systolic function. Am J Cardiol 2000, 86(8):863–867.
Maurer MS: Heart failure with a normal ejection fraction (HFNEF): embracing complexity. J Card Fail 2009, 15(7):561–564.
Zile MR, Baicu CF, Gaasch WH: Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004, 350(19):1953–1959.
Borlaug BA, Lam CS, Roger VL, et al.: Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 2009, 54(5):410–418.
Shah SJ, Schiller NB, Whooley MA: Abnormalities in left ventricular systolic function predict heart failure events despite normal baseline ejection fraction: data from the Heart and Soul Study [abstract]. J Am Coll Cardiol 2006, 49(Suppl 1):A122.
Maurer MS, King DL, El-Khoury Rumbarger L, et al.: Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail 2005, 11(3):177–187.
McManus DD, Shah SJ, Fabi MR, et al.: Prognostic value of left ventricular end-systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: data from the Heart and Soul Study. J Am Soc Echocardiogr 2009, 22(2):190–197.
Kass DA: Ventricular arterial stiffening: integrating the pathophysiology. Hypertension 2005, 46(1):185–193.
Kliger C, King DL, Maurer MS: A clinical algorithm to differentiate heart failure with a normal ejection fraction by pathophysiologic mechanism. Am J Geriatr Cardiol 2006, 15(1):50–57.
Borlaug BA, Melenovsky V, Russell SD, et al.: Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 2006, 114(20):2138–2147.
Phan TT, Nallur Shivu G, Abozguia K, et al.: Impaired Heart Rate Recovery and Chronotropic Incompetence in Patients with Heart Failure with Preserved Ejection Fraction. Circ Heart Fail 2009 Nov 16 (Epub ahead of print). This recent study confirmed earlier findings by Borlaug et al. [60] demonstrating the importance of chronotropic incompetence in the pathogenesis of HFpEF.
Mann T, Brodie BR, Grossman W, McLaurin LP: Effect of angina on the left ventricular diastolic pressure-volume relationship. Circulation 1977, 55(5):761–766.
Tschope C, Westermann D: Heart failure with normal ejection fraction. Pathophysiology, diagnosis, and treatment. Herz 2009, 34(2):89–96.
Sobue T, Yokota M, Iwase M, Ishihara H: Influence of left ventricular hypertrophy on left ventricular function during dynamic exercise in the presence or absence of coronary artery disease. J Am Coll Cardiol 1995, 25(1):91–98.
Arques S, Ambrosi P, Gelisse R, et al.: Prevalence of angiographic coronary artery disease in patients hospitalized for acute diastolic heart failure without clinical and electrocardiographic evidence of myocardial ischemia on admission. Am J Cardiol 2004, 94(1):133–135.
Arques S, Bonello L, Roux E, et al.: Angiographic coronary artery disease associated with hypertensive heart failure and normal ejection fraction. Insights from a prospective monocenter study. Int J Cardiol 2008, 130(1):75–77.
Kim C, Kwok YS, Heagerty P, Redberg R: Pharmacologic stress testing for coronary disease diagnosis: a meta-analysis. Am Heart J 2001, 142(6):934–944.
Lewis EF, Solomon SD, Jablonski KA, et al.: Predictors of heart failure in patients with stable coronary artery disease: a PEACE study. Circ Heart Fail 2009, 2(3):209–216.
Stevens SM, Farzaneh-Far R, Na B, et al.: Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: the Heart and Soul study. JACC Cardiovasc Imaging 2009, 2(1):11–20.
Holper EM, Blair J, Selzer F, et al: The impact of ejection fraction on outcomes after percutaneous coronary intervention in patients with congestive heart failure: an analysis of the National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry and Dynamic Registry. Am Heart J 2006, 151(1):69–75.
Bonow RO, Bacharach SL, Green MV, et al.: Impaired left ventricular diastolic filling in patients with coronary artery disease: assessment with radionuclide angiography. Circulation 1981, 64(2):315–323.
Gorcsan J 3rd, Diana P, Lee J, et al.: Reversible diastolic dysfunction after successful coronary artery bypass surgery. Assessment by transesophageal Doppler echocardiography. Chest 1994, 106(5):1364–1369.
Kramer K, Kirkman P, Kitzman D, Little WC: Flash pulmonary edema: association with hypertension and reoccurrence despite coronary revascularization. Am Heart J 2000, 140(3):451–455.
Poulsen SH, Jensen SE, Egstrup K: Longitudinal changes and prognostic implications of left ventricular diastolic function in first acute myocardial infarction. Am Heart J 1999, 137(5):910–918.
van Veldhuisen DJ, Cohen-Solal A, Bohm M, et al.: Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). J Am Coll Cardiol 2009, 53(23):2150–2158.
Cleland JG, Tendera M, Adamus J, et al.: The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006, 27(19):2338–2345.
Massie BM, Nelson JJ, Lukas MA, et al.: Comparison of outcomes and usefulness of carvedilol across a spectrum of left ventricular ejection fractions in patients with heart failure in clinical practice. Am J Cardiol 2007, 99(9):1263–1268.
Bakris GL, Fonseca V, Katholi RE, et al.: Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 2004, 292(18):2227–2236.
Yip GW, Wang M, Wang T, et al.: The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 2008, 94(5):573–580.
Fukuta H, Little WC: Observational studies of statins in heart failure with preserved systolic function. Heart Fail Clin 2008, 4(2):209–216.
Fukuta H, Sane DC, Brucks S, Little WC: Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation 2005, 112(3):357–363.
Stone PH: Ranolazine: new paradigm for management of myocardial ischemia, myocardial dysfunction, and arrhythmias. Cardiol Clin 2008, 26(4):603–614.
Ades PA: Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med 2001, 345(12):892–902.
Sossalla S, Wagner S, Rasenack EC, et al.: Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 2008, 45(1):32–43.
Hayashida W, van Eyll C, Rousseau MF, Pouleur H: Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther 1994, 8(5):741–747.
Moss AJ, Zareba W, Schwarz KQ, et al.: Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol 2008, 19(12):1289–1293.
Wilcox J, Shah A, Shah SJ: Electrocardiographic predictors of abnormal left ventricular diastolic function: importance of the QTc interval. J Card Fail 2009, 15(6 Suppl 1):S106.
Sabbah HN, Imai M, Cowart D, et al.: Hemodynamic properties of a new-generation positive luso-inotropic agent for the acute treatment of advanced heart failure. Am J Cardiol 2007, 99(2A):41A–46A.
Gheorghiade M, Blair JE, Filippatos GS, et al: Hemodynamic, echocardiographic, and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure. J Am Coll Cardiol 2008, 51(23):2276–2285.
Shah SJ, Blair JE, Filippatos GS, et al.: Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: results from the Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: a Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) trial. Am Heart J 2009, 157(6):1035–1041.
Beckett NS, Peters R, Fletcher AE, et al.: Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008, 358(18):1887–1898.
Davis BR, Kostis JB, Simpson LM, et al.: Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation 2008, 118(22):2259–2267.
Krum H, Schlaich M, Whitbourn R, et al.: Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009, 373(9671):1275–1281.
Schlaich MP, Sobotka PA, Krum H, et al.: Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009, 54(6):1195–1201.
Bisognano JD, de Leeuw PW, Bach DS: Improved cardiac structure and diastolic flow velocities in early-stage heart failure with chronic treatment using an implantable device: Results from European and United States trials of the Rheos system [abstract]. J Am Coll Cardiol 2009, 53:A188.
Curtis JP, Sokol SI, Wang Y, et al.: The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003, 42(4):736–742.
Guazzi M, Myers J, Arena R: Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol 2005, 46(10):1883–1890.
Gustafsson F, Torp-Pedersen C, Brendorp B, et al.: Long-term survival in patients hospitalized with congestive heart failure: relation to preserved and reduced left ventricular systolic function. Eur Heart J 2003, 24(9):863–870.
Kirk V, Bay M, Parner J, et al.: N-terminal proBNP and mortality in hospitalised patients with heart failure and preserved vs. reduced systolic function: data from the prospective Copenhagen Hospital Heart Failure Study (CHHF). Eur J Heart Fail 2004, 6(3):335–341.
MacCarthy PA, Kearney MT, Nolan J, et al.: Prognosis in heart failure with preserved left ventricular systolic function: prospective cohort study. BMJ 2003, 327(7406):78–79.
Macin SM, Perna ER, Cimbaro Canella JP, et al.: Differences in clinical profile and outcome in patients with decompensated heart failure and systolic dysfunction or preserved systolic function [in Spanish]. Rev Esp Cardiol 2004, 57(1):45–52.
McAlister FA, Teo KK, Taher M, et al.: Insights into the contemporary epidemiology and outpatient management of congestive heart failure. Am Heart J 1999, 138(1 Pt 1):87–94.
Ojeda S, Anguita M, Munoz JF, et al.: Clinical characteristics and medium-term prognosis of patients with heart failure and preserved systolic function. Do they differ in systolic dysfunction [in Spanish]? Rev Esp Cardiol 2003, 56(11):1050–1056.
Pernenkil R, Vinson JM, Shah AS, et al.: Course and prognosis in patients > or = 70 years of age with congestive heart failure and normal versus abnormal left ventricular ejection fraction. Am J Cardiol 1997, 79(2):216–219.
Smith GL, Masoudi FA, Vaccarino V, et al.: Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol 2003, 41(9):1510–1518.
Ghali JK, Kadakia S, Bhatt A, et al.: Survival of heart failure patients with preserved versus impaired systolic function: the prognostic implication of blood pressure. Am Heart J 1992, 123(4 Pt 1):993–997.
Kupari M, Lindroos M, Iivanainen AM, et al.: Congestive heart failure in old age: prevalence, mechanisms and 4-year prognosis in the Helsinki Ageing Study. J Intern Med 1997, 241(5):387–394.
Madsen BK, Hansen JF, Stokholm KH, et al.: Chronic congestive heart failure. Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J 1994, 15(3):303–310.
Andersson B, Hall C: N-terminal proatrial natriuretic peptide and prognosis in patients with heart failure and preserved systolic function. J Card Fail 2000, 6(3):208–213.
Acknowledgments
Dr. Shah is supported by an American Heart Association Scientist Development Grant, a Northwestern Memorial Foundation Eleanor Wood Prince Grant, a Northwestern Memorial Foundation Dixon Translational Research Award, and an Actelion Entelligence Investigator Award. His research also has been supported in the past by a Heart Failure Society of America Research Fellowship Award and an American Society of Echocardiography Career Development Award.
Disclosure
Dr. Shah has served on an advisory board for Gilead Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, S.J. Evolving Approaches to the Management of Heart Failure with Preserved Ejection Fraction in Patients with Coronary Artery Disease. Curr Treat Options Cardio Med 12, 58–75 (2010). https://doi.org/10.1007/s11936-009-0060-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-009-0060-2