Abstract
Purpose of Review
The leading cause of voiding dysfunction in older men is benign prostatic obstruction. In the setting of a grossly enlarged prostate (>80cm3), an open simple prostatectomy has been the gold standard for surgical treatment. Here, we will discuss the minimally invasive robot-assisted approach and compare it to the classic open approach and holmium laser enucleation of the prostate.
Recent Findings
Literature on robot-assisted simple prostatectomy, in concurrence with our institutional experience, has shown an overall lower morbidity, shorter hospital stay, and decreased indwelling catheter time, with equivalent functional outcomes compared to open simple prostatectomy. Similar operative times and hospital stays were found compared to holmium laser enucleation of the prostate, although a steep learning curve and cost of new equipment hinder the wide spread use of this transurethral approach.
Summary
On review of current literature in addition to our institutional experience, we favor robot-assisted simple prostatectomy over open simple, based on associated increased morbidity/catheter time/hospital stay, and holium laser enucleation of the prostate, based on steep learning curve and cost of new equipment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most common cause of adult male lower urinary tract symptoms (LUTS) is benign prostatic obstruction (BPO) caused by benign prostatic hyperplasia (BPH). Worldwide an estimated 1.1 billion men suffer with BPO-associated LUTS [1]. Treatment options include behavioral modification, medical therapy, catheterization (continuous or intermittent), and/or surgical intervention. Those treatment options for BPO have expanded dramatically over the last 20 years. Current medical therapies include the use of α1-andregenic antagonists, 5α-reductase antagonists, and phosphodiesterase type 5 inhibitors. Patients who have failed medical therapy and meet indications for surgical intervention can be considered for invasive intervention. Surgical indications include acute urinary retention; recurrent or persistent UTIs; significant symptoms not responsive to medical therapy; recurrent gross hematuria of prostatic origin; pathophysiologic changes of the kidneys, ureters, or bladder secondary to BPO, and bladder calculi secondary to obstruction. Surgical approach is usually dictated by prostatic size. The transurethral approach is generally recommended for prostates <80cm3; this includes transurethral incision of bladder neck (TUIBN), transurethral resection of prostate (TURP). The open simple prostatectomy (OSP), once considered the “Gold Standard” for treatment of high volume prostate (>80 ml) disease, is one of the oldest surgical procedures in the urologic armamentarium. Recently, advancements in minimally invasive procedures for BPO have been shown to be equal or improved in postoperative functional outcomes. This combined with overall lower morbidity, shorter hospital stays, decreased blood loss, and reduced indwelling foley catheter time more surgeons are leaning toward those transurethral and laparoscopic procedures over OSP [2,3,4]. Here the robot-assisted simple prostatectomy (RASP) will be discussed in detail and compared to transurethral holmium laser enucleation of the prostate [HoLEP] and OSP.
Procedural Origins
Eugene Fuller first performed the classically described OSP in 1894 via a suprapubic approach. Freyer and Proust popularized the transvesical approach in the early 1900s [5, 6]. They argued that by entering the bladder, one could treat concomitant pathologic findings including removal of bladder stones, large median lobes, or possible bladder diverticula. In contrast, the retropubic transcapsular approach described by Terrence Millin in 1945 removed undue morbidity by not entering the bladder at all. Instead, his technique focused on removing the prostatic adenoma at the apex, provided better exposure for hemostatic control and protecting the urethral sphincter [7].
The first reported laparoscopic radical prostatectomy, by Schuessler in 1991, paved the way for minimally invasive techniques involving the prostate. Schuessler used Millin’s open radical prostatectomy technique as his template for the new laparoscopic approach [8]. Guillonneau reported in 2002 a posterior approach for radical prostatectomies that started first by freeing the seminal vesicles, through the posterior vesical peritoneum, that produced evidence that subcapsular dissection of the adenoma was possible [9]. Known as the first surgeon to intentionally search for and use the retroadenomatous plane, Miradolino Mariano utilized a standard laparoscopic technique [10]. Sotelo first reported a RASP and demonstrated promising results attributable to the three-dimensional view and wristed instrumentation [11].
During the development and advancement of laparoscopic approaches for treatment of BPO, transurethral laser technology has evolved to treat larger volume BPO (>80cm3) once solely treated by OSP. According to the 2013 European Guidelines for Treatment and Follow-up of Non-neurogenic Male Lower Urinary Tract Symptoms Including of BPO, HoLEP was the “current standard/first choice” for transurethral treatment of BPO (>80cm3) [12]. Gilling reported his technique that was fashioned after the OSP. The holmium laser was used to incise along the prostatic capsule to carve out intact the median lobe and each lateral lobe in a retrograde fashion. Each intact lobe is pushed into the bladder. A mechanical morcellator is then used to remove the prostatic lobes [13, 14]. HoLEP has shown results that are comparable to OSP with shorter hospital stays, shorter catheter times, and decreased rate of transfusion [15,16,17].
Operative Planning
To assist in making the decision on which therapeutic approach to take, one must fully evaluate the patient with a full medical and surgical history, physical exam (including digital rectal exam, and focused neurologic exam to rule additional pathology that may be causing LUTS), PSA, and a urinalysis to evaluate for infection or hematuria. Symptom assessment is conducted via the AUA symptom survey or International Prostate Symptom Score (IPSS) questionnaire.
If considering invasive therapy one can consider the following diagnostic studies to assist with operative planning: urinary flow rate, post void residual, pressure flow urodynamics, cystoscopy, abdominal ultrasound, and transrectal ultrasound. Upper tract imaging is not recommended unless there is a presence of hematuria, renal insufficiency, history of upper tract surgery, or concern for calculi. Specific operative approach is dependent on patient preference, surgeon preference, medical and surgical history, and/or prostate size. The care provider must rule out any malignancy prior to proceeding with therapeutic option. Elevated PSA and/or abnormal DRE may warrant TRUS and needle biopsy. Upper tract imaging, cystoscopy and cytology are recommended for those men presenting with hematuria.
In preparation for the OR, the patient should undergo appropriate evaluation including labs, EKG, and clearance by primary care physician for significant preexisting medical conditions. Any medications that would affect the patient’s normal coagulation should be reviewed and discontinued if possible. We do not usually obtain a type and screen for patients undergoing RASP although this would be mandatory for OSP. Urinalysis and culture should also be completed to treat any UTI. Perioperative intravenous antibiotics are given as recommended. Appropriate DVT prophylaxis should also be performed. [18].
Technical Approach to Robot-Assisted Simple Prostatectomy
Prepping and Positioning
General anesthesia is administered. The patient is then secured to the operating table by cloth tape or security belt across his chest with arms tucked securely at the side. Care is taken to pad and protect bony prominences of the upper extremities. Next, the patient’s legs are secured in low lithotomy position in stirrups or spreader bars. After placing the patient in a 30° Trendelenburg position, the patient is prepped and draped in sterile fashion. A 18F foley catheter is placed, as well as an orogastric tube.
Insufflation and Port Placement
We prefer a Veress needle for initial insufflation for our transperitoneal approach. The ports are marked out in the same fashion as our robotic-assisted radical prostatectomy cases: supraumbilical camera port (8 mm), four ports (3–8 mm and one 12-mm lateral assistant port in the left lower quadrant) separated by 8 cm are laid out in a straight line across the lower abdomen, and a 5-mm assistant port that is placed in the upper left quadrant. Our usual da Vinci Surgical System (Sunnyvale, Ca) instrumentation includes monopolar cautery scissors, Maryland bipolar forceps and Prograsp forceps.
Operative Approach
Once the robot is docked, all adhesions are taken down as to fully expose the deep pelvis. The sigmoid colon is mobilized to allow the bladder to fully drop. The space of Retzius is developed after incising the peritoneum. The superficial dorsal vein is cauterized then divided and the anterior periprostatic fat is cleared and removed through the assistant port. The endopelvic fascia is incised bilaterally but in limited fashion in order to allow clear vision to assess the full size of the prostate. A running stitch of 3-0V-Lock suture is used to ligate the runoff of the main dorsal vein at the level of the anterior apex of the prostate.
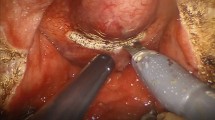
After changing the 0° camera for the 30° and the monopolar scissors for the permanent spatula cautery, the bladder neck dissection is then performed. We prefer to approach the adenoma in this fashion, as it is identical to our radical prostatectomy technique, rather than in a transvesical fashion. This dissection is commenced by incising the fat encasing the bladder neck lateral and proximal to the prostatovesical junction. This dissection allows precise visualization of the lateral edges of the bladder that can be traced distally and medially to spare the bladder neck. We feel this method consistently provides adequate bladder preservation regardless the shape of the intravesical lobe such that bladder neck reconstruction rarely needs to be performed. Once the bladder is entered, the foley catheter is retracted anteriorly with the Prograsp. In a similar fashion to a radical prostatectomy, we completely mobilize the bladder from the prostate except directly posteriorly toward the vasa. This dissection plane is carried distally along the adenoma to leave the vasa and seminal vesicles in place. The plane along the interior of prostatic capsule is established (Fig. 1). This plane is carefully continued laterally and distally, all the while checking the incision of the endopelvic fascia to ensure an adequate depth of dissection is being continued. Any bleeding that is encountered is usually easily controlled with spot monopolar or bipolar cautery. Thus, the blood loss during the case is usually very low. As judged by the contour of the prostate, the apex of the prostate is approached and the plane is carried anteriorly. The anterior commissure of the prostate is then divided carefully in a caudal direction so that the verumontanum can be seen. Using the verumontanum as a landmark, an incision is carried posteriorly through the apex of the prostate to the posterior plane to deliver the adenoma. The now detached adenoma is placed into a laparoscopic entrapment sac.
Utilizing the Prograsp, the median lobe is lifted anteriorly and the posterior adenoma (blue line) is dissected free of the capsule (green-dotted line with an arrow). Blunt dissection and cautery are used to develop plane interior to prostatic capsule. Note bladder is completely mobilized from prostate (yellow line)
Careful inspection of the bed of the adenoma is performed to ensure that there is no bleeding or unresected adenoma. Further dissection can still be easily performed if necessary. We take great care to ensure that the dissection plane does not go distal to the verumontanum even if some residual lateral apical tissue still remains. We feel that this tissue is very unlikely to be obstructive. The instruments are changed to needle drivers, and 3-0 Quill suture, composed of monocryl, is used to reanastomose the bladder neck to the remaining prostatic urethra. General bites are taken through the distal apical tissue to ensure a solid approximation. An 18 French 2-way foley catheter is placed and the bladder is irrigated to ensure there is no leak from the anastomosis. Continuous bladder irrigation is never required postoperatively. The anterior fat overlying the prostatovesical junction is then reattached to the anterior bladder wall using a 3–0 V-lock suture. Prior to specimen removal, we perform a transperitoneal transversus abdominis plane (TAP) block by injecting 0.5% Marcaine with a butterfly needle under direct vision. We have demonstrated that this provides improved postoperative pain control when compared to local anesthetic injected at each port site [19]. No drain is left. The ports are removed and the specimen is retrieved through the camera port. Patients are usually discharged the next day with planned catheter removal in 4 to 6 days.
Varying Approaches
Several techniques have been described in the literature. They can be roughly classified into transcapsular and transvesical (posterior and anterior). The transcapsular technique is simply described as a transverse capsular incision 1–2.5 cm distal to the anterior prostatovesical junction [20, 21]. Alternatively, transvesical approaches include vertical or horizontal cystotomies at or near the bladder dome or proximal to the prostatovesical junction, respectively. The vertical cystotomy can be performed, either anteriorly after entering the space of Retzius or posteriorly without bladder mobilization and can assist with bladder calculi removal and diverticulectomy. Descriptions of this technique utilized stay sutures at the cystotomy edges tacked laterally, to provide visualization [22]. The anterior horizontal transvesical approach was reported more often as it is very closely related to the classically described OSP [11, 21, 23,24,25,26]. After accessing the space of Retzius, access to the adenoma is completed through a wide transvesical transverse incision just proximal to the prostatovesical junction. Additionally, several descriptions reported using traction sutures placed into the lateral lobes [11, 21, 24]. With any approach, care must be taken to not inadvertently injure the ureteral orifices especially when encountering a large intravesical lobe.
Another point of variation across techniques was the management of the incised edge of the bladder neck. Retrigonization has been described as advancing the posterior bladder mucosa into the now empty prostatic pseudo-capsule. Interrupted absorbable sutures or continuous running urethrovesical anastomosis, as is our preference, can improve hemostasis obviating the need for CBI, greatly simplify catheter placement, and decrease rate of bladder neck contractions [2, 11]. Clavijo described use of a hemostatic agent into the prostatic fossa if troublesome post-resection bleeding persisted. [27]. Coelho reported on other modifications including a posterior prostatic capsule plication (similar to a Rocco stitch), a modified vanVelthoven continuous vesicourethral anastomosis and suturing of the anterior prostatic capsule to the anterior bladder surface. In his study of six patients, no blood transfusions or CBI were required, and hospital stays were shortened [23].
Extraperitoneal approaches have also been described. Joseph et al. reported that this approach can avoid intraabdominal adhesions and compartmentalize any potential hematoma or urinoma to the space of Retzius which may decrease morbidity [28]. Clavijo proposed an intrafascial approach, which results in a complete subcapsular prostatectomy. Although this approach may result in fewer cases of residual adenocarcinoma, this benefit would be counterbalanced against the increased handling of the neurovascular bundle and urethra, resulting in an increased risk of erectile dysfunction and incontinence [27].
Comparative Outcomes
Overall, there is a paucity of comparative studies between OSP and RASP surgical approaches. Recently, a comparative single institution non-randomized retrospective study was published comparing RASP to HoLEP [29]. HoLEP has been extensively studied over the past 20 years including numerous randomized controlled trials against OSP that revealed overall comparable functional outcomes but with reduced hospital stays, catheterization times, blood loss, and transfusion rates [14,15,16,17] (Table 1).
Learning Curve
Robert et al. examined the learning curve of surgeons during their adoption of HoLEP by conducting a prospective, multicenter clinical study. Participating surgeons had no experience with HoLEP but were experienced in TURP and OSP. Success was cumulatively defined as follows: complete enucleation and morcellation, <90 min duration, without conversion to TURP in four consecutive cases. After initial instruction (self study and two supervised cases), almost 50% of centers abandoned HoLEP. Those surgeons who successfully completed the procedure subjectively reported that around 50 cases were needed to “feel comfortable”. Several retrospective studies also concluded that approximately 50 cases were needed to see significant changes in efficiency rates of enucleation and morcellation [30, 31]. In conclusion, they recommended only a specialized, HoLEP-trained urologist to complete the procedure rather than a surgeon without dedicated training, especially with larger prostate glands [32]. In contrast with the widespread adoption of RARP, the learning curve for RASP is likely much shorter than described for HoLEP.
Operative Time
Multiple studies have reported on mean RASP operative times and these operative times range from 90 to 228 min [21,22,23, 28, 33]. When compared to OSP, several large multicenter studies showed the mean operative time ranged from 54 to 85 min [34,35,36,37]. Several large HoLEP studies showed mean operative times ranging from 73 to 115 mins, similar to RASP [15, 17, 29].
Estimated Blood Loss and Transfusion
Two large studies comparing OSP to minimally invasive simple prostatectomy (MISP) concluded that intraoperative blood loss and postoperative transfusions rates were much higher in the OSP patient population. The collective of studies on RASP report a transfusion rate of 0–5% [38]. Lucca et al. found a 20–25% rate of transfusion for OSP [22]. A Nationwide Inpatient Sample review indicated a 17% transfusion rate in OSPs done from 1998 to 2010 [21]. In two large HoLEP studies, no postoperative transfusions were required for intraoperative blood loss [17, 39]. Of note, El Tayeb et al. completed a comparative analysis of those patients undergoing HoLEP that required intermittent or continuous anticoagulation versus those not. Beside a slightly prolonged length of hospital stay and duration of CBI, no significant findings affecting the recovery of the patients were found. Given those findings, they suggest HoLEP is a safe option for patients on intermittent or continuous anticoagulation [40]. No such analysis has been done for RASP.
Length of Hospital Stay
Since Sotelo’s initial RASP publication in 2008, the published data reports a mean range of 1–4 days with an average of 2.3 days [11, 15, 16, 20,21,22, 24,25,26, 38, 41]. Gratzke et al. reported a mean of 11.9 days for hospital stay after surgery in men undergoing OSP. Naspro et al. reported a shorter length of stay at 2.7 days for patients undergoing HoLEP versus 5.4 days for OSP [15]. Compared to RASP, HoLEP studies revealed mean ranges of length of stay (LOS) from 1 to 2 days [17, 29, 39]. We routinely discharge our patients after RASP on postoperative day 1.
Length of Catheterization
Mean catheterization periods in RASP studies range from 3 to 9 days (11, 20, 35, 34, 39, 42). Kuntz et al. reported a mean catheter duration of 2.9 days following HoLEP [17]. Naspro et al. reported that when compared to HoLEP, OSP indwelling catheter was extended; 1.5 versus 4.1 days, respectively [15]. Indwelling catheter time is also variable between surgical techniques within each surgical approach. The need for continuous bladder irrigation is not required in the newer RASP techniques compared its widespread use with HoLEP and OSP [15, 23, 29].
Functional Outcomes
Lucca et al. reported MISP reduced IPSS scores by 17.2 points and improved Qmax improvement by 14.3 ml/s. No significant differences, in terms of outcomes, were found between OSP and MISP. This included preoperative and postoperative Qmax in addition to preoperative and postoperative IPSS between the two groups (42). Kuntz and Naspro showed comparable long-term functional outcomes (Qmax, PVR, AUA symptom score) for HoLEP versus OSP. Of significance, Naspro et al. revealed in his study that HoLEP had a higher rate of dysuria (68.2 vs 41%; P < 0.001) at 3 months postoperatively compared to OSP [15, 17]. Umari reported that both HoLEP and RASP showed improvements in flow rate, a reduction in post-void residuals, and improved IPSS scores, although multivariate analysis showed no statistically significant differences for these findings between surgical approaches [29].
Cost
Sutherland et al. performed a cost comparison that revealed that the average RASP cost was $5212 versus $2415 for OSP in 2011. Matei et al. reported a cost of €3840 ($4013 US Dollars) per RASP compared to €5000 ($5226 US Dollars) for OSP [25]. This difference could be accounted for in shorter hospital stays, lower transfusion rates, and lower rates of bladder irrigation. Salonia et al. reported a HoLEP cost of €2356 ($2462 US Dollars) in 2011 [16]. In spite of the lower case by case cost, this benefit could be mitigated for hospitals that already own a da Vinci system to utilize RASP rather than considering purchase of a high-power holmium laser and morcellator.
Conclusion
When considering surgical intervention in those men with >80cm3 prostate glands, RASP is an excellent choice for surgeons experienced in robotic surgery. When compared to OSP, RASP has improved perioperative morbidity, decreased hospital stay, lower rates of transfusion, and shorter indwelling catheter time. In comparison to HoLEP, the learning curve is not nearly as steep and access to already established robotic instrumentation may be more cost effective. In conclusion, given similar functional outcomes, ease of adapting techniques and utilizing already purchased equipment, RASP, when compared to OSP and HoLEP, becomes the more attractive approach for most patients, surgeons, and institutions alike.
Abbreviations
- LUTS:
-
Lower urinary tract symptoms
- BPO:
-
Benign prostatic obstruction
- BPH:
-
Benign prostatic hyperplasia
- OSP:
-
Open simple prostatectomy
- RASP:
-
Robotic-assisted simple prostatectomy
- HoLEP:
-
Holmium laser enucleation of the prostate
- MISP:
-
Minimally invasive simple prostatectomy
- DVC:
-
Dorsal venous complex
- LOS:
-
Length of stay
References
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–8.
Han M and Partin AW. Simple prostatectomy: open and robot-assisted laparoscopic approaches. Campbell-Walsh Urology, 106, 2535–2542. e1
Madersbacher S, Alivizatos G, Nordling J, et al. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol. 2004;46:547–54.
McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803.
Freyer PJ. A new method of performing prostatectomy. Lancet. 1900;1:774.
Freyer PJ. One thousand cases of total enucleation of the prostate for radical cure of enlargement of that organ. Br Med J. 1912;2:868.
Meier DE, Tarpley JL, et al. The outcome of suprapubic prostatectomy: a contemporary series in the developing world. Urology. 1995;46(1):40–4.
Schuessler WW, Schulam PG, Clayman RV, et al. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854.
Guillonneau B, Cathelineau X, Doublet JD, et al. Laparoscopic radical prostatectomy: assessment after 550 procedures. Crit Rev Oncol Hematol. 2002;43(2):123–33.
Mariano MD, Graziottin TM, Tefilli MV. Laparoscopic prostatectomy for vascular control for benign prostatic hyperplasia. J Urol. 2002;167(6):2528–9.
Sotelo R, Clavijo R, Carmona O, et al. Robotic simple prostatectomy. J Urol. 2008;179:513–5.
Gilling PJ, Fraundorfer MR. Holmium laser prostatectomy: a technique in evolution. Curr Opin Urol. 1998;8:11.
Tooher R, Sutherland P, Costello A, Gilling P, et al. A systematic review of holmium laser prostatectomy for benign prostatic hyperplasia. J Urol. 2004;171(Issue 5):1773–81.
Naspro R, Suardi N, Salonia A, et al. Holumium laser enucleation of the prostate versus open prostatectomy for prostates >70g; 24m follow up. Eur Urol 2006; 563–8
Salonia A, Suradi N, Naspro R, et al. Holumium laser enucleation versus open prostectomy for benign prostatic hyperplasia: an inpatient cost analysis. Urology. 2006;68:302–6.
Kuntz RM, Lehrich K, Ahyai S. Holmium laser enucleation of the prostate versus open prostatectomy for prostates greater than 100grams: 5 year follow-up results of a randomized clinical trial. Eur Urol. 2008;53:160–6.
Kaplan SA. Update on the American Urological Association Guidelines for treatment of benign prostatic hyperplasia. Rev Urol. 2006;8(Suppl 4):S10–7.
Yezdani M, Katz B, Yu S, Lee A, Lee DI. A novel transversus abdominal plane block during robotic assisted radical prostatectomy. Philadelphia: AUA Annual Meeting Moderated Poster Presentation; 2015.
Sutherland DE, Perez DS, Weeks DC. Robot-assisted simple prostatectomy for severe benign prostatic hyperplasia. J Endourol. 2011;25:641–4.
Banapour P, Patel N, Kane CJ, Cohen SA, Parsons JK. Robotic-assisted simple prostatectomy: a systematic review and report of a single institution case series. Prostate Cancer Prostatic Dis. 2014;17:1–5.
Leslie S, de Castro Abreu AL, Chopra S, et al. Transvesical robotic simple prostatectomy: initial clinical experience. Urol. 2014;66:321–9.
Coelho RF, Chauhan S, Sivaraman A, et al. Modified technique of robotic-assisted simple prostatectomy: advantages of a vesico-urethral anastomosis. BJU Int. 2012;109:426–33.
Umari P, Fossati N, Gandaglia G, et al. Robotic assisted simple prostatectomy (RASP) versus holmium laser enucleation of the prostate (HoLEP) for lower urinary tract symptoms in patients with large volume prostates (>100ml): a comparative analysis from a high volume center. J Urol. 2016; doi:10.1016/j.juro.2016.08.114.
Matei DV, Brescia A, Mazzoleni F, et al. Robot-assisted simple prostatectomy (RASP): does it make sense? BJU Int. 2012;110(11 Pt C):E972–9.
Vora A, Mittal S, Hwang J, et al. Robot-assisted simple prostatectomy: multi-institutional outcomes for glands larger than 100grams. J Endourol. 2012;26:499–502.
Clavijo R, Carmona O, et al. Robot-assisted simple prostatectomy: novel technique. J Endourol. 2013;27(3):328–32.
Joseph JV, Rosenbaum R, et al. Robotic extraperiotenal radical prostatectomy: an alternative approach. J Urol. 2006;175(3):945–51.
Shah HN, Mahajan AP, Sodha HS, Hegde S, Mohile PD, Bansal MB. Prospective evaluation of the learning curve for holmium laser enucleation of the prostate. J Urol. 2007;177:1468–74.
Matei DV, Spinelli MG, Nordio A, et al. Robotic simple prostatectomy. Eur Urol Suppl. 2008;9:337.
Placer J, Gelabert-Mas A, Vallmanya F, et al. Holmium laser enucleation of prostate: outcome and complications of self-taught learning curve. Urology. 2009;73:1042–8.
Robert G, Cornu JN, Fourmarier M, et al. Multicenter prospective evaluation of the learning curve of holmium laser enucleation of the prostate (HoLEP). BJU. 2016;117:495–9.
Pokorny M, et al. Robot-assisted simple prostatectomy for treatment of lower urinary tract symptoms secondary to benign prostatic enlargement: surgical technique and outcomes in a high-volume robotic centre. Euro Urol. 2015;68(3):451–7.
Uffort EE, Jensen JC. Robotic-assisted laparoscopic simple prostatectomy: an alternative minimal invasive approach for prostate adenoma. J Robotic Surg. 2010;4:7–10.
Varkarakis I, Kyriakakis Z, Delis A, Protogerou V, Deliveliotis C. Long-term results of open transvesical prostatectomy from a contemporary series of patients. Urology. 2004;64:306.
Adam C, Hofstetter A, Deubner J, Zaak D, Weitkunat R, Seitz M, et al. Retropubic transvesical prostatectomy for significant prostatic enlargement must remain a standard part of urology training. Scand J Urol Nephrol. 2004;38:472.
Tubaro A, Carter S, Hind A, Vicentini C, Miano L. A prospective study of the safety and efficacy of suprapubic transvesical prostatectomy in patients with benign prostatic hyperplasia. J Urol. 2001;166:172.
Autorino R, Zargar H, Mariano M, et al. Perioperative outcomes of robotic and laparoscopic simple prostatectomy: a European- American multi-institutional analysis. Eur Urol. 2015;68:86–94.
Humphreys MR, Miller NL, Handa SE, Terry C. Holium laser enucleation of the prostate—outcomes independent of prostate size? J Urol. 2008;180(6):2431–5.
El Tayeb MM, Jacob JM, Bhojani N, Bammerlin E, Lingeman JE. Holmium laser enucleation of the prostate in patients requiring anticoagulation. J Endourol. 2016;30(7):805–9.
Lucca I, Shariat SF, Hofbauer SL, et al. Outcomes of minimally invasive simple prostatectomy for benign prostatic hyperplasia: a systematic review and meta-analysis. World J Urol. 2015;33:563–70.
Gratzke C, Schlenker B, Seitz M et al. Complications and early postoperative outcomes after open prostatectomy in patients with benign prostatic enlargement: Results of a prospective multicenter study. J Urol 2007: 1419–1422
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ross Cockrell and David I. Lee declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Endourology
Rights and permissions
About this article
Cite this article
Cockrell, R., Lee, D.I. Robot-Assisted Simple Prostatectomy: Expanding on an Established Operative Approach. Curr Urol Rep 18, 37 (2017). https://doi.org/10.1007/s11934-017-0681-z
Published:
DOI: https://doi.org/10.1007/s11934-017-0681-z