Abstract
While initial implementations of prostate MRI suffered from suboptimal performance in tumor detection, technological advances over the past decade have allowed modern multi-parametric prostate MRI (mpMRI) to achieve high diagnostic accuracy for detection, localization, and staging and thereby impact patient management. A particular emerging application of mpMRI is in the pre-biopsy setting to allow for MRI-targeted biopsy, for instance, through real-time MRI/ultrasound fusion, which may help reduce the over-detection of low-risk disease and selectively detect clinically significant cancers, in comparison with use of standard systematic biopsy alone. mpMRI and MRI-targeted biopsy are spreading beyond the large academic centers to increasingly be adopted within small and community practices. Aims of this review article are to summarize the hardware and sequences used for performing mpMRI, explore patient specific technical considerations, delineate approaches for study interpretation and reporting [including the recent American College of Radiology Prostate Imaging Reporting and Data System (PI-RADS) version 2], and describe challenges and implications relating to the widespread clinical implementation of mpMRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common non-cutaneous cancer and second most common cause of cancer-related death among men in the USA [1]. It varies widely in clinical behavior, ranging from a non-aggressive, indolent disease, which can be followed by active surveillance, to an aggressive, rapidly progressive disease that can be treated by a growing number of options that include radical prostatectomy, external beam radiation, brachytherapy, and various forms of ablative therapy [2]. An important limitation of prostate cancer screening by prostate-specific antigen (PSA) and digital rectal examination (DRE) is their poor specificity [3]. However, a 12-core systematic transrectal ultrasound (TRUS) biopsy performed in response to an abnormal PSA or DRE has suboptimal sensitivity for significant cancer [4], leading to uncertainty regarding negative biopsy results, as well as frequent detection of insignificant cancer in a third of cases, leading to concerns of “over-detection” [5]. Therefore, there is growing enthusiasm for adoption of new diagnostic paradigms to reduce over-detection and over-treatment of low-risk disease while reliably diagnosing any high-risk disease that is present [6••].
Prostate MRI provides superb anatomic assessment of the prostate gland due to its excellent spatial and contrast resolution. Initial implementations of prostate MRI largely replied upon anatomic evaluation and had relatively low diagnostic performance. However, current prostate MRI protocols consist of a multi-parametric approach that complement standard anatomical sequences with sequences providing additional forms of contrast and improved tissue characterization. State-of-the-art multi-parametric prostate MRI has been shown to achieve high accuracy for tumor detection, localization, and staging, thereby facilitating management decisions [6••, 7]. While the use of prostate MRI was mostly limited to academic centers following its introduction in the 1980s, technological advances over the past decade have led to increasing clinical adoption in small and community practices. In this review, we aim to describe prostate MRI hardware and sequences, patient specific considerations, current approaches for interpretation and reporting of results, and clinical implications of prostate MRI.
Hardware
Prostate MRI examinations can be performed at a field strength of either 1.5 or 3.0 T. 3.0-T MRI provides increased signal to noise ratio (SNR), which allows for improved spatial resolution and potentially faster scan times [8]. Although clinically acceptable images can be obtained at both 1.5 and 3.0 T, it is generally recommended that a 3.0-T MRI be used when available [9••]. As metal-related susceptibility artifact increases at higher field strength and becomes particularly severe at 3.0 T, a field strength of 1.5 T may be deemed preferable in patients with bilateral hip prostheses [10].
Prostate MRI requires use of at least an external phased array coil laid across the patients’ pelvis. However, many centers elect to also use an endorectal coil (ERC). The ERC is physically positioned closer to the prostate, providing greater SNR that can be applied to increase spatial resolution. While the necessity of the ERC is controversial at both 1.5 and 3 T, its impact seems to be more advantageous at 1.5 T MRI [11]. As an example of the variable data, while Heijmink et al. demonstrated improved image quality, tumor localization, and better staging using the ERC compared to a phased array coil at 3.0 T [12]; more recently, Kim et al. reported equivalent staging accuracy between ERC and phased array coils at 3.0 T [13]. Ongoing improvements in MRI hardware and software design, including the development of higher performance multi-channel surface coils, are increasing the clinical feasibility of performing MRI at 1.5 T without an endorectal coil. In addition, some practices may elect to tailor the coil selection to the specific indication. For instance, the ERC may be used for local staging in patients awaiting prostatectomy, in which maximal spatial resolution helps optimize visualization of the neurovascular bundles, whereas surface coil imaging may be used in the pre-biopsy setting, in which sufficient resolution to guide targeted biopsy can be obtained using an external coil.
While the ERC provides improved SNR, there are several disadvantages, including potential patient discomfort, higher cost, increased scanner time, and potential gland deformation [14]. Rectal air insufflation to 80 mg is required for ERC imaging, which has been reported to rarely lead to complications such as rectal bleeding, erosion, and proctitis [14]. Furthermore, administration of an enema before ERC placement may decrease patient compliance with the examination. Additional workflow considerations must be addressed when using an ERC, including additional personnel and examination time needed for coil placement and removal. Given the advantages and disadvantages of the two approaches, as well as the contradictory studies regarding the added benefit of the ERC particularly at 3.0 T, the choice of ERC or phased array coil imaging continues to vary between practices based on local experience, expertise, and preference.
Sequences
T2-Weighted Imaging
High-resolution T2-weighted images are the mainstay of prostate MRI protocols, providing, among imaging approaches, the best anatomical depiction of the prostate including assessment of the prostatic margin, internal structures, and zonal anatomy. Furthermore, this sequence aids tumor staging by facilitating evaluation of extra-prostatic extension, seminal vesicle invasion, and neurovascular bundle invasion by tumor. The peripheral zone of the prostate usually exhibits high signal on T2-weighted images. In contrast, peripheral zone tumors generally appear as round or oval foci of decreased signal using this sequence. However, in patients with inflammation or prostatitis, the peripheral zone may be heterogeneous with patchy areas of decreased signal, making tumor detection more difficult. Furthermore, low-grade tumors may be iso-intense to the high signal peripheral zone and therefore not well visualized using T2-weighted imaging. A meta-analysis incorporating 14 studies investigated the diagnostic performance of T2-weighted images alone in prostate tumor localization, demonstrating a sensitivity and specificity of 0.57–0.62 and 0.74–0.78, respectively [15]. This moderate performance highlights the need for use of additional sequences to achieve accurate tumor detection and localization (Figs. 1 and 2).
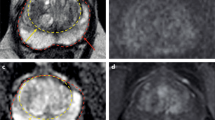
Prostate cancer in an 81-year-old male with a Gleason score of 4 + 3 and a PSA level of 6.4 ng/ml. Imaging of the prostate using a phased array coil at 3.0 T was performed. a Axial T2-weighed (T2W) image demonstrates an area of decreased T2 signal (arrow) within the left mid-gland peripheral zone. b High b value diffusion-weighted image (DWI) demonstrates high signal in this region. c Apparent diffusion coefficient (ADC) map from DWI shows corresponding low ADC. d Dynamic contrast-enhanced (DCE) MR imaging shows increased enhancement in the region of signal abnormality on T2-weighted and diffusion-weighted images
Prostate cancer in a 62-year-old male with a Gleason score of 4 + 3 and a PSA level of 13 ng/ml, who had undergone a previous standard 12-core biopsy which was negative for tumor. a Axial T2-weighed (T2W) image demonstrates an area of decreased T2 signal (arrow) within the anterior apex transition zone. b High b value diffusion-weighted image (DWI) demonstrates high signal in this region. c Apparent diffusion coefficient (ADC) map from DWI shows corresponding low ADC. d Early dynamic contrast-enhanced (DCE) MR imaging shows rapid enhancement in the region of signal abnormality on T2-weigthed and diffusion-weighted images
Diffusion-Weighted Imaging
Diffusion-weighted imaging (DWI) generates image contrast based on the ability of water molecules to move freely within tissues [16]. In simple fluid, water molecules demonstrate relatively free diffusivity. However, water movement demonstrates increasing restriction of diffusion as the cellular density of tissue increases, as is encountered in cellular tumors. The degree of diffusion weighting of a given acquisition is reflected by the b values, which in turn is influenced by the strength of diffusion-sensitizing gradients that are applied during sequence acquisition. The tissue contrast generated at low b values mainly represent water motion due to capillary perfusion, while contrast at high b values is heavily influenced by barriers to water motion from cell membranes [16, 17]. There is a debate regarding the most appropriate b value to be used for DWI of the prostate. Historically, b values up to 800–1000 s/mm2 were used. However, recent studies report improved lesion detection using even higher b values up to 2000 s/mm2 [18–20]. In addition, the actual diffusion coefficient of water cannot be directly measured by MRI. Rather, diffusion-weighted images must be acquired using at least two different b values, from which a diffusion coefficient can be calculated. The most widely applied coefficient in clinical practice is the apparent diffusion coefficient (ADC) [21]. The ADC map better reflects the diffusivity of water molecules than the directly acquired b value images themselves, which in fact reflect a combination of both diffusion weighting and T2 weighting.
The impact of diffusion-weighted imaging in prostate MRI protocols has been firmly established [22–27]. Prostate tumors generally demonstrate increased signal on high b value DWI as well as decreased ADC, given the association between tumor cellularity and restricted diffusion. Identification of these imaging features can greatly improve tumor detection compared with T2-weighted imaging along. A meta-analysis incorporating 27 studies evaluated the diagnostic performance of DWI alone in detecting prostate cancer and demonstrated a sensitivity and specificity of 0.62 and 0.90, respectively [28]. The diagnostic performance is improved when DWI is combined with T2-weighted images, as demonstrated in an additional meta-analysis which pooled 10 studies, reporting a sensitivity and specificity of 0.76 and 0.82, respectively [27]. Further advantages of DWI include its short acquisition time, ease of acquisition and post-processing, and lack of use of intravenous contrast. Currently, the combination of T2-weighted images and DWI is needed to meet minimal technical standards for prostate MRI [29].
Dynamic Contrast-Enhanced Imaging
Many practices routinely obtain dynamic contrast-enhanced MRI (DCE-MRI) as part of their standard prostate MRI protocol, although its added value remains controversial. Prostate tumors release factors that promote vessel formation and capillary permeability [30, 31], thereby leading to more rapid enhancement than surrounding normal tissue [32]. DCE-MRI entails serial rapid 3D T1-weighted acquisitions of the prostate after the intravenous administration of a gadolinium-based contrast agent. Typically, images are acquired sequentially at a rate of at least every 10 seconds and for a total duration of at least 2 minutes [32]. Some authors suggest that the temporal resolution should be even faster, at least every 5 seconds [33, 34]. While multiple prior studies have demonstrated the usefulness of DCE-MRI in prostate tumor detection [35–38], others have suggested that the combination of T2-weighted imaging and DWI alone are sufficient [39].
Timing of MRI After Biopsy
Prostate biopsy, which in many practices is performed prior to consideration of obtaining a prostate MRI, leads to varying amounts of hemorrhage within the prostate [40]. This hemorrhage often leads to decreased T2 signal in the peripheral zone, therefore mimicking or obscuring an area of tumor and hindering tumor detection and localization [40]. To address this issue, it is common to recommend a delay between any prior prostate biopsy and MRI, although with varying recommendations regarding the needed time interval [41–44]. Although a suggested delay of approximately 8 weeks is common, such a delay between prostate biopsy and MRI theoretically may prolong management decisions and definitive treatment. Furthermore, the resolution of prostate hemorrhage is variable between patients and is often still present to some extent even after 8 weeks [45]. Therefore, radiologists should be prepared to interpret prostate MRI examinations when hemorrhage is present. One imaging finding that may assist such assessment is the “hemorrhage exclusion sign,” in which hemorrhage spares the region of tumor. In one study, application of this finding improved accuracy for tumor detection in the setting of hemorrhage [46].
It is possible that careful evaluation of multi-parametric sequences may mitigate the negative influence of hemorrhage on diagnostic accuracy compared with T2-weighted images alone [40, 45]. For instance, Tamada et al. demonstrated good performance of mpMRI for tumor detection in the presence of hemorrhage [40], and a more recent investigation demonstrated neither extensive post-biopsy hemorrhage nor delay in biopsy less than 4 weeks to negatively impact detection when using multi-parametric evaluation [45]. Despite these encouraging results, it is still possible for hemorrhage to mask small or subtle tumors. Some practices initially perform a T1-weighted sequence and then reschedule the examination if significant hemorrhage is identified. This approach, however, requires monitoring of the examination by the radiologist as well as close consultation with the referring urologist in the event of a delayed examination. In addition, this approach leads to greater patient inconvenience in the event of a rescheduled examination. In summary, while most practices advise a delay between biopsy and MRI, the length of delay and other logistical aspects of implementing the delay vary among institutions.
Patient Preparation
Practices differ in their requirements for patient preparation prior to prostate MRI. Stool and gas within the rectum may exacerbate artifacts on DWI, causing anatomic warping that can manifest as alterations in the contour of the prostate on high b value DWI compared to T2-weighted imaging [47•]. Furthermore, artifacts related to rectal motion may impair all sequences [47•, 48]. Use of an enema prior to prostate MRI to empty the rectum of stool and gas, even for non-ERC exams, as well as an antispasmodic agent to decrease bowel motion have been proposed to address these issues [29]. However, there is a lack of supporting data as well as general consensus among experts regarding the value of such measures. A cleansing enema entails time and cost, while itself potentially leading to increased bowel peristalsis [9••], and antispasmodic agents are prone to adverse drug reactions. Furthermore, studies have suggested no significant effect on overall image quality or tumor detection with or without the administration of a cleansing enema [49] or antispasmodic agent [50] prior to prostate MRI. Nonetheless, even in the absence of such measures, a reasonable and commonly applied step is to instruct the patient to evacuate the rectum prior to the examination. If initial MR images show a large amount of air within the rectum, further considerations include laying the patient prone or removing the air from the rectum using a suction catheter [9••]. Lastly, some practices instruct patients to refrain from ejaculation for approximately 3 days prior to the examination to allow for maximum distension of the seminal vesicles [51]. However, as with the other previously noted approaches to patient preparation, increased benefit of this measure on prostate and seminal vesicle tumor detection has not been formally reported.
Interpretation
There is a steep learning curve among radiologists for prostate MRI interpretation. Prior publications have demonstrated significant inter-observer variability in prostate MRI interpretation, with varying levels of reader experience being a key contributor [52, 53]. Formal education programs including individual feedback, case reviews, and follow-up of pathology results are of paramount importance in attaining expertise and maximizing one’s diagnostic performance. In a study by Garcia-Reyes et al., radiology fellows evaluated prostate MRIs before and after a dedicated education program that included didactic lectures and case correlation with pathology findings, demonstrating significantly increased diagnostic accuracy of index cancer and anterior cancers compared to pre-education values [54]. In a similar study, radiology fellows’ average accuracy in detecting peripheral and transition zone tumors increased significantly after expert radiologist led weekly interactive tutorials which included quiz cases [55]. Regular multidisciplinary meetings among urologists, radiologists, and pathologists to perform group evaluation of earlier interpretations are also helpful in optimizing a center’s prostate MRI readings. Existing literature indicates that MRI performed and interpreted with high quality achieves a sensitivity of over 90 % for clinically significant cancer [35, 56–61] and that missed cancers are overwhelmingly small and insignificant [62].
Reporting
Standardized reporting of prostate MRI is of critical importance for facilitating communication between the radiologist and the urologist, as well as for ensuring that important information is conveyed in an actionable fashion in the report. The European Society of Urogenital Radiology (ESUR) Prostate Imaging and Reporting Archiving Data System (PI-RADS) expert panel prostate MRI guidelines were published in 2012 and aimed to standardize reporting by rating lesions on each sequence using standardized criteria [29]. However, this system lacked consistent instruction on how to determine an overall suspicion score [63] and had limited application among clinical practices. The more recent American College of Radiology (ACR) PI-RADS version 2 represents a more complete and comprehensive reporting system. PI-RADS version 2 provides explicit criteria for assessing lesions using each sequence as well as for deriving an overall 1–5 assessment category. These assessment categories indicate the likelihood of significant cancer, ranging from very low to very high probability [9••]. This scoring system is intended to assist in adjusting patient risk profiles and facilitating decisions about the need for targeted biopsy. It is anticipated that PI-RADS version 2 will undergo broad implementation, both nationally and internationally.
Targeted Biopsy
Improvements in the accuracy of MP-MRI have supported the development of MRI-targeted biopsy. Although initial studies largely described in-bore targeting of lesions within the MR gantry, growing literature demonstrates the effectiveness of lesion targeting by “fusion” biopsy in which the MP-MRI images are electronically superimposed in real time upon TRUS images [6••, 64•]. MRI/US fusion biopsy can be readily performed by the urologist during an office visit. Targeted biopsy has strong potential to address the inherent limitations of TRUS biopsy [65]. Siddiqui et al. demonstrated that performing fusion targeted biopsy solely of MRI targets would significantly increase the detection of high-risk prostate cancer while decreasing the detection of low-risk cancer compared to performing solely a standard systematic biopsy [6••]. Therefore, targeted biopsy could significantly change the distribution of risk in men with newly diagnosed prostate cancer toward diagnosis of more high-risk disease [6••]. Given that the majority of patients in their study cohort had a previous prostate biopsy, further validation of these findings in a biopsy-naïve population is required to validate this diagnostic paradigm [6••]. Due to the decreased detection of low-risk prostate cancer when compared to standard biopsy, a targeted biopsy approach may have further beneficial economic and psychological implications. De Rooij et al. demonstrated improved cost-effectiveness and quality of life for men with clinical suspicion of prostate cancer using MP-MRI followed by MR-guided biopsy than using conventional systematic biopsy for diagnosis [66]. While there is currently a surge of interest in MRI-targeted prostate biopsy, further studies including large prospective multi-center trials are still awaited prior to a large-scale change to the present status of systematic biopsy as the standard of care.
Future Directions
Over the past decade, prostate MRI has exhibited substantially improved clinical performance, and the examination is being embraced by a growing fraction of the urologist community. However, impediments to widespread clinical use still exist. For instance, there is a compelling need for uniformly high-quality images and image interpretation in order for prostate MRI to be of practical value. Dedicated training courses, mini-fellowships, enhanced educational materials, and certification pathways may serve as various approaches for facilitating widespread competence among radiologists. In addition, in order for urologists to consistently offer prostate MRI to their patients, insurance coverage is needed. Such coverage will require continued studies examining impact on clinical outcomes and cost-effectiveness. In addition, studies providing more focused assessment of prostate MRI and MRI-targeted biopsy in biopsy-naïve patients are needed. Ultimately, if MRI-targeted biopsy were to replace systematic biopsy as the standard of care, this would have profound implications regarding the relative detection of high-risk and low-risk disease [6••]. In conclusion, MP-MRI has become established as the most accurate imaging test for prostate cancer evaluation and is now being routinely used in many practices to assist tumor detection, biopsy guidance, and treatment planning. Nonetheless, continued technical optimization and further prospective investigations are anticipated prior to its widespread adoption.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49.
Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90(10):766–71.
Schroder FH, Carter HB, Wolters T, et al. Early detection of prostate cancer in 2007. Part 1: PSA and PSA kinetics. Eur Urol. 2008;53(3):468–77.
Mufarrij P, Sankin A, Godoy G, Lepor H. Pathologic outcomes of candidates for active surveillance undergoing radical prostatectomy. Urology. 2010;76(3):689–92.
Heijnsdijk EA, der Kinderen A, Wever EM, Draisma G, Roobol MJ, de Koning HJ. Overdetection, overtreatment and costs in prostate-specific antigen screening for prostate cancer. Br J Cancer. 2009;101(11):1833–8.
Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–7. Siddiqui et al. demonstrated that MR/US fusion targeted biopsy of MRI targets significantly increased the detection of high-risk prostate cancer while decreasing the detection of low-risk cancer compared to performing solely a standard systematic biopsy in a cohort of 1003 men. Therefore, targeted biopsy could significantly change the distribution of risk in men with newly diagnosed prostate cancer toward diagnosis of more high-risk disease.
Wang L, Hricak H, Kattan MW, Chen HN, Scardino PT, Kuroiwa K. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology. 2006;238(2):597–603.
Chang KJ, Kamel IR, Macura KJ, Bluemke DA. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics. 2008;28(7):1983–98.
American College of Radiology. Prostate Imaging and Reporting and Data System: Version 2. 2015 [April 2, 2015]; Available from: http://www.acr.org/∼/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf. The American College of Radiology Prostate Imaging and Reporting and Data System Version 2 provides guidelines for the performance and interpretation of prostate MRI, developed by a multicenter export panel, including a standardized scheme for providing risk assessment categories for identified lesions.
Mazaheri Y, Vargas HA, Nyman G, Akin O, Hricak H. Image artifacts on prostate diffusion-weighted magnetic resonance imaging: trade-offs at 1.5 Tesla and 3.0 Tesla. Acad Radiol. 2013;20(8):1041–7.
Shah ZK, Elias SN, Abaza R, et al. Performance comparison of 1.5-T endorectal coil MRI with 3.0-T nonendorectal coil MRI in patients with prostate cancer. Acad Radiol. 2015;22(4):467–74.
Heijmink SW, Futterer JJ, Hambrock T, et al. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T—comparison of image quality, localization, and staging performance. Radiology. 2007;244(1):184–95.
Kim BS, Kim TH, Kwon TG, Yoo ES. Comparison of pelvic phased-array versus endorectal coil magnetic resonance imaging at 3 Tesla for local staging of prostate cancer. Yonsei Med J. 2012;53(3):550–6.
Lee SH, Park KK, Choi KH, et al. Is endorectal coil necessary for the staging of clinically localized prostate cancer? Comparison of non-endorectal versus endorectal MR imaging. World J Urol. 2010;28(6):667–72.
Tan CH, Paul Hobbs B, Wei W, Kundra V. Dynamic contrast-enhanced MRI for the detection of prostate cancer: meta-analysis. AJR Am J Roentgenol. 2015;204(4):W439–48.
Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188(6):1622–35.
Gupta RT, Kauffman CR, Polascik TJ, Taneja SS, Rosenkrantz AB. The state of prostate MRI in 2013. Oncology (Williston Park). 2013;27(4):262–70.
Kim CK, Park BK, Lee HM, Kwon GY. Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Investig Radiol. 2007;42(12):842–7.
Kitajima K, Kaji Y, Kuroda K, Sugimura K. High b-value diffusion-weighted imaging in normal and malignant peripheral zone tissue of the prostate: effect of signal-to-noise ratio. Magn Reson Med Sci. 2008;7(2):93–9.
Lim HK, Kim JK, Kim KA, Cho KS. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection—a multireader study. Radiology. 2009;250(1):145–51.
Koo JH, Kim CK, Choi D, Park BK, Kwon GY, Kim B. Diffusion-weighted magnetic resonance imaging for the evaluation of prostate cancer: optimal B value at 3T. Korean J Radiol. 2013;14(1):61–9.
Delongchamps NB, Beuvon F, Eiss D, et al. Multiparametric MRI is helpful to predict tumor focality, stage, and size in patients diagnosed with unilateral low-risk prostate cancer. Prostate Cancer Prostatic Dis. 2011;14(3):232–7.
Isebaert S, Van den Bergh L, Haustermans K, et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J Magn Reson Imaging. 2013;37(6):1392–401.
Chen M, Dang HD, Wang JY, et al. Prostate cancer detection: comparison of T2-weighted imaging, diffusion-weighted imaging, proton magnetic resonance spectroscopic imaging, and the three techniques combined. Acta Radiol. 2008;49(5):602–10.
Tamada T, Sone T, Higashi H, et al. Prostate cancer detection in patients with total serum prostate-specific antigen levels of 4–10 ng/mL: diagnostic efficacy of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T2-weighted imaging. AJR Am J Roentgenol. 2011;197(3):664–70.
Tan CH, Wei W, Johnson V, Kundra V. Diffusion-weighted MRI in the detection of prostate cancer: meta-analysis. AJR Am J Roentgenol. 2012;199(4):822–9.
Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199(1):103–10.
Jie C, Rongbo L, Ping T. The value of diffusion-weighted imaging in the detection of prostate cancer: a meta-analysis. Eur Radiol. 2014;24(8):1929–41.
Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–57.
Brawer MK, Deering RE, Brown M, Preston SD, Bigler SA. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer. 1994;73(3):678–87.
Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143(2):401–9.
Verma S, Turkbey B, Muradyan N, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol. 2012;198(6):1277–88.
Franiel T, Ludemann L, Rudolph B, et al. Evaluation of normal prostate tissue, chronic prostatitis, and prostate cancer by quantitative perfusion analysis using a dynamic contrast-enhanced inversion-prepared dual-contrast gradient echo sequence. Investig Radiol. 2008;43(7):481–7.
Rosenkrantz AB, Geppert C, Grimm R, et al. Dynamic contrast-enhanced MRI of the prostate with high spatiotemporal resolution using compressed sensing, parallel imaging, and continuous golden-angle radial sampling: preliminary experience. J Magn Reson Imaging. 2015;41(5):1365–73.
Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186(5):1818–24.
Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology. 2010;255(1):89–99.
Ocak I, Bernardo M, Metzger G, et al. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. AJR Am J Roentgenol. 2007;189(4):849.
Kim JK, Hong SS, Choi YJ, et al. Wash-in rate on the basis of dynamic contrast-enhanced MRI: usefulness for prostate cancer detection and localization. J Magn Reson Imaging. 2005;22(5):639–46.
Rais-Bahrami S, Siddiqui MM, Vourganti S, et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU Int. 2015;115(3):381–8.
Tamada T, Sone T, Jo Y, et al. Prostate cancer: relationships between postbiopsy hemorrhage and tumor detectability at MR diagnosis. Radiology. 2008;248(2):531–9.
White S, Hricak H, Forstner R, et al. Prostate cancer: effect of postbiopsy hemorrhage on interpretation of MR images. Radiology. 1995;195(2):385–90.
Ramchandani P, Schnall MD. Magnetic resonance imaging of the prostate. Semin Roentgenol. 1993;28(1):74–82.
Ikonen S, Kivisaari L, Vehmas T, et al. Optimal timing of post-biopsy MR imaging of the prostate. Acta Radiol. 2001;42(1):70–3.
Qayyum A, Coakley FV, Lu Y, et al. Organ-confined prostate cancer: effect of prior transrectal biopsy on endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenol. 2004;183(4):1079–83.
Rosenkrantz AB, Mussi TC, Hindman N, et al. Impact of delay after biopsy and post-biopsy haemorrhage on prostate cancer tumour detection using multi-parametric MRI: a multi-reader study. Clin Radiol. 2012;67(12):e83–90.
Barrett T, Vargas HA, Akin O, Goldman DA, Hricak H. Value of the hemorrhage exclusion sign on T1-weighted prostate MR images for the detection of prostate cancer. Radiology. 2012;263(3):751–7.
Rosenkrantz AB, Taneja SS. Radiologist, be aware: ten pitfalls that confound the interpretation of multiparametric prostate MRI. AJR Am J Roentgenol. 2014;202(1):109–20. This article reviews 10 pitfalls that impact the interpretation of multi-parametric prostate MRI. These include normal anatomic structures or benign processes that may be misinterpreted as tumor, as well as technical issues that may hinder interpretation. Strategies to overcome these challenges are also suggested.
Murphy G, Haider M, Ghai S, Sreeharsha B. The expanding role of MRI in prostate cancer. AJR Am J Roentgenol. 2013;201(6):1229–38.
Lim C, Quon J, McInnes M, Shabana WM, El-Khodary M, Schieda N. Does a cleansing enema improve image quality of 3T surface coil multiparametric prostate MRI? J Magn Reson Imaging. 2014.
Wagner M, Rief M, Busch J, et al. Effect of butylscopolamine on image quality in MRI of the prostate. Clin Radiol. 2010;65(6):460–4.
Medved M, Sammet S, Yousuf A, Oto A. MR imaging of the prostate and adjacent anatomic structures before, during, and after ejaculation: qualitative and quantitative evaluation. Radiology. 2014;271(2):452–60.
Ruprecht O, Weisser P, Bodelle B, Ackermann H, Vogl TJ. MRI of the prostate: interobserver agreement compared with histopathologic outcome after radical prostatectomy. Eur J Radiol. 2012;81(3):456–60.
Mullerad M, Hricak H, Wang L, Chen HN, Kattan MW, Scardino PT. Prostate cancer: detection of extracapsular extension by genitourinary and general body radiologists at MR imaging. Radiology. 2004;232(1):140–6.
Garcia-Reyes K, Passoni NM, Palmeri ML, et al. Detection of prostate cancer with multiparametric MRI (mpMRI): effect of dedicated reader education on accuracy and confidence of index and anterior cancer diagnosis. Abdom Imaging. 2015;40(1):134–42.
Akin O, Riedl CC, Ishill NM, Moskowitz CS, Zhang J, Hricak H. Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur Radiol. 2010;20(4):995–1002.
Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259(2):453–61.
Hugosson J, Carlsson S. Overdetection in screening for prostate cancer. Curr Opin Urol. 2014;24(3):256–63.
Abd-Alazeez M, Ahmed HU, Arya M, et al. The accuracy of multiparametric MRI in men with negative biopsy and elevated PSA level—can it rule out clinically significant prostate cancer? Urol Oncol. 2014;32(1):45.e17–22.
Ouzzane A, Puech P, Villers A. How accurately can MRI detect indolent disease? Curr Opin Urol. 2014;24(3):264–9.
Pepe P, Garufi A, Priolo G, Pennisi M. Can 3-Tesla pelvic phased-array multiparametric MRI avoid unnecessary repeat prostate biopsy in patients with PSA < 10 ng/mL? Clin Genitourin Cancer. 2015;13(1):e27–30.
Da Rosa MR, Milot L, Sugar L, et al. A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging. 2015;41(1):220–5.
Rosenkrantz AB, Mendrinos S, Babb JS, Taneja SS. Prostate cancer foci detected on multiparametric magnetic resonance imaging are histologically distinct from those not detected. J Urol. 2012;187(6):2032–8.
Bomers JG, Barentsz JO. Standardization of multiparametric prostate MR imaging using PI-RADS. Biomed Res Int. 2014;431680(10):9.
Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66(2):343–51. This article compares outcomes of MRI-targeted biopsy between MRI-ultrasound fusion (MRF-TB) and visual targeting (VE-TB), demonstrating a trend toward improved cancer detection using MRF-TB. Additionally, MRF-TB was more often histologically informative and improved targeting in smaller lesions when compared to VE-TB.
Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66(1):22–9.
de Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. Eur Urol. 2014;66(3):430–6.
Compliance with Ethics Guidelines
Conflict of Interest
Andrea S. Kierans, Samir S. Taneja, and Andrew B. Rosenkrantz each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Office Urology
Rights and permissions
About this article
Cite this article
Kierans, A.S., Taneja, S.S. & Rosenkrantz, A.B. Implementation of Multi-parametric Prostate MRI in Clinical Practice. Curr Urol Rep 16, 56 (2015). https://doi.org/10.1007/s11934-015-0530-x
Published:
DOI: https://doi.org/10.1007/s11934-015-0530-x