Abstract
Imaging for urothelial cancer, particularly that located in the bladder, has generally been based on computed tomography (CT). However in more recent times the role of positron emission tomography-CT (PET-CT) has emerged and increasingly magnetic resonance (MR) imaging has become utilised. This concise review will outline the role of these modalities when dealing with muscle-invasive bladder cancer (MIBC).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urothelial cancer of the bladder (bladder carcinoma) is the most frequent type of tumour of the urinary tract and is most prevalent in the fifth to seventh decade of life [1]. More than 90 % of bladder cancers are urothelial (transitional cell) carcinomas, 5 % are squamous cell carcinomas, and less than 2 % are adenocarcinomas. Approximately 70 % of bladder cancers present as superficial tumours, which tend to recur, and 30 % present as muscle-invasive bladder cancer (MIBC) associated with a high risk of death from distant metastases [2, 3••].
Role of Imaging in Invasive Bladder Cancer
Imaging of MIBC imaging should be divided into diagnosis and staging whereby finding the urothelial cancer as a targeted investigation for haematuria for diagnosis is vastly different to localised staging of a known tumour and for distant staging (and re-staging) of non- urothelial viscera, nodes and bones.
Diagnosis of bladder cancer is generally made due to investigation of haematuria with a cystoscopy or a renal tract ultrasound for investigation of haematuria. Further, increasingly incidental lesions are detected when cross-sectional imaging of the pelvis is undertaken for non-urological reasons. Generally a renal tract ultrasound is done. In terms of other modalities to investigate haematuria, computed tomography intravenous urography (CTIVU) is an excellent modality for detecting both upper and lower urinary tract lesions with a filling defect noted and depth of invasion recognisable in some cases. Diagnosis is unlikely to be made using positron emission tomography-CT (PET-CT) and; hence, it only has a role in staging, re-staging and monitoring of chemotherapy responses. However, no imaging modality can readily diagnose subtle lesions (which may indeed be muscle invasive) nor carcinoma-in-situ. Hence cystoscopy remains in every algorithm for investigation of haematuria. Ultrasound is largely used for screening and diagnosis and will not be discussed further.
Staging of MIBC involves imaging of the chest, abdomen and pelvis. Occasionally a bone scan may be required if history or a raised alkaline phosphatease suggests bony disease.
Nodal disease prior to visceral disease and bony disease occur in that order and frequency.
International Guidelines have made recommendations regarding staging. According to the most recent European Association of Urology (EAU guidelines) [4] muscle invasive bladder EAU guidelines, patients with confirmed muscle-invasive BCa should be staged by computed tomography (CT) scans of the chest, abdomen, and pelvis, if available.
The National Comprehensive Cancer Network (NCCN) [5] suggests a computed tomography or MR to investigate muscle invasive bladder cancer with chest imaging not specified (chest roentography or CT).
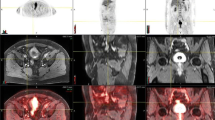
The situation is more clear-cut for loco-regional staging and is best conducted by CT (Figs. 1 and 2) or MR for depth of invasion into the muscle and surrounding perivesical tissues and adjacent organs (e.g., prostate in men).
Re-staging including post chemotherapy is a distinct area (Fig. 3). Increasingly neoadjuvant chemotherapy is accepted as a valuable tool in the treatment of MIBC. In particular patients with nodal disease (and sometimes perivesical fat invasive [T3] disease), they should be offered neoadjuvant chemotherapy [4, 5]. CT and more recently MR and PET-CT have emerged as the tools of choice for accurate staging of NMIBC. In particular with PET-CT, which will be discussed, the ability to distinguish metabolically active disease rather than that based on size criterion alone is essential in selecting patients.
However, despite advances in imaging, incorrect clinical staging and especially understaging remains a serious problem in MIBC, and improvements in all steps of the staging process are needed to achieve more accuracy and improved care for MIBC patients [6].
Imaging Modalities
The imaging modalities will now each be discussed in more depth.
Computed Tomography
Around thirty years old and entrenched in the past decade, CT and CTIVU are now the most common tools for assessing the urothelium where NMIBC has been diagnosed or is suspected. Assessing the urothelium was once the domain of coronal plane imaging via IVU. Although still valid and inexpensive with minimal radiation burden, it is laborious and has been largely abandoned in the Western world for assessing upper tract and even bladder urothelium. Nevertheless, as with IVU, for CTIVU the filling defect is still important as is the appearance of any abnormal structures in the ureter or surrounding tissues.
Staging of UC is really where CT has excelled. In one examination the chest, retroperitoneum abdominal and pelvic cavity as well as the groin regions are easily imaged. Equivocal nodal enlargement of just over a cm remains just that- equivocal. Extravesical spread is predicted in certain cases but is by no means accurate in all, particularly if a recent surgical artefact remains.
A summary of the results of CT are presented in Table 1.
Magnetic Resonance Imaging
Magnetic Resonance (MR) imaging is an imaging modality with excellent soft-tissue contrast. Dynamic contrast enhanced MR (DCE-MR) and MR spectroscopy (MRS) techniques complement conventional imaging in these areas. Further combinations of these MR techniques can also guide treatment selection and planning, and has been found to have an incremental value to assessment of local stage but bladder cancer has few studies [7•, 8, 9•].
MR for the staging of MIBC is somewhat limited due to access and cost. However, there is reasonable data that excellent imaging of both the upper and lower tracts is possible. Furthermore, the retroperitoneum and pelvis may be assessed without the burden of radiation. Criticisms of subtle findings with older machines are being overcome with the faster 3T incarnations.
With radiation burden and better access UC staging and re-staging with MR may increase in the coming decade. Issues of intravenous contrast still remain despite the initial hope that gadolinium posed fewer or no risks, but such has not been realised. However, compared to CT, which in many centres is with its multi- plane reconstructions the gold standard for staging and re- staging, MR compares very favourably for staging (Table 1) [9•].
Furthermore, MR remains useful to reduce radiation burden in younger patients, to help define pelvic anatomy particularly for surgical planning where, for example, in females, bladder neck or urethral involvement is suspected. Data on pelvic nodes is growing but no head-to-head comparisons versus CT have been done. Diffusion weighted MR is becoming more commonplace but little data exist [6, 9•, 10].
Overall, the optimal management of patients with MIBC is dependent on accurate staging and detection of metastatic disease. Current imaging techniques including ultrasonography, CT and MR have not proven to be highly accurate (Table 1) [11, 12].
Positron-Emission Tomography Combined with Computed Tomography (PET-CT)
As with all urologic oncology, imaging continues to evolve and clinicians should be aware of the role, advantages and limitations of each. Further, costs and critical analysis of the value of each modality for individual patients must be weighed against access and expertise at any one center.
F-fluorodeoxyglucose (FDG) remains the most common PET radiotracer used in prostate and bladder cancer evaluation (Fig. 4), but its role is hampered by a generally low glucose metabolic rate in primary prostate carcinoma, and physiologic excretion of FDG through the urinary system masking FDG uptake in primary bladder carcinoma. The use of diuresis strategies facilitates the identification of primary bladder cancer, and may be useful in staging the extravesical spread of disease. FDG PET may also be useful in patients with ureteric and urethral cancers. New PET tracers are showing promise in the staging and biologic characterisation of prostate cancer, which can assist with therapeutic decision making in patients undergoing radiotherapy of primary disease, and in the assessment of metastatic disease [3••].
Detection and local staging in MIBC using FDG PET has not been widely undertaken due to physiologic FDG activity excreted through the urinary system interferes with the visualisation of primary bladder cancer and loco regional lymph nodes. Adequate hydration, delayed pelvic images after oral hydration and forced diuresis with frusemide administration are amongst the various mechanisms that have been looked at to improve the diagnostic capabilities of FDG PET [11, 13, 14]. Table 2 outlines the studies of relevant F-FDG PET radiotracers in bladder carcinoma [11]. C-acetate PET/CT is also being explored with reasonable results but only small numbers (Table 2). However 11C-acetate was disappointing for nodal disease post chempotherapy due to granulomatous disease [15].
A recent meta-analysis of FDG PET in the staging and restaging of bladder cancer found that the pooled sensitivity was 82 % and pooled specificity was 89 % [11]. Impressively the global measure of diagnostic accuracy was 0.92. The results of this meta-analysis suggest that FDG PET or PET/CT provides good diagnostic accuracy of lymph nodes; however, the application of FDG PET or PET/CT in diagnosis and localised staging of MIBC is in some instances restricted by radiotracer urinary excretion. Further, in the same meta-analysis, there were four studies which used intravenous injection of frusemide, [13, 16–18] with two using delayed pelvic images after oral hydration and diuretic administration [13, 17]. A more complete summary of the role of PET-CT in staging of bladder cancer has recently been published by Lee et al. [3••].
Conclusion
Imaging of urothelial carcinoma (TCC) arising in the bladder has undergone rapid change in the past decade (Table 3). Ultrasound has significantly improved in terms of diagnostic capabilities whilst CT and CTIVU have become the cornerstone of staging with IVU largely obsolete in the Western world. In recent years MR and PET-CT are increasingly being investigated as tools that add to the management of select UC patients for staging with a recent prospective analysis of supporting little difference between these and CT [7•].
PET-CT remains a staging tool to help select patients suitable for surgery, chemotherapy and even radiation and is increasingly utilised as a problem- solving tool. However, it will become increasingly a prognostic and management directive tool as more data emerges. There have been numerous studies which have looked at the utility of FDG in various lower genitourinary carcinomas, most commonly in prostate and bladder carcinoma. Whilst general principles of FDG excretion in the urine can impose a high degree of difficulty in scan interpretation in these malignancies, there is still a role in selected patient populations, such as in the staging and restaging of advanced prostate cancer. There is promising evolving evidence for the use of new non-FDG PET radiotracers which would provide greater biological characterisation, which may be able to assist in the management decisions in these patients [3••].
Thus in the management directive sense, selection and response to chemotherapy may be possible as well as moving appropriate patients to trials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49.
•• Lee ST, Lawrentschuk N, Scott AM. Pet in prostate and bladder tumors. Semin Nucl Med. 2012;42:231–46. A comprehensive analysis on the limitations of staging as it currently stands for muscle invasive bladder cancer including with contemporary imaging.
Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS. The updated eau guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009;55:815–25.
National Comprehensive Cancer Network (NCCN). Nccn clinical practice guidelines in oncology (nccn guidelines®)-bladder cancer 2012 (Version 1, 2013).
Bostrom PJ, van Rhijn BWG, Fleshner N, Finelli A, Jewett MA, Thoms J, et al. Staging and staging errors in bladder cancer. Eur Urol Suppl. 2010;9:2–9.
• Vargas HA, Akin O, Schoder H, Olgac S, Dalbagni G, Hricak H, et al. Prospective evaluation of mri, (11)c-acetate pet/ct and contrast-enhanced ct for staging of bladder cancer. Eur J Radiol. 2012;81:4131–7. One of the few prospective studies examining CT, PET-CT and MR in a prospective fashion to stage bladder cancer.
Purysko AS, Leao Filho HM, Herts BR. Radiologic imaging of patients with bladder cancer. Semin Oncol. 2012;39:543–58.
• Green DA, Durand M, Gumpeni N, Rink M, Cha EK, Karakiewicz PI, et al. Role of magnetic resonance imaging in bladder cancer: Current status and emerging techniques. BJU Int. 2012;110:1463–70. A comprehensive analysis of the benefits and pitfalls of MR in bladder cancer as a staging tool for bladder cancer with a call for more prospective studies.
Giannarini G, Petralia G, Thoeny HC. Potential and limitations of diffusion-weighted magnetic resonance imaging in kidney, prostate, and bladder cancer including pelvic lymph node staging: a critical analysis of the literature. Eur Urol. 2012;61:326–40.
Lu YY, Chen JH, Liang JA, Wang HY, Lin CC, Lin WY, Kao CH. Clinical value of fdg pet or pet/ct in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol. 2011.
Paik ML, Scolieri MJ, Brown SL, Spirnak JP, Resnick MI. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000;163:1693–6.
Anjos DA, Etchebehere EC, Ramos CD, Santos AO, Albertotti C, Camargo EE. 18f-fdg pet/ct delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med. 2007;48:764–70.
Kosuda S, Kison PV, Greenough R, Grossman HB, Wahl RL. Preliminary assessment of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with bladder cancer. Eur J Nucl Med. 1997;24:615–20.
Schoder H, Ong SC, Reuter VE, Cai S, Burnazi E, Dalbagni G, et al. Initial results with (11)c-acetate positron emission tomography/computed tomography (pet/ct) in the staging of urinary bladder cancer. Mol Imaging Biol. 2012;14:245–51.
Drieskens O, Oyen R, Van Poppel H, Vankan Y, Flamen P, Mortelmans L. Fdg-pet for preoperative staging of bladder cancer. Eur J Nucl Med Mol Imaging. 2005;32:1412–7.
Harkirat S, Anand S, Jacob M. Forced diuresis and dual-phase f-fluorodeoxyglucose-pet/ct scan for restaging of urinary bladder cancers. Indian J Radiol Imaging. 2010;20:13–9.
Kibel AS, Dehdashti F, Katz MD, Klim AP, Grubb RL, Humphrey PA, et al. Prospective study of [18f]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27:4314–20.
Bachor R, Kotzerke J, Reske SN, Hautmann R. Lymph node staging of bladder neck carcinoma with positron emission tomography. Urologe A. 1999;38:46–50.
Apolo AB, Riches J, Schoder H, Akin O, Trout A, Milowsky MI, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol. 2010;28:3973–8.
Jadvar H, Quan V, Henderson RW, Conti PS. [f-18]-fluorodeoxyglucose pet and pet-ct in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol. 2008;13:42–7.
Disclosure
No conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawrentschuk, N., Lee, S.T. & Scott, A.M. Current Role of PET, CT, MR for Invasive Bladder Cancer. Curr Urol Rep 14, 84–89 (2013). https://doi.org/10.1007/s11934-013-0308-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11934-013-0308-y