Abstract
Much progress has been made in the use of imaging as a diagnostic tool in giant cell arteritis (GCA), which assists in the management of patients where the initial diagnosis is unclear. This includes patients with atypical cranial symptoms, or with predominantly systemic, constitutional or limb symptoms. Ultrasound and magnetic resonance imaging are capable of visualising both the cranial and extracranial large vessel circulation, with vessel wall thickening and stenotic lesions being visualised. Computed tomographic angiography is helpful in visualising the aorta for aneurysm complicating GCA but can also detect vessel wall thickening in established large vessel vasculitis. PET-CT is a very sensitive test for early vascular inflammation in extracranial large vessel vasculitis, before aneurysmal or stenotic lesions have developed, of use in the patient with unexplained constitutional symptoms. The place of imaging in the follow-up of GCA is being investigated, and repeated imaging may be useful in select cases. Generally, vascular abnormalities become less defined once glucocorticoid treatment has been started, and therefore, imaging studies must be conducted early as part of a GCA fast-track assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Classical cases of cranial giant cell arteritis (GCA) are easily diagnosed. However, when GCA presents as large vessel vasculitis (LVV) in the form of refractory polymyalgia, isolated aortitis or more widespread large vessel involvement without cranial symptoms, imaging becomes essential to ascertaining the diagnosis and optimal management.
The complications of untreated GCA include ischaemic complications of sight loss and stroke. Sight loss has been reported to occur in between 15 and 25 % of patients at presentation [1]. Extracranial involvement is frequent [2–5] and can be complicated by aortic aneurysm formation and dissection, reported as occurring at a rate of 18 % in a report from the Mayo clinic, along with large artery stenosis at a rate of 13 % [6].
The traditional ‘gold standard’ test for GCA is biopsy of a temporal artery (TA), but biopsy positivity may be no greater than 40 % and mainly useful in cranial manifestations of the disease (as embodied in the 1990 ACR Classification criteria). Hence, imaging plays an increasingly important role in diagnosis and follow-up in GCA and indicated for the following reasons:
-
1.
Early diagnosis—ultrasound and cranial magnetic resonance imaging (MRI) versus TA biopsy
-
2.
Differentiation of mimics from GCA
-
3.
Assessment of refractory cases of GCA or polymyalgia rheumatica (PMR)
-
4.
Diagnosis of LVV in the context of a systemic syndrome (constitutional symptoms, anaemia, raised inflammatory markers)
-
5.
Differentiation from other causes of LVV such as retroperitoneal fibrosis, IgG4 disease, isolated aortitis, inflammatory aortic aneurysms
-
6.
Assessment of disease activity and outcomes in evaluation of response to therapy
There is crossover in the applicability of imaging techniques and genuine overlap of GCA with other large vessel vasculitides, including Takayasu’s arteritis and IgG4-related disease. While reference will be made below to imaging in these diseases, these will not be included in this review.
This review will consider the use of ultrasound, computed tomography (CT), MRI and fluorodeoxyglucose positron emission tomography (FDG PET), with angiography briefly reviewed for historical interest. The modalities are discussed with regard to the purposes of imaging in GCA as stated above.
Ultrasound
The use of colour Doppler ultrasound for the assessment of LVV mirrors the technological development of the equipment and probes available. Reports on the use of Doppler ultrasonography for studying arterial flow patterns appeared from the late 1970s. In 1990, long segments of circumferential thickening of the carotid arteries were found to be characteristic of Takayasu’s arteritis [7], later described as the “macaroni” sign [8].
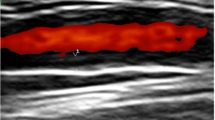
Probes capable of studying superficial temporal arteries allowed demonstration of equivalent abnormalities in these vessels [9], described as ‘hypoechoic halos’ surrounding vessel lumen, due to an oedematous thickened artery wall (Fig. 1). This is different from the focal hyperechoic wall thickening seen in atherosclerosis. The addition of Doppler studies have identified areas of stenosis and occlusion indicating damage due to arteritis, though this is not a specific finding of GCA [10].
The technique also allows examination of other cranial (occipital and facial) and extra-cranial (axillary, subclavian) arteries (Fig. 2). Ultrasound of the extracranial arteries improves the sensitivity of the test. The axillary arteries are easily accessible, and involvement is found in 53 % of patients diagnosed with GCA [11], although only 60 % of patients with large vessel involvement in GCA have temporal arteritis. Diamantopoulous et al. reported that additional scanning of the carotid arteries improves the sensitivity of the test further [12]. The total time for examination is 30–40 min.
A probe with frequency greater than 15 MHz should be used (corresponding to an image resolution of 0.1 mm) with gain and dynamic range setting that allows vessel wall to be differentiated from the vessel lumen. With this, a resolution much greater than that achieved by other techniques can be obtained. The pulse repetition frequency during colour Doppler should range between 2 and 3.5 kHz depending on the calibre of vessel being examined, with inappropriately high settings giving the false appearance of a halo. Dynamic techniques such as the ‘compression sign’ described by Aschwanden et al. mitigate against operator experience [13•].
Ultrasound assessment of LVV is operator dependent. High sensitivity and specificity are obtained in the hands of an experienced operator, but very heterogeneous results have been reported in the various studies of this technique, with test sensitivities ranging from 35 to 86 % [14, 15]. Standardisation of technique and equipment, probe settings, image acquisition and examination of a number of cases, both normal and abnormal, before applying the test in practice, is required. Ultrasound may only detect transmural inflammatory involvement; limited histopathological abnormalities consistent with GCA may be difficult to detect [16].
A fast track pathway [17] that reduces sight loss with immediate treatment and assessment of GCA relies heavily on urgent diagnostic evaluation with ultrasound and, if indicated, a temporal artery biopsy. There is some evidence that sensitivity of ultrasound declines within a few days of GC therapy (discussed below). However, it makes biopsy unnecessary in unequivocally positive or negative cases [18].
Ultrasound can identify vasculitis in any region, including the lower limbs [19], abdominal aorta [20] and even the thoracic aorta by transesophageal echocardiography [21].
Computed Tomography and Computed Tomographic Angiography
Computed tomographic angiography (CTA) is useful in the diagnosis of extracranial LVV. Mural thickening, as well as wall enhancement in the venous phase, are considered signs of active large vessel disease, correlating with activity on PET-CT [22••, 23]. Evidence of aortitis may be seen in up to 65 % of patients with newly diagnosed GCA [24•]. CTA is more traditionally used to assess the lumen, and can therefore also detect stenotic and aneurysmal lesions that can complicate GCA [25]. Aneurysmal lesions at onset may indicate resistant disease and worse vascular prognosis [3]. Unusual patterns of stenosis not characteristic of atherosclerosis may alert a radiologist to the possibility of LVV.
Of likely greater clinical relevance, however, is the role of contrast-enhanced thoracic CT in the routine investigation of patients with inflammation of unknown origin. This is a simple, fast and easily accessible test, in which mural thickening of the aorta and its major branches can often be well demonstrated as an incidental finding (Fig. 3) [26] (personal data). Identification of this sign requires a vigilant radiologist who will often be primed to look for evidence of infection or malignancy by the requesting physician. We expect that, with greater awareness of the frequent extracranial large vessel involvement in GCA, the discovery of “incidental” LVV will increase. However, CT may not reliably detect mural thickening in early stages of LVV.
A 76-year-old female underwent routine CT scan (with contrast of her chest, abdomen, pelvis) for evaluation of weight loss, polymyalgia and anaemia and raised CRP. The scan revealed extensive aortitis and subsequent ultrasound evaluation confirmed ‘halos’ in the axillary artery. The temporal artery biopsy was positive indicating a diagnosis of ‘polymyalgia arteritica’. a Temporal artery halo before treatment. b, c PET-CT evidence of axillary artery vasculitis after starting treatment. di–iii Aortic wall thickening noted on contrast-enhanced thoracic CT
Magnetic Resonance Imaging
MRI is used to demonstrate vascular luminal anatomy of stenosis and dilatation, and measure mural thickening and enhancement, thought to correlate with disease activity.
In 1998, Mitomo et al. reported a case where MRA using a 1.5 T scanner appeared to demonstrate luminal narrowing, in a patient already diagnosed with GCA clinically and by biopsy [27]. An MRI of 1 T was found to be insensitive to vessel wall changes, being unable to provide sufficient resolution to examine the temporal artery wall in detail [28]. Bley et al. reported the use of 1.5 T contrast-enhanced MRI to evaluate mural inflammation of temporal arteries [29], followed by a series of 7 cases where the cranial vessels were successfully imaged using 3 T MRI, combining MRA with contrast-enhanced MRI to identify delayed mural enhancement [30]. In a subsequent study of 64 patients with suspected GCA, the technique was found to have a sensitivity of 85.7 % and specificity of 95.5 % with 10 days or less steroid treatment [30]. A further refinement is the use of T2-weighted BLADE imaging that can identify severe inflammation without contrast and reliably measure vessel wall thickness as a surrogate for inflammatory change, though contrast-enhanced MRI remains more sensitive [31]. Recently, a multicentre trial reported a sensitivity of approximately 80 % and specificity approaching 90 % in patients treated for 5 days or less, but sensitivity reducing to 72.7 % with treatment for 6–14 days [32•].
Other groups have reported cases of MRI being used in cranial GCA [33, 34]. The use of a 32 channel surface coil appears to offer no advantage to a 12 channel coil, which potentially allows combination with neck and body coils for a more comprehensive vascular assessment [35].
MRI of 3 T has been used to investigate the involvement of intracranial, deep ophthalmic and deep temporal arteries [36–38]. The clinical correlation of abnormal findings in this context is not yet established.
ECG gated 1.5 and 3 T MRI, with appropriate surface coils, allows imaging of the aorta, iliac vessels and major upper body branches, making this suited to the evaluation of extracranial LVV. Hence, there has been much focus on this technique in the context of Takayasu’s arteritis, where the younger patient population makes reduced radiation exposure advantageous and the pattern of vessel involvement is predominantly extracranial. However, its use in GCA has been investigated [39], including the possibility of simultaneously combining cranial imaging [40].
Stenotic lesions are common in GCA and often asymptomatic. They may signify damage rather than active disease. In a report of 28 patients, 19 were found to have subclavian or axillary artery involvement, but only 6 had signs or symptoms [41]. Mural changes were not evaluated. The study of Meller et al. used 1.5 T MRI to study 14 patients with LVV. Thirteen patients were found to be positive on MRI. Interestingly, no abnormalities were found on MRA. Forty seven of seventy six territories concorded between MRI and PET, with the authors of the opinion PET was a more reliable investigation [42].
FDG PET and PET-CT
There are many case reports illustrating the use of PET and PET-CT with FDG in the diagnosis of GCA. Blockmans et al. reported a prospective study of 35 patients followed with serial PET [43]. The degree of FDG uptake appeared to correlate with CRP [44]. These findings not only established PET-CT as a potential diagnostic tool for GCA but confirmed GCA as a systemic disease with frequent extracranial involvement. A meta-analysis has determined an overall sensitivity of 80 % and specificity of 89 % for PET and PET-CT in the diagnosis of GCA when compared to reference clinical criteria [45]. However, ensuring that PET is performed while patients are still steroid naïve can improve sensitivity [46].
Metabolically active lesions, which may not be specific to vasculitis, can introduce challenges in interpretation. Atherosclerotic lesions, being metabolically and immunologically active, take up FDG [47]. Blockmans et al. noted a large number of “control” patients with non-vasculitic diagnoses had FDG uptake in lower limb vessels [48]. The combination of PET with CT images improves the current limited resolution of PET and allows for better morphological identification of atheromatous plaques.
Most clinicians and reports have relied on the trained eye of a reporting radiologist, with smooth uptake of FDG along a length of aorta and its branches being considered characteristic of LVV (Fig. 3). However, a number of methods have been employed to provide more accurate quantification, making use of the measured standardised uptake value (SUV) of an area of interest. One approach has been to compare vascular wall uptake against background liver uptake [45]. The Meller scale [42] uses a semiquantitative 0–3 scale, with 0 (no uptake) and 1 (uptake less than liver background) being negative, and 2 (equal to liver) and 3 (higher than liver) being positive. However, systemic inflammation may also involve the liver, so that it does not serve as true reference standard. A comparison of arterial wall uptake to venous blood pool SUVmax (maximum measured SUV in the area of interest) was found to be superior [49•], but this has not been confirmed by others or compared to visual analysis.
However, although a semiquantitative approach may increase the objectivity of findings, it does not necessarily increase diagnostic accuracy. Limitations are the difficulty in precisely defining areas of interest that do not overlap with adjacent structures, and the frequent presence of atherosclerotic lesions in the population affected by GCA that would contribute to false positive results. Lehmann et al. found that a purely quantitative approach increased the sensitivity of analysis greatly, at the expense of much reduced specificity [50]. Further, Meller noted differences in measured background uptake depending on whether a dedicated or hybrid PET scanner was used, implying that variability is introduced by different scanning equipment [42].
Imaging the cranial arteries by PET is technically difficult due to the high FDG uptake by the brain and limited camera resolution. Available technology continues to improve, and Muto et al. were able to identify temporal artery inflammation on PET-CT images [22••].
Despite its usefulness, the applicability of PET-CT is limited by its cost and the amount of radiation exposure involved. The use of immunosuppression at the time of PET has the potential to reduce sensitivity (from 99.6 to 52.9 % in one report [51]), limiting it to patients with atypical or subclinical vasculitis due to the emphasis in current guidance on early empirical treatment in cranial GCA. Glycaemic levels should be well controlled, although whether this is as relevant in inflammatory conditions as it is in malignancy is not firmly established [52, 53]
Conventional Angiography
Conventional angiography is of historic interest. Gillanders [54] described a series of 15 patients where what were thought to be characteristic changes of GCA were seen in affected patients which were then used as a guide to biopsy location. The findings appeared to be replicated by the references [55, 56, 57]. However, in later studies, Layfer [58] found that the purported characteristic changes were not specific, while Sewell et al. [59] determined that angiography was not an alternative to biopsy.
Angiography of the aorta or limbs can indicate a diagnosis of extracranial LVV and delineate extent of vessel involvement [57]. Angiographic abnormalities form part of the ACR 1990 criteria for the classification of Takayasu’s arteritis. Occasionally, angiography can establish disease of the limb vessels in isolation [60]. However, the test is limited by only being able to demonstrate areas of stenosis and dilatation, which are likely signs of damage rather than active inflammation.
Imaging Findings After Initiating Treatment and During Follow-Up
Imaging findings of cranial vasculitis appear to disappear rapidly with treatment. Schmidt et al. noted in a longitudinal study that, on average, appearances resolved after a mean of 16 days of treatment [9], with Karahaliou et al. noting a similar mean time of 22 days to resolution [10]. Hauenstein et al. noted sensitivity of 88 % within the first day of treatment, reducing to 50 % after more than 4 days of treatment [61••]. Disappearance of the halo sign has been reported after only 2 days of treatment [62], making early ultrasound necessary for the test to be discriminative.
Similar findings have been reported for cranial MRI, where Hauenstein et al. noted an initial sensitivity of 88 % reducing to 56 % after 4 days [61••]. In a large prospective series, the sensitivity reduced from approximately 80 to 72.7 % with treatment for 6–14 days [32•]. MRI abnormalities have been reported to disappear after 16 months of successful treatment [63].
De Miguel et al. reported much longer times for resolution, with a mean time of 11 weeks till halo disappearance [64], and Perez-Lopez et al. have noted persistence in half of a cohort of 26 halo-positive patients at 6 weeks [65]. Persistence up to 7 months has also been reported [66]. A possible explanation for longer lasting halos could be intimal proliferation with fibrosis that is often histologically reported as healed arteritis.
Resolution of aortic wall thickening with treatment has been reported after a mean interval of 6.3 months [22••]. In cases of limb arteritis imaged by ultrasound, improvement, but not necessarily complete resolution, is noted after mean intervals of 21.9 [67] and 40 months [68]. However, a report by Meller et al. did not show reduction of vessel wall thickening after a median interval of 19 months when studied by MRI [42].
FDG uptake on PET also reduces with treatment but does not necessarily normalise (Fig. 3). Blockmans [43] reported that, although there was reduction in FDG uptake between onset and 3 months, residual uptake persisted to 6 months, suggesting that vascular lesions remain metabolically active after they are treated. Muto et al. similar found improvement, but not resolution, of PET findings after mean interval of 4.6 months [22••]. The place of repeat PET in suspected relapse is therefore unclear without interval scanning. Despite this, serial PET has been used as an outcome measure in response to treatment, albeit with PET results apparently lagging some months behind clinical response [69].
Large vessel wall enhancement on MRI is considered equivalent to FDG uptake on PET and would be expected to improve with treatment. However, this finding has not been prospectively studied in GCA. More extensive studies in Takayasu’s arteritis have shown inconsistent findings during follow-up.
Delayed wall enhancement may persist in inactive disease [70]. This has been attributed to the distribution of gadolinium contrast media into fibrotic as well as inflamed tissue. This property of gadolinium is well described in the context of cardiac MRI, in Takayasu’s arteritis [71]. Contrast agents that interact with plasma proteins have prolonged intravascular persistence and reduced interstitial distribution [72]. However, even oedema-weighted imaging was found to demonstrate oedema in 56 % of cases in clinical remission based on National Institutes of Health criteria [73]. A further report complicates the picture by suggesting mural thickening correlates with disease activity at diagnosis, but that reduction in mural enhancement more reliably reflects reduced disease activity at 6 months [74].
A possible explanation is that these findings may reflect true subclinical disease activity, increasingly recognised in supposedly quiescent GCA. Furthermore, although the two diseases share histopathological features, they remain separate entities, which can reflect in imaging differences [75].
Apart from suggestions that patients with large vessel involvement are younger and at perhaps lower risk of ischaemic complications [68, 76, 77], the place of imaging appearances in determining prognosis is unclear.
Czihal et al. noted that patients with both cranial and upper limb involvement by USS appeared to have more resistant disease [76]. Initial PET findings did not appear to predict risk of relapse [43]. However, aortic FDG uptake correlates with later aneurysm formation [78].
The place of imaging in monitoring and relapse is not yet defined. The sensitivity of the halo sign for identifying relapse is undetermined. However, the specificity of the halo sign and its rapid resolution after treatment suggests its utility for monitoring patients with continuing or recurring symptoms (Fig. 1).
Both et al. found that MRI and PET in the context of difficult to control vasculitis do not agree with each other, with MRI apparently being overly sensitive and perhaps less specific than PET [79].
Surveillance for Aortic Aneurysm
An unresolved issue remains surveillance for aortic aneurysm formation. In a longitudinal study conducted over 50 years, an incidence of aneurysm and/or dissection of 18.7 per 1000 person-years at risk was found, with thoracic aortic dissection occurring at a rate of 5.4 per 1000 person-years at risk [6]. Evidence regarding how to conduct screening is lacking. A recent systematic review found that between 5 and 10 patients would need to be screened to identify a thoracic aneurysm [80•].
The best timing and method for screening is undefined. Ultrasound examination of the abdominal aorta is a simple non-invasive test that can easily be implemented in a screening programme. However, imaging of the thoracic aorta is more involved. A chest X-ray is a simple test that detect mediastinal enlargement but is not sensitive. Alternatives include CTA or MRA, or echocardiography of the aortic root and proximal aorta. Currently, British Society of Rheumatology guidance is to perform a chest X-ray every 2 years to monitor for development of thoracic aneurysm [81], while European League Against Rheumatism recommendations state that aortic imaging should be considered, especially if an aortic insufficiency murmur is heard [82].
Imaging as a Clue to a Non-GCA Diagnosis
Imaging may play a vital role in determining a non-GCA mimic diagnosis in the context of fast track clinics that are referred early cases of suspected GCA. Cross-sectional imaging techniques particularly CT and MRI can incidentally note pathology or find alternative causes of patient symptomatology, including potentially serious disease. This is of particular relevance in the context of GCA Fast Track clinics where the consultation may be the first clinical opportunity for detection (Fig. 4).
A 78-year-old female was referred to ENT at Southend Hospital, UK, with severe jaw pain and tenderness, pain radiating to the temple, raised ESR, CRP and weight loss. She was started on prednisolone 60 mg daily and referral re-directed to rheumatology “fast track” for urgent evaluation. Ultrasound of temporal arteries and branches and axillary arteries was normal. Further imaging undertaken with CT scan head and neck and MRI of the throat revealed a large posterior tongue tumor with metastatic in mandibular lymph node
Discussion
In many rheumatic diseases, there has been a shift in emphasis to early diagnostic criteria to enter the window of opportunity for effective treatment before damage. In the context of GCA, this includes cranial ischaemic complications, aortic aneurysm formation and extended periods of generally debilitating illness. The 1990 ACR criteria for classification focus on the cranial manifestations of GCA, while we need strategies for the assessment of extracranial involvement in diagnosis and ongoing management. Meanwhile, cranial GCA may not declare itself and is often imitated by other conditions, making cranial imaging helpful in this context as well.
Imaging has become an integral part of diagnosis and assessment in GCA and the related condition of polymyalgia rheumatica. The halo sign on ultrasound is a critical sign in Fast track GCA clinics. Specific signs of LVV activity on ultrasound, CTA, MRI or FDG-PET complement laboratory assessments and supply vital information (Table 1). In addition, signs of damage in the form of stenotic or aneurysmal complications can be identified.
A consistent finding in all investigative procedures for cranial GCA is that results normalise rapidly with corticosteroids. Due to the obligation to start early treatment, this means that imaging investigations must be available early in the assessment process. “Fast-track” treatment pathways are therefore required with rapid access to imaging investigations for these to be worthwhile.
The role of imaging in follow-up, particularly in relapsing and non-responding patients, and especially in those without classical symptoms, has not been delineated. In general, imaging abnormalities resolve with treatment, but the frequent reports of persisting abnormalities in patients clinically in remission mean that findings in individual patients can be difficult to interpret. Further longitudinal studies are required.
With current techniques, it is now possible to fully characterise the extent and activity of vessel involvement in newly diagnosed patients. However, the relationship between these parameters and treatment required or the ischaemic risk posed has not been defined. Large vessel involvement may cause low ischaemic risk to limb circulation, whereas aortic aneurysms and vascular insufficiency in other areas may occur due to concomitant arteriosclerosis in this patient age group.
Each imaging investigation has its relative strengths and weaknesses, but in general, all have a place in initial diagnosis and follow-up (Table 1). Comparable test performances [83] would suggest that the practical approach should develop a combination of imaging modalities in complementary fashion based on local resources and expertise. However, the frequent use of contrast-enhanced CT and vascular ultrasound also means that practitioners should be aware of the signs of vasculitis where they are seen incidentally.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dasgupta B, Patil, Karia N. Giant cell arteritis: a review. Eye Brain. 2013.
Agard C, Ponge T, Fradet G, Baron O, Sagan C, Masseau A, et al. Giant cell arteritis presenting with aortic dissection: two cases and review of the literature. Scand J Rheumatol. 2006;35(3):233–6.
Espitia O, Neel A, Leux C, Connault J, Espitia-Thibault A, Ponge T, et al. Giant Cell Arteritis with or without Aortitis at Diagnosis. A Retrospective Study of 22 Patients with Longterm Followup. J Rheumatol. 2012;39(11):2157–62.
Evans JM, Bowles CA, Bjornsson J, Mullany CJ, Hunder GG. Thoracic aortic aneurysm and rupture in giant cell arteritis. A descriptive study of 41 cases. Arthritis Rheum. 1994;37(10):1539–47.
García-Martínez A, Hernández-Rodríguez J, Arguis P, Paredes P, Segarra M, Lozano E, et al. Development of aortic aneurysm/dilatation during the followup of patients with giant cell arteritis: a cross-sectional screening of fifty-four prospectively followed patients. Arthritis Rheum. 2008;59(3):422–30.
Nuenninghoff DM, Hunder GG, Christianson TJH, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48(12):3522–31.
Bond JR, Charboneau JW, Stanson AW. Takayasu’s arteritis. Carotid duplex sonographic appearance, including color Doppler imaging. J Ultrasound Med Off J Am Inst Ultrasound Med. 1990;9(11):625–9.
Maeda H, Handa N, Matsumoto M, Hougaku H, Ogawa S, Oku N, et al. Carotid lesions detected by B-mode ultrasonography in Takayasu’s arteritis: “macaroni sign” as an indicator of the disease. Ultrasound Med Biol. 1991;17(7):695–701.
Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337(19):1336–42.
Karahaliou M, Vaiopoulos G, Papaspyrou S, Kanakis MA, Revenas K, Sfikakis PP. Colour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res Ther. 2006;8(4):R116.
Czihal M, Zanker S, Rademacher A, Tatò F, Kuhlencordt PJ, Schulze-Koops H, et al. Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol. 2012;41(3):231–6.
Diamantopoulos AP, Haugeberg G, Hetland H, Soldal DM, Bie R, Myklebust G. Diagnostic Value of Color Doppler Ultrasonography of Temporal Arteries and Large Vessels in Giant Cell Arteritis: A Consecutive Case Series: US in GCA. Arthritis Care Res. 2014;66(1):113–9.
Aschwanden M, Daikeler T, Kesten F, Baldi T, Benz D, Tyndall A, et al. Temporal artery compression sign--a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med Stuttg Ger 1980. 2013;34(1):47–50. The temporal artery halo compression sign is an important technique to help reduce interobserver variability.
Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord. 2010;11(1):44.
Ball EL, Walsh SR, Tang TY, Gohil R, Clarke JMF. Role of ultrasonography in the diagnosis of temporal arteritis. Br J Surg. 2010;97(12):1765–71.
Muratore F, Boiardi L, Restuccia G, Macchioni P, Pazzola G, Nicolini A, et al. Comparison between colour duplex sonography findings and different histological patterns of temporal artery. Rheumatology. 2013;52(12):2268–74.
Patil P, Williams M, Maw WW, Achilleos K, Elsideeg S, Dejaco C, Borg F, Gupta S, Dasgupta B. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol. 2015;33(2 Suppl 89):103–6.
Alberts M. Temporal arteritis: improving patient evaluation with a new protocol. Perm J. 2013;17(1):56–62.
Sigl M, Hsu E, Scheffel H, Haneder S, Rümenapf G, Amendt K. Lower extremity vasculitis in giant cell arteritis: Important differential diagnosis in patients with lower limb claudication. Vasa. 2014;43(5):326–36.
Agard C, Hamidou M-A, Said L, Ponge T, Connault J, Chevalet P, et al. Screening of abdominal aortic involvement using Doppler sonography in active giant cell (temporal) arteritis at the time of diagnosis. A prospective study of 30 patients]. Rev Médecine Interne Fondée Par Société Natl Francaise Médecine Interne. 2007;28(6):363–70.
Stengl KL, Buchert R, Bauknecht H, Sobesky J. A hidden giant: Wallenberg syndrome and aortal wall thickening as an atypical presentation of a giant cell arteritis. Case Rep. 2013;2013(mar01 1):bcr2012006994–bcr2012006994.
Muto G, Yamashita H, Takahashi Y, Miyata Y, Morooka M, Minamimoto R, et al. Large vessel vasculitis in elderly patients: early diagnosis and steroid-response evaluation with FDG-PET/CT and contrast-enhanced CT. Rheumatol Int. 2014;34(11):1545–54. An important paper demonstrating the usefulness of both CT and PET-CT in the evaluation of patients with inflammation of unknown origin. The characteristic findings of LVV should be recalled when interpreting these imaging tests, particularly when considering the ubiquitous use of CT in the diagnostic work-up of these patients.
Polachek A, Pauzner R, Levartovsky D, Rosen G, Nesher G, Breuer G, et al. The fine line between Takayasu arteritis and giant cell arteritis. Clin Rheumatol. 2015;34(4):721–7.
Prieto-Gonzalez S, Arguis P, Garcia-Martinez A, Espigol-Frigole G, Tavera-Bahillo I, Butjosa M, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012;71(7):1170–6. This study in biopsy proven patients demonstrated both the utility of CT angiography in diagnosis, as well as the high frequency of large vessel involvement in newly diagnosed patients.
Rodríguez-Caulo EA, Velázquez CJ, García-Borbolla M, Barquero JM. Mega-Aorta Syndrome Development in Giant Cell Arteritis. A Same Entity? Ann Vasc Surg. 2011;25(8):1141.e1–3.
Cabero Moyano J, Andreu Magarolas M, Castañer González E, Gallardo Cistaré X, Belmonte Castan E. Nonurgent aortic disease: clinical-radiological diagnosis of aortitis. Radiología. 2013;55(6):469–82.
Mitomo T, Funyu T, Takahashi Y, Murakami K, Koyama K, Kamio K. Giant cell arteritis and magnetic resonance angiography. Arthritis Rheum. 1998;41(9):1702.
Ghinoi A, Zuccoli G, Nicolini A, Pipitone N, Macchioni L, Bajocchi GL, et al. 1T magnetic resonance imaging in the diagnosis of giant cell arteritis: comparison with ultrasonography and physical examination of temporal arteries. Clin Exp Rheumatol. 2008;26(3 Suppl 49):S76–80.
Bley TA, Weiben O, Uhl M, Vaith P, Schmidt D, Warnatz K, et al. Assessment of the cranial involvement pattern of giant cell arteritis with 3T magnetic resonance imaging. Arthritis Rheum. 2005;52(8):2470–7.
Bley TA, Uhl M, Carew J, Markl M, Schmidt D, Peter H-H, et al. Diagnostic value of high-resolution MR imaging in giant cell arteritis. AJNR Am J Neuroradiol. 2007;28(9):1722–7.
Geiger J, Bley T, Uhl M, Frydrychowicz A, Langer M, Markl M. Diagnostic value of T2-weighted imaging for the detection of superficial cranial artery inflammation in giant cell arteritis. J Magn Reson Imaging JMRI. 2010;31(2):470–4.
Klink T, Geiger J, Both M, Ness T, Heinzelmann S, Reinhard M, et al. Giant cell arteritis: diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis-results from a multicenter trial. Radiology. 2014;273(3):844–52. A multicentre trial confirming the validity of MRI in GCA diagnosis even in different testing centres with varying equipment.
Al-Zubidi N, Mai C, Haykal HA, Lee AG. Temporal artery enhancement on cranial magnetic resonance imaging. Can J Ophthalmol J Can Ophtalmol. 2014;49(3):e63–5.
Carra-Dalliere C, Menjot de Champfleur N, Heroum C, Bonafe A, Arquizan C. Giant cell arteritis with severe bilateral involvement of the intracranial arteries. J Neuroradiol J Neuroradiol. 2014;41(2):144–7.
Franke P, Markl M, Heinzelmann S, Vaith P, Bürk J, Langer M, et al. Evaluation of a 32-channel versus a 12-channel head coil for high-resolution post-contrast MRI in giant cell arteritis (GCA) at 3T. Eur J Radiol. 2014;83(10):1875–80.
Geiger J, Ness T, Uhl M, Lagreze WA, Vaith P, Langer M, et al. Involvement of the ophthalmic artery in giant cell arteritis visualized by 3T MRI. Rheumatology. 2008;48(5):537–41.
Siemonsen S, Brekenfeld C, Holst B, Kaufmann-Buehler A-K, Fiehler J, Bley TA. 3T MRI Reveals Extra- and Intracranial Involvement in Giant Cell Arteritis. Am J Neuroradiol. 2015;36(1):91–7.
Veldhoen S, Klink T, Geiger J, Vaith P, Glaser C, Ness T, et al. MRI displays involvement of the temporalis muscle and the deep temporal artery in patients with giant cell arteritis. Eur Radiol. 2014;24(11):2971–9.
Narváez J, Narváez JA, Nolla JM, Sirvent E, Reina D, Valverde J. Giant cell arteritis and polymyalgia rheumatica: usefulness of vascular magnetic resonance imaging studies in the diagnosis of aortitis. Rheumatol Oxf Engl. 2005;44(4):479–83.
Bley TA, Wieben O, Uhl M, Miehle N, Langer M, Hennig J, et al. Integrated head-thoracic vascular MRI at 3 T: assessment of cranial, cervical and thoracic involvement of giant cell arteritis. Magma N Y N. 2005;18(4):193–200.
Koenigkam-Santos M, Sharma P, Kalb B, Oshinski JN, Weyand CM, Goronzy JJ, et al. Magnetic resonance angiography in extracranial giant cell arteritis. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2011;17(6):306–10.
Meller J, Strutz F, Siefker U, Scheel A, Sahlmann CO, Lehmann K, et al. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003;30(5):730–6.
Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A prospective study of 35 patients. Arthritis Rheum. 2006;55(1):131–7.
Walter MA, Melzer RA, Schindler C, Müller-Brand J, Tyndall A, Nitzsche EU. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32(6):674–81.
Besson FL, Parienti J-J, Bienvenu B, Prior JO, Costo S, Bouvard G, et al. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38(9):1764–72.
Papathanasiou ND, Du Y, Menezes LJ, Almuhaideb A, Shastry M, Beynon H, et al. 18F-Fludeoxyglucose PET/CT in the evaluation of large-vessel vasculitis: diagnostic performance and correlation with clinical and laboratory parameters. Br J Radiol. 2012;85(1014):e188–94.
Yun M, Jang S, Cucchiara A, Newberg AB, Alavi A. 18F FDG uptake in the large arteries: a correlation study with the atherogenic risk factors. Semin Nucl Med. 2002;32(1):70–6.
Blockmans D, Maes A, Stroobants S, Nuyts J, Bormans G, Knockaert D, et al. New arguments for a vasculitic nature of polymyalgia rheumatica using positron emission tomography. Rheumatol Oxf Engl. 1999;38(5):444–7.
Besson FL, de Boysson H, Parienti J-J, Bouvard G, Bienvenu B, Agostini D. Towards an optimal semiquantitative approach in giant cell arteritis: an (18)F-FDG PET/CT case–control study. Eur J Nucl Med Mol Imaging. 2014;41(1):155–66. The aortic to blood pool SUVmax comparison semiquantitative method described is likely to be preferred in subsequent research studies, though in clinical practice an expert radiologist's visual impression will probably still be used in many centres.
Lehmann P, Buchtala S, Achajew N, Haerle P, Ehrenstein B, Lighvani H, et al. 18F-FDG PET as a diagnostic procedure in large vessel vasculitis—a controlled, blinded re-examination of routine PET scans. Clin Rheumatol. 2011;30(1):37–42.
Fuchs M, Briel M, Daikeler T, Walker UA, Rasch H, Berg S, et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39(2):344–53.
Rabkin Z, Israel O, Keidar Z. Do hyperglycemia and diabetes affect the incidence of false-negative 18F-FDG PET/CT studies in patients evaluated for infection or inflammation and cancer? A Comparative analysis. J Nucl Med Off Publ Soc Nucl Med. 2010;51(7):1015–20.
Zhuang HM, Cortés-Blanco A, Pourdehnad M, Adam LE, Yamamoto AJ, Martínez-Lázaro R, et al. Do high glucose levels have differential effect on FDG uptake in inflammatory and malignant disorders? Nucl Med Commun. 2001;22(10):1123–8.
Gillanders LA, Strachan RW, Blair DW. Temporal arteriography. A new technique for the investigation of giant cell arteritis and polymyalgia rheumatica. Ann Rheum Dis. 1969;28(3):267–9.
Hunder GG, Baker HL, Rhoton AL, Sheps SG, Ward LE. Superficial temporal arteriography in patients suspected of having temporal arteritis. Arthritis Rheum. 1972;15(6):561–70.
Moncada R, Baker D, Rubinstein H, Shah D, Love L. Selective temporal arteriography and biopsy in giant cell arteritis: polymyalgia rheumatica. Am J Roentgenol Radium Ther Nucl Med. 1974;122(3):580–5.
Stanson AW, Klein RG, Hunder GG. Extracranial angiographic findings in giant cell (temporal) arteritis. AJR Am J Roentgenol. 1976;127(6):957–63.
Layfer LF, Banner BF, Huckman MS, Grainer LS, Golden HE. Temporal arteriography. Analysis of 21 cases and a review of the literature. Arthritis Rheum. 1978;21(7):780–4.
Sewell JR, Allison DJ, Tarin D, Hughes GR. Combined temporal arteriography and selective biopsy in suspected giant cell arteritis. Ann Rheum Dis. 1980;39(2):124–8.
Berti A, Campochiaro C, Cavalli G, Pepe G, Praderio L, Sabbadini MG, et al. Giant cell arteritis restricted to the limb arteries: An overlooked clinical entity. Autoimmun Rev. 2015;14(4):352–7.
Hauenstein C, Reinhard M, Geiger J, Markl M, Hetzel A, Treszl A, et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology. 2012;51(11):1999–2003. This study confirms that both ultrasound and MRI findings normalise rapidly within days and in parallel to each other, necessitating assessement pathways that allow for early scanning.
Santoro L, D’Onofrio F, Bernardi S, Gremese E, Ferraccioli G, Santoliquido A. Temporal ultrasonography findings in temporal arteritis: early disappearance of halo sign after only 2 days of steroid treatment. Rheumatology. 2013;52(4):622.
Bley TA, Markl M, Schelp M, Uhl M, Frydrychowicz A, Vaith P, et al. Mural inflammatory hyperenhancement in MRI of giant cell (temporal) arteritis resolves under corticosteroid treatment. Rheumatol Oxf Engl. 2008;47(1):65–7.
De Miguel E, Roxo A, Castillo C, Peiteado D, Villalba A, Martín-Mola E. The utility and sensitivity of colour Doppler ultrasound in monitoring changes in giant cell arteritis. Clin Exp Rheumatol. 2012;30(1 Suppl 70):S34–8.
Pérez López J, Solans Laqué R, Bosch Gil JA, Molina Cateriano C, Huguet Redecilla P, Vilardell Tarrés M. Colour-duplex ultrasonography of the temporal and ophthalmic arteries in the diagnosis and follow-up of giant cell arteritis. Clin Exp Rheumatol. 2009;27(1 Suppl 52):S77–82.
Diamantopoulos AP, Myklebust G. Long-term inflammation in the temporal artery of a giant cell arteritis patient as detected by ultrasound. Ther Adv Musculoskelet Dis. 2014;6(3):102–3.
Czihal M, Piller A, Schroettle A, Kuhlencordt PJ, Schulze-Koops H, Hoffmann U. Outcome of giant cell arteritis of the arm arteries managed with medical treatment alone: cross-sectional follow-up study. Rheumatology. 2013;52(2):282–6.
Schmidt WA, Moll A, Seifert A, Schicke B, Gromnica-Ihle E, Krause A. Prognosis of large-vessel giant cell arteritis. Rheumatology. 2008;47(9):1406–8.
Henes JC, Mueller M, Pfannenberg C, Kanz L, Koetter I. Cyclophosphamide for large vessel vasculitis: assessment of response by PET/CT. Clin Exp Rheumatol. 2011;29(1 Suppl 64):S43–8.
Eshet Y, Pauzner R, Goitein O, Langevitz P, Eshed I, Hoffmann C, et al. The limited role of MRI in long-term follow-up of patients with Takayasu’s arteritis. Autoimmun Rev. 2011;11(2):132–6.
Schneeweis C, Schnackenburg B, Stuber M, Berger A, Schneider U, Yu J, et al. Delayed contrast-enhanced MRI of the coronary artery wall in takayasu arteritis. PLoS One. 2012;7(12), e50655.
Papa M, De Cobelli F, Baldissera E, Dagna L, Schiani E, Sabbadini M, et al. Takayasu arteritis: intravascular contrast medium for MR angiography in the evaluation of disease activity. AJR Am J Roentgenol. 2012;198(3):W279–84.
Tso E, Flamm SD, White RD, Schvartzman PR, Mascha E, Hoffman GS. Takayasu arteritis: Utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum. 2002;46(6):1634–42.
Sun Y, Ma L, Ji Z, Zhang Z, Chen H, Liu H, et al. Value of whole-body contrast-enhanced magnetic resonance angiography with vessel wall imaging in quantitative assessment of disease activity and follow-up examination in Takayasu’s arteritis. Clin Rheumatol. 2015
Furuta S, Cousins C, Chaudhry A, Jayne D. Clinical features and radiological findings in large vessel vasculitis: are Takayasu arteritis and giant cell arteritis 2 different diseases or a single entity? J Rheumatol. 2015;42(2):300–8.
Czihal M, Piller A, Schroettle A, Kuhlencordt P, Bernau C, Schulze-Koops H, et al. Impact of cranial and axillary/subclavian artery involvement by color duplex sonography on response to treatment in giant cell arteritis. J Vasc Surg. 2015;61(5):1285–91.
Schmidt WA, Krause A, Schicke B, Kuchenbecker J, Gromnica-Ihle E. Do temporal artery duplex ultrasound findings correlate with ophthalmic complications in giant cell arteritis? Rheumatology. 2009;48(4):383–5.
Blockmans D, Coudyzer W, Vanderschueren S, Stroobants S, Loeckx D, Heye S, et al. Relationship between fluorodeoxyglucose uptake in the large vessels and late aortic diameter in giant cell arteritis. Rheumatol Oxf Engl. 2008;47(8):1179–84.
Both M, Ahmadi-Simab K, Reuter M, Dourvos O, Fritzer E, Ullrich S, et al. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteritis. Ann Rheum Dis. 2008;67(7):1030–3.
Mackie SL, Hensor EMA, Morgan AW. Pease CT. Should I send my patient with previous giant cell arteritis for imaging of the thoracic aorta? A systematic literature review and meta-analysis. Ann Rheum Dis. 2014;73(1):143–8. An important systematic review illustrating the paucity of evidence regarding the optimal strategy for monitoring of patients for the development of aortic aneurysms.
Dasgupta B, Borg FA, Hassan N, Alexander L, Barraclough K, Bourke B, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatol Oxf Engl. 2010;49(8):1594–7.
Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68(3):318–23.
Bley TA, Reinhard M, Hauenstein C, Markl M, Warnatz K, Hetzel A, et al. Comparison of duplex sonography and high-resolution magnetic resonance imaging in the diagnosis of giant cell (temporal) arteritis. Arthritis Rheum. 2008;58(8):2574–8.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Asad Khan and Bhaskar Dasgupta declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Khan, A., Dasgupta, B. Imaging in Giant Cell Arteritis. Curr Rheumatol Rep 17, 52 (2015). https://doi.org/10.1007/s11926-015-0527-y
Published:
DOI: https://doi.org/10.1007/s11926-015-0527-y