Abstract
Circulating antinuclear autoantibodies contribute to the diagnosis of systemic sclerosis (SSc) and correlate with disease-specific organ manifestations. Recent findings show the induction of interstitial lung disease and obliterative vasculopathy by transfer of IgG from SSc patients in healthy mice indicating a contribution of antibodies to SSc pathogenesis. Several functional or agonistic autoantibodies have been described in SSc, thus putting autoimmunity into a new spotlight. Autoantibodies against the angiotensin II receptor type-1 and the endothelin1 receptor type-A are associated with severe disease and provide new insights into its pathogenesis. They link the hallmarks of SSc, vasculopathy, immune activation, and fibrosis. At present, the contribution of the specific antibodies to disease manifestations remains to be examined. However; functional autoantibodies could represent a significant piece in the puzzle of SSc pathogenesis and may open new gateways and opportunities for therapeutic intervention. This review focuses on the features of functional autoantibodies in SSc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is an autoimmune disorder with limited therapeutic options and high morbidity and mortality. Autoimmunity plays a significant role in the pathogenesis of SSc. Antinuclear autoantibodies are seen in 85–99 % of SSc patients and are associated with specific disease subtypes and organ manifestations. Their presence predicts disease onset prior to diagnosis [1–7]. Although antinuclear autoantibodies do not affect the pathogenesis of SSc to our present knowledge, they suggest a contribution of autoimmunity in the triad-complex of SSc pathogenesis of inflammation, vascular injury, and fibrosis.
The concept of functional autoantibodies (Ab) is continuously emerging in the endeavor to understand the complexity of SSc pathogenesis. Detailed study of Ab functionality could significantly improve our current understanding of molecular pathway activation. In addition, functional Ab could lead the way to discovering yet unknown molecular pathways and thus opening the way for new potential targets and effective therapeutic options in SSc. In this review, functional Ab (summarized in Table 1) and our current understanding of their effects in SSc are summarized.
Anti-endothelial Cell Antibodies
Anti-endothelial cell antibodies (AECA) were first identified in the early 1970s in sera of patients with different rheumatic diseases [21]. Due to variable detection methods, AECAs are found in approximately 22–86 % of SSc patients [22–26]. However, AECAs are also found in other systemic rheumatic diseases such as in systemic lupus erythematosus (SLE), Sjögren’s syndrome, or in patients with rheumatoid arthritis (RA) [22, 27–29]. Their presence in SSc has been connected to more severe disease manifestations and organ/systemic involvement [30, 31]. The presence of AECA is associated with perivascular, vascular, and lung involvement including digital ulcers, severity of peripheral vascular injury detected by nail fold capillaroscopy and PAH [25, 32–34]. AECAs have been demonstrated to induce endothelial cell activation: AECA-positive IgG from SSc patients led to an upregulation of adhesion molecules on endothelial cell surface such as vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), or E-selectin [8]. In vitro, AECA-positive sera induced apoptosis in cultured human dermal microvascular cells [9] and human dermal endothelial cells (HDECs) [10] by activation of the caspase-3 pathway and the expression of fibrillin-1. AECA-positive sera stimulate expression of the adhesion molecules VCAM-1, ICAM-1, and E-selectin on human endothelial cells. Moreover, endothelial cells pretreated with AECA-positive sera showed increased adhesion of histiocytic lymphoma U937 cell lines and expressed interleukin-1 (IL-1) [8]. The authors concluded that AECAs could actively participate in SSc pathogenesis by activation of endothelial cells. In vivo, transfer of AECAs from UCD-200 chickens, resembling human SSc features, resulted in induction of apoptosis upon binding to endothelial cells in healthy chicken embryos [11]. The molecular target of AECAs remained unknown until a quantitative immunoblotting technique identified the nuclear and ubiquitous protein CENT-B as the main target in patients with lcSSc [31].
Agonsitic Anti-ICAM-1 Autoantibodies
Recently, AECAs specifically targeting ICAM-1 were identified in 24 out of 60 SSc patients [12]. The authors developed an ELISA-assay to detect anti-ICAM-1 antibodies in SSc serum samples. Their measurements revealed that sera of SSc patients contain significantly higher levels of IgG and IgM anti-ICAM-1 Ab in the diffuse cutaneous SSc (dSSc) and in the limited cutaneous (lSSc) subset of SSc compared to healthy controls. Furthermore, purified anti-ICAM-1 antibodies showed agonistic properties when tested in vitro on human umbilical vein endothelial cells (HUVECs). Here, binding of anti-ICAM-1 antibodies to ICAM-1 increased production of reactive oxygen species (ROS). Furthermore, increased levels of VCAM-1 protein were detected when HUVECs were treated with anti-ICAM-1 antibodies [12]. These findings demonstrate the induction of pro-inflammatory cascades by anti-ICAM-1 antibodies in HUVECs. Moreover, these results demonstrate that AECAs can also activate endothelial cells by binding to molecules expressed on the cell surface, while previous results mainly indicated nuclear located antigens. These interesting findings demonstrate the complex nature of AECAs and future experiments could elucidate the mechanisms and the impact of AECAs in the pathogenesis of SSc.
Anti-fibroblast Antibodies
Anti-fibroblast antibodies (AFAs) were detected in SSc sera using a cell-based ELISA [13]. They were found in 58 % of the SSc sera with higher prevalence in dcSSc than lcSSc [13]. Antibodies to fibroblasts have also been found in patients with idiopathic and scleroderma-associated pulmonary hypertension [35]. AFA-positive SSc sera induced proadhesive and proinflammatory phenotypic changes in fibroblasts by upregulating ICAM-1 surface expression, interleukin-6 (IL-6) production, and induced enhanced adhesion of U937 cells [13]. The glycolytic enzyme alpha-enolase was found to be a primary target of AFAs [36]. Anti-alpha-enolase antibodies were associated with antitopoisomerase 1 antibodies and showed an association to the prevalence of interstitial lung disease (ILD) [36].
Anti-PDGF Receptor Autoantibodies
Functional Ab reactive to the platelet-derived growth factor receptor (PDGFR) have been detected in 46 out of 46 SSc patients [14]. The presence of anti-PDGFR autoantibodies was demonstrated in a functional bioassay, and stimulatory activity was shown on fibroblasts. Anti-PDGFR autoantibodies induced tyrosine phosphorylation in normal fibroblasts and increased ROS levels. Higher levels of alpha-SMA and type I collagen were detected in normal fibroblasts. The authors concluded that anti-PDGFR autoantibodies could be a specific feature of SSc with an active role in disease pathogenesis due to their stimulatory activity. However, when anti-PDGFR-alpha autoantibodies were measured by an immunobiological assay, they were not specific for SSc since they were also detected in normal subjects [37]. Another study reported the presence of anti-PDGFR-alpha autoantibodies in approximately one-third of the tested sera from SSc patients, but they were also found in similar frequency in normal subjects. This group could not observe any biologic activity of anti-PDGFR-alpha autoantibodies [38].
This example shows that slight differences in the handling of cellular systems can lead to crucial differences in the results. Standardized screening procedures for functionally active human anti-PDGFR antibodies are required. Search for epitopes and generation of synthetic polyclonal and monoclonal antibodies are among the priority tasks to verify the specific role of anti-PDGFR-alpha autoantibodies and their specific biologic activity in systemic sclerosis [39]. In addition, their role as biomarker or diagnostic marker remains to be evaluated.
Autoantibodies Against Matrix Metalloproteinase-1 and Matrix Metalloproteinase-3
Excessive accumulation of extracellular matrix components (ECM) is a feature of fibrosis in SSc. The degradation of ECM is induced by matrix metalloproteinases (MMPs), and MMP activity is regulated by tissue inhibitors of metalloproteinases (TIMPs). An imbalance of MMP and TIMP regulation could contribute to excessive ECM deposition [40]. MMP-1 is involved in the degradation of type I–III collagens that are major components of ECM in affected and normal skin in SSc [41, 42]. MMP-3 degrades type V collagen, elastin, and fibrillin among others. SSc fibroblasts display a reduced MMP-1 activity compared to normal fibroblasts [15].
The authors hypothesized that antibodies reactive to MMP-1 and MMP-3 could be involved in ECM accumulation by inhibiting MMP activity. And indeed, anti-MMP-1 antibodies and anti-MMP-3 antibodies were detected by ELISA in two studies [16, 17]. Forty-nine percent of all SSc patients and in 75 % of dSSc patients were positive for anti-MMP-1 Ab. Similarly, anti-MMP-3 Ab were measured in 52 % of all SSc patients and in 71 % of dSSc patients. Anti-MMP-1 Ab levels correlated with the extent of fibrosis in skin, lung, and in renal blood vessels. They inhibited MMP-1 activity, while anti-MMP-3 antibodies inhibited the activity of MMP-3.
Therefore, anti-MMP-1 and anti-MMP-3 Ab may contribute to fibrosis by reduction of MMP activity and could be involved in SSc pathogenesis. The authors concluded that anti-MMP-1 antibody could represent a link between autoimmunity and fibrosis in SSc. However, the authors also point out that the significance of anti-MMP-3 antibodies remains to be clarified.
Anti-AT1R and Anti-ETAR Autoantibodies
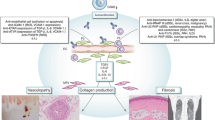
Simultaneous presence of autoantibodies reactive to the angiotensin II type 1 receptor (AT1R), and the endothelin-1 type A receptor (ETAR) was described in patients with SSc [18]. The antibody levels strongly correlate with each others, which is probably related to cross reactivity [18]. These autoantibodies were detected by a solid phase assay using membrane extracts overexpressing the native AT1R or ETAR. Anti-AT1R and anti-ETAR Ab are detected in approximately 85 % of SSc patients. High levels of anti-AT1R and anti-ETAR Ab are associated with severe SSc manifestations and complications such as digital ulcers, pulmonary arterial hypertension, lung fibrosis, and death. They predict mortality, cardiovascular complications such as PAH, and mortality in the presence of SSc-PAH [18, 19]. Anti-AT1R/ETAR Ab are not specific for SSc. However, they identify patients at risk for certain organ complication and their response to therapy, e.g., for PAH [19]. Functional activities were tested in isolated IgG fractions positive for anti-AT1R and anti-ETAR autoantibodies in vitro by using different cells in which the receptors are present. In human endothelial cells, the antibodies induce TGFß, VCAM-1, interleukin-8 (IL-8, CXCL8), IL-6, and CCL2. They increase intracellular Ca2+ concentrations and neutrophil transendothelial migration and reduce regenerative capacity of endothelial cells [43]. Similarities between the effects of anti-AT1R/ETAR antibodies and those observed for AECAs suggest that AECAs at least partially act via AT1R and ETAR activation. In peripheral blood cells, the Ab induced expression of IL-8 and CCL18, a marker for alternative monocytic activation. Both IL-8 and CCL18 were shown to predict progressive interstitial lung disease. For all the in vitro experiments, activation via AT1R and ETAR was proven by the use of specific receptor blockers [44]. As shown by our in vitro experiments, the effects of the anti-AT1R/ETAR Ab are dependent on the antibody concentrations, the disease manifestation of the SSc donors, and the disease duration. The effects were strongest when the donor suffered from early and severe disease. In addition, receptor expression of AT1R, ETAR, and their counter playing receptors AT2R and ETBR seems to be important and varies among the SSc patients [44]. Figure 1 shows the published effects by the anti-AT1R/ETAR antibodies.
Effects of Ab-positive IgG fractions were also tested by passive transfer into naïve C57BL6/6J mice. Elevated levels of neutrophils were detected in bronchoalveolar lavage fluids (BALF) after a single IgG transfer and structural alterations of lung architecture after repeated IgG transfers [43]. All these experimental findings suggest the ability of anti-AT1R and anti-ETAR Ab to induce inflammatory and fibrotic mechanisms in vitro and probably in vivo, although further studies are necessary to identify the specific contribution of anti-AT1R/ETAR Ab of the effects induced by SSc-IgG in vivo. In addition, the results demonstrate the complex nature of anti-AT1R and anti-ETAR autoantibodies and their intricate agonistic effects. These findings strongly indicate the importance of abnormal AT1R and ETAR activity, not only by modification of expression and regulation by natural ligands, but also by autoantibodies in the pathogenesis of SSc [19, 44].
Antibodies to Estrogen Receptor-α
SSc is more frequently diagnosed in women than in men. Female sex hormones could therefore play a role in the disease pathogenesis. Up to today, the impact of estrogens on SSc is not well examined and therefore, the role of estrogens in SSc still remains unclear. Estrogens and 17β-estradiol in particular, can modulate immune function by activation of estrogen receptors (ER) ERα and ERβ. Furthermore, estrogens are also known players in immune-mediated rheumatic diseases [45]. ERα and ERβ are located in the nucleus, and their function is modulated by estrogens via transcription factor regulation in the nucleus. However, ERα expression was also detected in the cytoplasm of human peripheral blood lymphocytes [45]. Moreover, Ab specific to ERα (anti-ERα antibodies) were recently described in patients with SLE, where these antibodies showed functional properties such as cell activation and T-lymphocyte proliferation [46]. A recent study found, anti-ERα Ab to be present in the sera of 42 % of 86 analyzed SSc patients [47]. Furthermore, associations between anti-ERα antibody levels and clinical manifestations such as disease subtype and SSc activity were observed. Anti-ERα antibodies are among others associated with diffuse cutaneous involvement, a high EScSG activity index, and late stage pattern on nailfold capillaroscopy [48]. Furthermore, anti-ERα antibody positivity correlated with increased T-lymphocyte apoptotic susceptibility and alterations in regulatory T-lymphocyte (Treg) homeostasis. Therefore, the authors suggested that anti-ERα Ab are markers of SSc progression and display functional activity.
Functional Autoantibodies to Methionine Sulfoxide Reductase
Oxidative stress is known to play an important role in SSc. Increased cellular release of reactive oxygen species (ROS) has been detected in SSc monocytes, and the maintenance of a fibrotic phenotype of SSc fibroblasts due to oxidative stress was reported [20, 49]. Methionine sulfoxide reductase A (MSRA) is one of the antioxidant repair enzymes. Antibodies to MSRA (anti-MSRA Ab) have been detected in 33 % of SSc patients by using an ELISA assay with recombinant MSRA. Levels of anti-MSRA Ab were higher in SSc compared to healthy controls especially in SSc patients suffering from pulmonary fibrosis and cardiac involvement as well as in patients with decreased total antioxidant power [50]. Furthermore, serum levels of anti-MSRA Ab correlated negatively with vital capacity (VC) and diffusion capacity for carbon monoxide (DLco) and positive with renal vascular damage [50]. In addition, levels of anti-MSRA Ab positively correlated with markers of oxidative and cellular stress, such as 8-isoprostane and heat shock protein 70 (Hsp 70). Functional effects on MSRA activity were tested by IgG fractions positive for anti-MSRA Ab. Upon treatment, the enzymatic activity of MSRA was inhibited. The authors concluded altogether that anti-MSRA Ab are useful serologic markers for disease severity and could via inhibition of MSRA enzymatic activity enhance oxidative stress and thus cause vascular damage.
Muscarinic-3 Acetylcholine Receptor Autoantibodies
Gastrointestinal tract (GIT) involvement affects approximately 90 % of SSc patients [51]. Fibrosis of smooth muscle cells could account for GIT dysmotility [52]. However, intrinsic neurons predominantly control GIT motility. The involvement of autoantibodies reactive to the muscarinic-3 receptor and their role in the pathogenesis of GIT involvement was investigated recently [53]. GIT motility is regulated predominantly via the activation of the M3R that is activated by the neurotransmitter acetylcholine. An inactivation of the M3R by an anti-M3R antibody could therefore be involved in GIT dysmotility in SSc. The presence of anti-M3R Ab was investigated using an enzyme immunoassay (EIA) in serum samples. Significantly higher anti-M3R antibody levels were found in SSc patients with severe GIT involvement compared to SSc patients without GIT involvement in the first 2 years of the disease. Here, elevated anti-M3R antibody levels were detected in 64 % of SSc patients with severe GIT involvement. These findings suggest a connection of anti-M3R antibody to severe GIT involvement at an early stage. Moreover, the authors suggest that patients with anti-M3R antibodies could exhibit more severe GIT involvement compared to patients without anti-M3R antibodies. However, it still remains unclear whether anti-M3R Ab can exhibit functional activity and whether this activity inhibits the effects of M3R. Anti-M3R Ab could be involved in pathogenesis of GIT involvement in SSc. Nonetheless, experiments on the effects of anti-M3R antibody are necessary to clarify its role in SSc.
Conclusions
The interplay of autoimmunity, vascular injury, and fibrosis in SSc remains incompletely understood. Functional autoimmunity is an emerging aspect in the complex picture of SSc pathogenesis. The described features of functional autoantibodies in SSc suggest a link between autoimmunity and vasculopathy and fibrosis. A detailed understanding of functional autoimmunity could on the one hand represent a new paradigm in SSc pathogenesis. On the other hand, functional autoantibodies could open new therapeutic options for the treatment of SSc and could improve the currently limited possibilities.
References
Mierau R et al. Frequency of disease-associated and other nuclear autoantibodies in patients of the German Network for Systemic Scleroderma: correlation with characteristic clinical features. Arthritis Res Ther. 2011;13:R172.
Bunn CC, Denton CP, Shi-Wen X, Knight C, Black CM. Anti-RNA polymerases and other autoantibody specificities in systemic sclerosis. Br J Rheumatol. 1998;37:15–20.
Jacobsen S et al. Clinical features and serum antinuclear antibodies in 230 Danish patients with systemic sclerosis. Br J Rheumatol. 1998;37:39–45.
Meyer OC, Fertig N, Lucas M, Somogyi N, Medsger Jr TA. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J Rheumatol. 2007;34:104–9.
Hamaguchi Y et al. The clinical relevance of serum antinuclear antibodies in Japanese patients with systemic sclerosis. Br J Dermatol. 2008;158:487–95.
Denton CP et al. Demographic, clinical and antibody characteristics of patients with digital ulcers in systemic sclerosis: data from the DUO Registry. Ann Rheum Dis. 2012;71:718–21.
Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42.
Carvalho D, Savage CO, Black CM, Pearson JD. IgG antiendothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro. Induction of adhesion molecule expression and involvement of endothelium-derived cytokines. J Clin Invest. 1996;97:111–9.
Sgonc R et al. Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum. 2000;43:2550–62.
Ahmed SS, Tan FK, Arnett FC, Jin L, Geng YJ. Induction of apoptosis and fibrillin 1 expression in human dermal endothelial cells by scleroderma sera containing anti-endothelial cell antibodies. Arthritis Rheum. 2006;54:2250–62.
Worda M et al. In vivo analysis of the apoptosis-inducing effect of anti-endothelial cell antibodies in systemic sclerosis by the chorionallantoic membrane assay. Arthritis Rheum. 2003;48:2605–14.
Wolf SI, Howat S, Abraham DJ, Pearson JD, Lawson C. Agonistic anti-ICAM-1 antibodies in scleroderma: activation of endothelial pro-inflammatory cascades. Vasc Pharmacol. 2013;59:19–26.
Chizzolini C et al. Autoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosis. Arthritis Rheum. 2002;46:1602–13.
Baroni SS et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–76.
Takeda K, Hatamochi A, Ueki H, Nakata M, Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Investig Dermatol. 1994;103:359–63.
Sato S, Hayakawa I, Hasegawa M, Fujimoto M, Takehara K. Function blocking autoantibodies against matrix metalloproteinase-1 in patients with systemic sclerosis. J Investig Dermatol. 2003;120:542–7.
Nishijima C et al. Autoantibody against matrix metalloproteinase-3 in patients with systemic sclerosis. Clin Exp Immunol. 2004;138:357–63.
Riemekasten G et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70:530–6.
Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, Witzenrath M, Kühl AA, Heidecke H, Ghofrani HA, Tiede H, Schermuly RT, Nickel N, Hoeper MM, Lukitsch I, Gollasch M, Kuebler WM, Bock S, Burmester GR, Dragun D, Riemekasten G. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med. 2014;190(7):808–17.
Sambo P et al. Monocytes of patients with systemic sclerosis (scleroderma spontaneously release in vitro increased amounts of superoxide anion. J Investig Dermatol. 1999;112:78–84.
Lindqvist KJ, Osterland CK. Human antibodies to vascular endothelium. Clin Exp Immunol. 1971;9:753–60.
Hebbar M et al. Assessment of anti-endothelial cell antibodies in systemic sclerosis and Sjogren’s syndrome. Ann Rheum Dis. 1997;56:230–4.
Renaudineau Y et al. Anti-endothelial cell antibodies in systemic sclerosis. Clin Diagn Lab Immunol. 1999;6:156–60.
Ihn H et al. Characterization of autoantibodies to endothelial cells in systemic sclerosis (SSc): association with pulmonary fibrosis. Clin Exp Immunol. 2000;119:203–9.
Salojin KV et al. Antiendothelial cell antibodies: useful markers of systemic sclerosis. Am J Med. 1997;102:178–85.
Wusirika R et al. The assessment of anti-endothelial cell antibodies in scleroderma-associated pulmonary fibrosis. A study of indirect immunofluorescent and western blot analysis in 49 patients with scleroderma. Am J Clin Pathol. 2003;120:596–606.
Renaudineau Y, Dugue C, Dueymes M, Youinou P. Antiendothelial cell antibodies in systemic lupus erythematosus. Autoimmun Rev. 2002;1:365–72.
Salih AM, Nixon NB, Dawes PT, Mattey DL. Soluble adhesion molecules and anti-endothelial cell antibodies in patients with rheumatoid arthritis complicated by peripheral neuropathy. J Rheumatol. 1999;26:551–5.
Belizna C, Duijvestijn A, Hamidou M, Tervaert JW. Antiendothelial cell antibodies in vasculitis and connective tissue disease. Ann Rheum Dis. 2006;65:1545–50.
Tamby MC et al. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax. 2005;60:765–72.
Servettaz A et al. Anti-endothelial cell antibodies from patients with limited cutaneous systemic sclerosis bind to centromeric protein B (CENP-B). Clin Immunol. 2006;120:212–9.
Pignone A et al. Anti-endothelial cell antibodies in systemic sclerosis: significant association with vascular involvement and alveolo-capillary impairment. Clin Exp Rheumatol. 1998;16:527–32.
Negi VS, Tripathy NK, Misra R, Nityanand S. Antiendothelial cell antibodies in scleroderma correlate with severe digital ischemia and pulmonary arterial hypertension. J Rheumatol. 1998;25:462–6.
Sgonc R et al. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996;98:785–92.
Tamby MC et al. Antibodies to fibroblasts in idiopathic and scleroderma-associated pulmonary hypertension. Eur Respir J. 2006;28:799–807.
Terrier B et al. Antifibroblast antibodies from systemic sclerosis patients bind to {alpha}-enolase and are associated with interstitial lung disease. Ann Rheum Dis. 2010;69:428–33.
Balada E et al. Anti-PDGFR-alpha antibodies measured by non-bioactivity assays are not specific for systemic sclerosis. Ann Rheum Dis. 2008;67:1027–9.
Loizos N et al. Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor alpha in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum. 2009;60:1145–51.
Dragun D, Distler JH, Riemekasten G, Distler O. Stimulatory autoantibodies to platelet-derived growth factor receptors in systemic sclerosis: what functional autoimmunity could learn from receptor biology. Arthritis Rheum. 2009;60(4):907–11.
Jinnin M. Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol. 2010;37:11–25.
Lovell CR, Nicholls AC, Duance VC, Bailey AJ. Characterization of dermal collagen in systemic sclerosis. Br J Dermatol. 1979;100:359–69.
Jimenez SA, Hitraya E, Varga J. Pathogenesis of scleroderma. Collagen. Rheum Dis Clin N Am. 1996;22:647–74.
Kill A et al. Autoantibodies to angiotensin and endothelin receptors in systemic sclerosis induce cellular and systemic events associated with disease pathogenesis. Arthritis Res Ther. 2014;16:R29.
Gunther J et al. Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Res Ther. 2014;16:R65.
Cutolo M et al. The immunomodulatory effects of estrogens: clinical relevance in immune-mediated rheumatic diseases. Ann N Y Acad Sci. 2010;1193:36–42.
Pierdominici M et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett. 2010;132:79–85.
Colasanti T et al. Autoantibodies to estrogen receptor alpha interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2012;64:778–87.
Giovannetti A et al. Autoantibodies to estrogen receptor alpha in systemic sclerosis (SSc) as pathogenetic determinants and markers of progression. PLoS One. 2013;8:e74332.
Sambo P et al. Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum. 2001;44:2653–64.
Ogawa F et al. Autoantibody against one of the antioxidant repair enzymes, methionine sulfoxide reductase A, in systemic sclerosis: association with pulmonary fibrosis and vascular damage. Arch Dermatol Res. 2010;302:27–35.
Domsic R, Fasanella K, Bielefeldt K. Gastrointestinal manifestations of systemic sclerosis. Dig Dis Sci. 2008;53:1163–74.
Rose S, Young MA, Reynolds JC. Gastrointestinal manifestations of scleroderma. Gastroenterol Clin N Am. 1998;27:563–94.
Kawaguchi Y et al. Muscarinic-3 acetylcholine receptor autoantibody in patients with systemic sclerosis: contribution to severe gastrointestinal tract dysmotility. Ann Rheum Dis. 2009;68:710–4.
Compliance with Ethics Guidelines
Conflict of Interest
Angela Kill and Gabriela Riemekasten declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Kill, A., Riemekasten, G. Functional Autoantibodies in Systemic Sclerosis Pathogenesis. Curr Rheumatol Rep 17, 34 (2015). https://doi.org/10.1007/s11926-015-0505-4
Published:
DOI: https://doi.org/10.1007/s11926-015-0505-4