Abstract
Giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are two closely related diseases in people aged 50 years and older, which are more frequently observed in Western countries. Despite being common entities, concern still exists about the epidemiology, pathogenesis, and diagnosis of both entities. New imaging techniques, such as 18 fluorodeoxyglucose-positron emission tomography, have proved to be useful in detecting large-vessel involvement in GCA. Corticosteroids are the cornerstone of the therapy in GCA and PMR. Relapses are frequent in these conditions. Unlike methotrexate and tumor necrosis factor-α antagonists, anti-interleukin-6 receptor therapy appears to be useful in patients with GCA and PMR who are refractory to corticosteroids. This review summarizes recent studies on GCA and PMR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are common and frequently overlapping conditions affecting individuals aged 50 years and older [1, 2]. GCA affects large and middle-sized blood vessels with predisposition to the involvement of cranial arteries derived from the carotid artery [1, 2]. PMR is characterized by pain and stiffness involving the shoulder girdle, proximal aspects of the arms, the neck, and the pelvic girdle [1, 2]. Approximately 40–60 % of individuals with GCA have PMR manifestations. In some cases, PMR may also be the presenting symptom of GCA [2].
An English language literature review was conducted using the PubMed database. It was mainly focused on studies published in the last 2 years. Recent data on epidemiology, pathogenesis, diagnosis and clinical manifestations, in particular those derived from large-vessel arteritis involvement and new drugs used in the management of these conditions, are discussed in the review.
Epidemiology
Both GCA and PMR are more common in Western countries, in particular in white individuals of Scandinavian background [1]. PMR is two to three times more common than GCA. A north-south gradient in the incidence of these conditions was seen in Europe with high rates in Sweden and Norway (generally more than 20 new cases of GCA per 100,000 individuals aged 50 and older per year) and lower incidence in Southern Europe (annual incidence rate 10 cases per 100,000 people aged 50 years and older in northwestern Spain) [3]. A recent study that showed information from the UK Clinical Practice Research Datalink supported information from previous studies that showed a higher incidence of GCA in women, in particular in the 70–79-year-old age group [4•]. This study also confirmed that GCA is often associated with PMR [4•]. Incidence of GCA and PMR is lower in Hispanic, Asian, and African-American populations [1]. As the population throughout the world continues to age, an increased prevalence of the disease should be expected. A recent study described for the first time information on the incidence of biopsy-proven GCA in Australia [5•]. According to results from this survey, age at onset and female predominance (female to male ratio 2.3) were similar to those reported in other Western countries [1, 3, 5•]. Another study focused on the epidemiology of GCA in Mexican mestizos confirmed the claim that GCA is uncommon in Latin America [6•].
A genetic component, in particular in patients with biopsy-proven GCA, has been reported [7]. In this regard, a strong association with HLA-DRB*04 alleles was found [7]. New studies also indicate that GCA is a polygenic disease. Gene polymorphisms located outside the MHC region that have been implicated in the pathogenesis of different autoimmune diseases, such as PTPN22, may also account for the increased risk of GCA [8••]. A recent review article emphasized the potential relevance of gene polymorphisms that influence the immune and inflammatory responses (including cytokines, adhesion molecules, and regulators of innate immunity) as crucial players in the development and progression of GCA [9••].
Pathogenesis
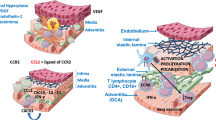
Both the humoral and cellular immune systems have been implicated in the pathogenesis of GCA. The inflammatory infiltrate in the affected vessels is predominantly composed of lymphocytes and macrophages and also frequently contains multinucleated giant cells of the Langerhans or foreign body type (often in close proximity to the fragmented internal elastic lamina) [10]. The majority of lymphocytes are CD4-positive T cells. Identical T cell clones have been isolated from multiple vasculitic sites, thereby suggesting a response to a specific antigen [10]. CD8-positive T cells may also be involved. NKG2D is an activating receptor found on NK cells and CD8 T cells. Dejaco et al. disclosed that NKG2D is functionally expressed on CD4CD28(−) and CD8 T cells in GCA and PMR. According to these authors, NKG2D ligands that are present in temporal arteries of these patients may be implicated in the pathogenesis of these conditions as they may co-stimulate NKG2D-expressing T cells [11•]. Samson et al. confirmed the implication of Th1 and Th17 lymphocytes in the pathogenesis of PMR and GCA. These entities are characterized by a significant shift in the Th17 cell/Treg cell balance toward an increased Th17 cell response [12••]. Also, Treg cells are decreased in patients with these conditions [12••]. Another recent study has disclosed that distribution of B cells is disturbed in GCA and PMR and that B cells likely contribute to the enhanced IL-6 response in both diseases [13••]. In newly diagnosed patients with GCA or PMR, B cell numbers inversely correlated with erythrocyte sedimentation rates, C-reactive protein, and serum BAFF levels. In these patients, tumor necrosis factor (TNF)-α-positive effector B cells, but not interleukin-10-positive regulatory B cells, are decreased. Interestingly, circulating numbers of effector B cells normalize following corticosteroid therapy. The presence of B cells in temporal artery biopsy (TAB) specimens from GCA patients was scarce [13••].
Diagnosis
A TAB is the gold standard test for the diagnosis of GCA [14]. TAB is generally performed on the most symptomatic size [14]. However, due to the segmental inflammatory involvement of the temporal artery, a contralateral biopsy may be considered to confirm a pathologic diagnosis of this vasculitis in patients in whom clinical suspicion of GCA is high [14]. Ultrasonography (US) of the temporal artery is another useful tool to make a diagnosis of GCA. High-resolution color Doppler US can show both the vessel wall and the lumen of the temporal artery. A concentric hypoechogenic mural thickening, dubbed “halo” that is the result of vessel wall edema, is a very specific and sensitive sign for GCA [15]. Other positive findings in the temporal artery of patients with GCA are the presence of occlusion and stenosis [15]. A meta-analysis supported the diagnostic value of the US-derived halo sign, the dark hypoechoic circumferential thickening around the artery lumen, indicating vasculitic wall edema, in GCA [16]. This meta-analysis disclosed a sensitivity of 68 % and a specificity of 91 % for the unilateral halo sign, as well as 43 and 100 %, respectively, for the bilateral halo sign in temporal artery US for GCA diagnosis, when the 1990 American College of Rheumatology classification criteria were used as the reference standard. Therefore, the halo sign in US is useful to make a diagnosis of GCA [16].
Due to the high specificity for GCA when a hypoechoic halo around the lumen of the temporal artery is observed, an issue of potential interest was to determine whether color duplex US-guided TAB performed at the site of the halo could prevent false-negative histological results and be useful for the diagnosis of GCA. To address this question, Germanò et al. compared color duplex US-guided TAB with standard TAB in a prospective cohort of unselected patients with suspected GCA [17••]. They assessed 105 patients seen at the Rheumatology Unit of the Reggio Emilia Hospital for suspected GCA in whom a TAB had been performed over a 3-year period [17••]. The study revealed that color duplex US-guided TAB does not result in a higher frequency of patients with positive TAB [17••]. Based on their results, in the presence of a positive halo sign at color duplex US, the probability of a positive TAB is equally high, regardless of whether the biopsy is guided by this technique or by a physical examination [17••]. A TAB performed at the site of halo did not provide a greater yield of positive TAB compared to standard TAB [17••]. These results are in keeping with a former study that disclosed that the presence of abnormalities of temporal arteries on the physical examination is associated with high predictive value for a positive TAB [18].
Although the disruption of the internal elastic lamina along with granulomatous inflammation with a mononuclear infiltrate and the presence of multinucleated giant cells are typical pathologic findings in the TAB of patients with GCA, multinucleated giant cells are only observed in 40–50 % of cases [14]. In this regard, the inflammatory infiltrate found in a TAB is broad, and the differences may have clinical implications. Of note, inflammatory infiltration of the TAB may be observed in patients with other conditions different from GCA such as ANCA-associated vasculitis or primary amyloidosis [19•].
With regard to the use of US in PMR, a comprehensive systematic review of the literature on this issue confirmed that US of the shoulders is a useful tool in the management of patients with PMR [20•]. Shoulder abnormalities obtained by US, in particular when findings such as bursitis and tenosynovitis of the long head of the biceps tendon are found in both shoulders, occur more frequently in patients with PMR than in controls. However, in the absence of suggestive clinical features, the presence of isolated US abnormalities should not lead to a diagnosis of PMR [20•].
Inflammation of the aorta and its branches may occur in patients with GCA, although symptoms of aortic involvement including aneurysms generally appear years after the diagnosis of this vasculitis [21]. Therefore, a systematic evaluation of patients by imaging techniques such as magnetic resonance imaging angiography and positron emission tomography (PET) may be useful to disclose the actual clinical impact of the extracranial involvement in patients with GCA as it may be more relevant than it was previously thought. It is particularly true in cases of aortitis involving the thoracic aorta or its main branches, which often present without cranial ischemic manifestations and are frequently associated with a negative TAB.
Over the past decade, 18 fluorodeoxyglucose (FDG)-PET has proved to be useful for detecting large-vessel arteritis in the setting of GCA, which can involve the larger thoracic, abdominal, and peripheral arteries [22]. It may be of particular relevance for the diagnosis of GCA patients with uncommon features such as fever of unknown origin or those presenting with asthenia, anorexia, and weight loss without cranial ischemic manifestations. With respect to this, experts suggest that PET may be more sensitive than other imaging techniques to detect vessel inflammation in patients with large-vessel vasculitides in an early stage of the disease [22, 23••]. Although other imaging techniques such as Doppler ultrasonography, computed tomography, and magnetic resonance imaging are able to demonstrate anatomical changes in the affected vessels (mural thickening, dilatation and aneurysms, and enhancement of perivascular connective tissue) when the inflammatory process is well established, they are not sensitive enough to diagnose early inflammatory changes that may be potentially reversible [22, 23••].
FDG-PET has been found to be useful to disclose the presence of large-vessel vasculitis in some patients presenting with PMR features [24]: (a) patients with PMR who do not achieve adequate corticosteroid response, (b) patients with PMR and persistently elevated inflammatory markers, and (c) patients with PMR and severe constitutional symptoms. In addition, recent reports have revealed that FDG-PET is also very useful in disclosing the presence of aortitis/large-vessel involvement in well-defined conditions different from GCA or isolated PMR [25, 26].
A review article indicates that the qualitative analysis of 18F-FDG uptake is the most widely adopted method to assess the presence and grading the activity of GCA-related vascular inflammation on 18F-FDG PET scan [23••]. This qualitative analysis is useful in performing dichotomous assessment (i.e., confirm or exclude the presence of vascular inflammation) and in grading the severity of vascular involvement according to ordinal scales, with the 18F-FDG uptake of the vessel wall being visually analyzed or compared with that of a reference structure [23••]. Qualitative methods appear to be more specific than semiquantitative ones, but they have lower sensitivity [23••]. Interestingly, a recent prospective study that included 43 consecutive patients seen at a medicine department from a teaching hospital because of suspected large-vessel vasculitis confirmed the presence of aortitis by a semiquantitative analysis of PET-CT in 25 of them [27••]. The authors found that images acquired 180 min after 18F-FDG injection and a target to background ratio index of 1.34 had very high accuracy and were strongly recommended for the diagnosis of aortitis in the clinical setting [27••].
Another issue of potential interest may be to establish whether biomarkers of GCA and PMR would be useful as diagnostic markers of these conditions. Baerlecken et al. disclosed that autoantibodies against ferritin were more common in patients with GCA and with PMR than in other vasculitic disorders, blood donors, and controls [28•]. In this regard, these authors found autoantibodies against the N-terminal 27 amino acids of the human ferritin heavy chain in 92 % of untreated GCA and PMR patients at disease onset and in up to 69 % of corticosteroid-treated patients with still active disease [28•]. More recently, the same group of investigators pointed out that the potential diagnostic test for GCA/PMR can be improved by combining three human ferritin peptide antibodies [29•].
Clinical Manifestations and Clinical Course
A recent review performed by Weyand and Goronzy highlights key clinical points to be kept in mind when dealing with GCA and PMR patients [30••]. They emphasized the need for prompt diagnosis and treatment of anterior optic neuritis as it may lead to permanent visual loss. They also mentioned that the aorta and its major branches may be involved in one quarter of GCA patients as well as the high frequency of relapses in GCA and PMR when corticosteroid dose is tapered [30••]. A recent study disclosed a high frequency of visual manifestations in an Italian cohort of GCA patients [31•]. The study also confirmed that once blindness is established, it is usually permanent and this risk makes GCA an ophthalmological emergency [31•, 32].
With the use of new imaging techniques such as FDG-PET, large-vessel involvement has increasingly been recognized in patients with GCA and less often in those with PMR [33••]. Aortic involvement in early GCA has been described in approximately one half to two thirds of patients using FDG-PET scan [33••]. The recognition of this complication is of main importance due to the risk of large-vessel complications, mainly aortic aneurysm and dissection [21]. Muratore et al. compared baseline variables, treatment, and outcome of patients with large-vessel GCA primarily involving the upper extremities, defined as radiographic evidence of subclavian vessel involvement, with those with cranial GCA [34••]. Patients with large-vessel involvement were younger and had a longer duration of symptoms at the time of GCA diagnosis than those with typical cranial GCA. They had more commonly PMR but less commonly cranial symptoms and visual loss than those presenting with typical cranial manifestations of GCA [34••]. They also had more relapses and required a longer duration of treatment [34••]. In keeping with these findings, in a study that included patients with a suspicion of GCA seen in two Italian centers in the last two decades, patients with “isolated aortitis,” defined as increased FDG uptake in the aorta not explained by atherosclerosis in the absence of FDG uptake in other large vessels, were younger than those with cranial GCA, with a predominance of men, and none of them presented at any time the typical cranial symptoms of this vasculitis [35•].
With regard to the lumbar pain observed in patients with PMR, a recent study using magnetic resonance imaging disclosed evidence of interspinous lumbar bursitis in 9 of 10 patients. Interestingly, moderate to marked lumbar bursitis occurred more frequently in patients with PMR than in the control group that included patients with spondyloarthritis, osteoarthritis, and rheumatoid arthritis [36•].
A retrospective population-based study of uniformly treated patients with biopsy-proven GCA disclosed relapses in 71 (40.8 %) of 174 patients [37]. The duration of corticosteroid therapy was significantly longer in those GCA patients who had relapses of the disease. The median dose of prednisone and the median duration of corticosteroid treatment at the time of the first relapse were 5 mg/day and 16 months, respectively. Headache (52 %) was the most common feature at the first relapse. PMR manifestations occurred in 30 % of the patients at that time. However, none of them developed visual loss [37]. A series of 106 GCA patients that were longitudinally followed also disclosed a high frequency of relapses (64 % had at least one relapse) [38•]. In this series, the first relapse consisted of PMR in 51 %, cranial symptoms in 31 %, and systemic complaints in 18 %. Relapses appeared predominantly, but not exclusively, within the first 2 years of treatment, and only 1 patient developed visual loss [38•]. Relapses are also common in patients diagnosed with isolated PMR [39•].
Treatment
Corticosteroids are the cornerstone of the therapy in GCA and PMR [14, 24, 30••]. Its use has dramatically reduced the frequency of severe visual ischemic complications in patients with GCA. In this vasculitis, the initial dose of prednisone or its equivalent ranges between 40 and 60 mg/day, either as single or divided dose for 3–4 weeks [14]. In isolated PMR, the initial dose of prednisone is lower than in GCA, generally 15–20 mg/day [30••]. GCA patients without severe ischemic complications experience rapid disease improvement with an initial prednisone dose of 40 mg/day. However, higher corticosteroid doses, generally an initial dose of 60 mg/prednisone/day or methylprednisolone pulse therapy (1 g daily for 3 consecutive days), are used in GCA patients who present with any of the severe ischemic manifestations, in particular with visual loss [24]. There are data showing that not the absolute dose but the time from the onset of symptoms to the first administration of corticosteroids is predictive of improvement of visual loss. Visual improvement, generally partial, was only observed in 8 (28 %) of 29 GCA patients who developed loss of vision closely to their admission at the hospital [32]. The main factor influencing the response to corticosteroids was the therapeutic delay [32]. In this regard, 7 of 12 patients treated within the first 24 h after the onset of visual loss had visual improvement. However, when corticosteroid therapy was delayed for more than 1 day, only 1 of the remaining 17 patients had some visual recovery [32]. These observations indicate that corticosteroid therapy should not be delayed under any circumstances in GCA patients with suspected visual impairment. In patients with visual ischemic manifestations such as amaurosis fugax or visual loss, intravenous methylprednisolone pulse therapy (1 g daily for 3 consecutive days), followed by 60 mg/prednisone/day for 3–4 weeks, is usually recommended [14, 24]. A question that needs further investigation is whether intravenous pulse methylprednisolone therapy may be better than high-dose oral prednisone in reducing the incidence of irreversible visual loss in GCA patients presenting with recent visual ischemic manifestations. In our experience, the efficacy of high-dosage oral prednisone was comparable to intravenous pulse corticosteroid therapy [32]. However, larger series of patients are needed to clarify this issue.
Most GCA manifestations other than blindness usually begin to improve within 24 to 72 h after the onset of corticosteroid therapy. Also, normalization of the routine laboratory parameters of inflammation (erythrocyte sedimentation rate and C-reactive protein) occurs within 2–4 weeks after onset of the therapy [24]. In patients with isolated PMR, rapid response to prednisone is achieved within a week after the onset of corticosteroid therapy, generally within the first 48–72 h [30••]. Afterward, corticosteroid dose can be tapered.
Alternative corticosteroid-sparing drugs are considered in patients with GCA or PMR who experience severe corticosteroid-related side effects and/or in those patients who require prolonged corticosteroid therapy due to relapses of the disease. However, a recent meta-analysis of published data indicates that in most cases, prednisolone/prednisone combined with adjunctive immunosuppression is not superior to prednisolone/prednisone alone in terms of efficacy and safety in GCA [40•]. With respect to this, there was no clear benefit of anti-TNF-α therapy (infliximab or adalimumab) in these patients [40•]. A former meta-analysis of three randomized placebo-controlled trials performed to reevaluate the efficacy and safety of adjunctive low-dose methotrexate (MTX) in GCA had already disclosed a modest role of MTX (10–15 mg/week) in reducing relapse rate and lowering the cumulative corticosteroid dose [41]. Although in a small series that included 12 patients with PMR and 11 with GCA leflunomide appeared to be effective as a corticosteroid-sparing agent in difficult-to-treat patients, further studies on this drug are necessary to confirm its usefulness in the management of these conditions [42•]. The humanized monoclonal anti-interleukin-6 receptor tocilizumab appears to be a biologic agent effective and relatively safe in patients with inflammatory aortitis refractory to corticosteroids or to other biologic immunosuppressive drugs [43•]. This drug led to rapid improvement in a series of 16 patients with aortitis who had failed to respond to corticosteroids and in most cases to MTX [43•]. Tocilizumab both as monotherapy and in association with corticosteroids also appears to be effective and safe in the treatment of patients with PMR [44•].
Outcome
Previous population-based studies did not show an increased incidence of cancer in GCA [45, 46]. Although a systematic review meta-analysis suggested that a low but statistically significant increased risk of cancer might exist among patients with GCA and PMR, when a study with potential selection bias was excluded from the meta-analysis, the new pooled risk ratio did not achieve statistical significance [47•].
Patients with biopsy-proven GCA and traditional cardiovascular risk factors or permanent visual loss have an increased risk of suffering strokes, in particular in the vertebrobasilar territory [48]. A recent study indicates that GCA is associated with increased risks for myocardial infarction, cerebrovascular accidents, and peripheral vascular disease [49•]. However, there were important limitations in this study such as lack of information on TAB and a substantial amount of missing data on cardiovascular risk factors [49•]. Another recent study suggests that patients using statins are less likely to develop GCA compared with patients not using statins. However, among patients diagnosed with GCA, the presenting clinical features and acute-phase reactants were similar in patients receiving statins compared with those not on statin therapy [50•].
Conclusion
GCA and PMR are common conditions in individuals aged 50 years and older. A TAB is the gold standard test for the diagnosis of this vasculitis. FDG-PET is useful in disclosing the presence of large-vessel vasculitis in patients with GCA. Corticosteroids are the cornerstone of the therapy in GCA and PMR. However, relapses are common. Unlike MTX and TNF-α antagonists, anti-IL-6 receptor therapy appears to be a promising therapy to be considered in the management of patients with GCA and PMR who are refractory to corticosteroids.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009;61:1454–612.
Gonzalez-Gay MA. Giant cell arteritis and polymyalgia rheumatica: two different but often overlapping conditions. Semin Arthritis Rheum. 2004;33:289–93.
Gonzalez-Gay MA, Miranda-Filloy JA, Lopez-Diaz MJ, Perez-Alvarez R, Gonzalez-Juanatey C, Sanchez-Andrade A, et al. Giant cell arteritis in northwestern Spain: a 25-year epidemiologic study. Medicine (Baltimore). 2007;86:61–88.
Petri H, Nevitt A, Sarsour K, Napalkov P, Collinson N. Incidence of giant cell arteritis and characteristics of patients: data-driven analysis of comorbidities. Arthritis Care Res (Hoboken). 2014. doi:10.1002/acr.22429. The incidence of GCA is higher in women, in particular in the 70 to 79 years old age group.
Dunstan E, Lester SL, Rischmueller M, Dodd T, Black R, Ahern M, et al. Epidemiology of biopsy-proven giant cell arteritis in South Australia. Intern Med J. 2014;44:32–9. Clinical features is Australian individuals with GCA is similar to that previously reported in Western countries.
Alba MA, Mena-Madrazo JA, Reyes E, Flores-Suárez LF. Giant cell arteritis in Mexican patients. J Clin Rheumatol. 2012;18:1–7. The incidence of GCA in Hispanic is low.
González-Gay MA, Amoli MM, Garcia-Porrua C, Ollier WE. Genetic markers of disease susceptibility and severity in giant cell arteritis and polymyalgia rheumatica. Semin Arthritis Rheum. 2003;33:38–48.
Serrano A, Márquez A, Mackie SL, Carmona FD, Solans R, Miranda-Filloy JA, et al. Identification of the PTPN22 functional variant R620W as susceptibility genetic factor for giant cell arteritis. Ann Rheum Dis. 2013;72:1882–6. PTPN22 R620W functional variant has been implicated in the susceptibility to several autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus. The present study also confirms its implication in GCA. These findings indicate that PTPN22 may represent a common pathway for different autoimmune diseases.
Carmona FD, González-Gay MA, Martín J. Genetic component of giant cell arteritis. Rheumatology (Oxford). 2014;53:6–18. Besides the well-known association with genes located in the HLA region, other gene polymorphisms located outside this region also account for the susceptibility to GCA.
Weyand CM, Goronzy JJ. Arterial wall injury in giant cell arteritis. Arthritis Rheum. 1999;42:844.
Dejaco C, Duftner C, Al-Massad J, Wagner AD, Park JK, Fessler J, et al. NKG2D stimulated T-cell autoreactivity in giant cell arteritis and polymyalgia rheumatica. Ann Rheum Dis. 2013;72:1852–9. This study highlights the relevance of T cells and the implication of NKG2D in the pathogenesis of GCA and PMR.
Samson M, Audia S, Fraszczak J, Trad M, Ornetti P, Lakomy D, et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum. 2012;64:3788–98. Although the suppressive activity of circulating Treg cells is not altered, the number of circulating Treg cells is decreased in patients with GCA or PMR. The numbers of Th17 lymphocytes are significantly increased in patients with GCA and PMR.
van der Geest KS, Abdulahad WH, Chalan P, Rutgers A, Horst G, Huitema MG, et al. Disturbed B cell homeostasis in newly diagnosed giant cell arteritis and polymyalgia rheumatica. Arthritis Rheumatol. 2014;66:1927–38. Patients newly diagnosed as having GCA or PMR exhibit decreased numbers of circulating B cells compared to healthy controls. B cell numbers recover following therapy in patients with GCA and PMR.
Gonzalez-Gay M. The diagnosis and management of patients with giant cell arteritis. J Rheumatol. 2005;32:1186–8.
Schmidt WA, Kraft HE, Vorpahl K, Volker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337:1336–42.
Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord. 2010;11:44.
Germanò G, Muratore F, Cimino L, Lo Gullo A, Possemato N, Macchioni P, et al. Is colour duplex sonography-guided temporal artery biopsy useful in the diagnosis of giant cell arteritis? A randomized study. Rheumatology (Oxford) 2014 Jun 17. pii: keu241. [Epub ahead of print] Interesting study that indicates that color duplex US of the temporal artery does not improve the sensitive of TAB to make a diagnosis of GCA. The results, rather than reducing the potential usefulness of color duplex US for the diagnosis of GCA, highlight the relevance of an adequate clinical examination to proceed to TAB when GCA is suspected.
Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, Gonzalez-Louzao C, Rodriguez-Ledo P. Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum. 2001;30:249–56.
Cavazza A, Muratore F, Boiardi L, Restuccia G, Pipitone N, Pazzola G, et al. Inflamed temporal artery: histologic findings in 354 biopsies, with clinical correlations. Am J Surg Pathol. 2014;38:1360–70. Study on 322 temporal artery biopsies. It discloses the wide spectrum of inflammatory lesions found in TAB. It also confirmed that some conditions, in particular systemic vasculitides different from GCA, may cause inflammation of the temporal artery.
Sakellariou G, Iagnocco A, Riente L, Ceccarelli F, Carli L, Di Geso L, et al. Ultrasound imaging for the rheumatologist XLIII. Ultrasonographic evaluation of shoulders and hips in patients with polymyalgia rheumatica: a systematic literature review. Clin Exp Rheumatol. 2013;31:1–7. Very elegant review article that indicates that in patients with suggestive clinical features of PMR, a shoulder US, in particular when findings are bilateral, is useful to support a diagnosis of PMR.
Gonzalez-Gay MA, Garcia-Porrua C, Piñeiro A, Pego-Reigosa R, Llorca J, Hunder GG. Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: a population-based study. Medicine (Baltimore). 2004;83:335–41.
Pipitone N, Versari A, Salvarani C. Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update. Rheumatology (Oxford). 2008;47:403–8.
Puppo C, Massollo M, Paparo F, Camellino D, Piccardo A, Shoushtari Zadeh Naseri M, et al. Giant cell arteritis: a systematic review of the qualitative and semiquantitative methods to assess vasculitis with 18F-fluorodeoxyglucose positron emission tomography. Biomed Res Int. 2014;2014:574248. 18 F-FDG- PET is an effective tool for the diagnosis, grading, and follow-up of patients affected by GCA involving the aorta and its proximal branches. In this exhaustive review the authors assessed the qualitative and quantitative methods used for the assessment of vasculitis by 18-FDG-PET. They concluded that the qualitative methods may be more specific than semiquantitative ones, but they have lower sensitivity.
Gonzalez-Gay MA, Martinez-Dubois C, Agudo M, Pompei O, Blanco R, Llorca J. Giant cell arteritis: epidemiology, diagnosis, and management. Curr Rheumatol Rep. 2010;12:436–42.
Bejerano C, Blanco R, González-Vela C, Pérez-Martín I, Martinez-Rodriguez I, Jimenez-Bonilla J, et al. Polymyalgia rheumatica as presenting manifestation of vasculitis involving the lower extremities in a patient with ulcerative colitis. Clin Exp Rheumatol. 2012;30(1 Suppl 70):S110–3.
Bejerano C, Blanco R, González-Vela C, Agüero R, Carril JM, González-Gay MA. Refractory polymyalgia rheumatica as presenting manifestation of large-vessel vasculitis associated to sarcoidosis. Successful response to adalimumab. Clin Exp Rheumatol. 2012;30(1 Suppl 70):S94–7.
Martínez-Rodríguez I, Martínez-Amador N, Banzo I, Quirce R, Jiménez-Bonilla J, De Arcocha-Torres M, et al. Assessment of aortitis by semiquantitative analysis of 180-min (18)F-FDG PET/CT acquisition images. Eur J Nucl Med Mol Imaging. 2014;41(12):2319–24. Prospective study that included 43 consecutive patients assessed by semiquantitative analysis of PET-CT because of suspected large vessel vasculitis. The authors found that images acquired 180 min after 18 F-FDG injection and a target to background ratio index of 1.34 showed very high accuracy and are strongly recommended for the diagnosis of aortitis in the clinical setting.
Baerlecken NT, Linnemann A, Gross WL, Moosig F, Vazquez-Rodriguez TR, Gonzalez-Gay MA, et al. Association of ferritin autoantibodies with giant cell arteritis/polymyalgia rheumatica. Ann Rheum Dis. 2012;71:943–7. Ferritin autoantibodies may be useful as a diagnostic and activity marker of PMR and GCA.
Große K, Schmidt RE, Witte T, Baerlecken NT. Epitope mapping of antibodies against ferritin heavy chain in giant cell arteritis and polymyalgia rheumatica. Scand J Rheumatol. 2013;42:215–9. Potential diagnostic test for GCA and PMR can be improved by combining three human ferritin peptide antibodies.
Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371:50–7. Review article that discussed preexisting information on the pathophysiology, diagnosis, clinical features and treatment of GCA. This review also points out some areas of uncertainty in GCA and PMR.
Figus M, Talarico R, Posarelli C, d’Ascanio A, Elefante E, Bombardieri S. Ocular involvement in giant cell arteritis. Clin Exp Rheumatol. 2013;31(1 Suppl 75):S96. Cohort study that confirms the high frequency of visual manifestations in patients with GCA. Early recognition and rapid onset of corticosteroid therapy is of main importance to prevent irreversible visual loss.
Gonzalez-Gay MA, Blanco R, Rodriguez-Valverde V, Martínez-Taboada VM, Delgado-Rodriguez M, Figueroa M, et al. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: predictors and response to treatment. Arthritis Rheum. 1998;41:1497–504.
Muratore F, Pazzola G, Pipitone N, Boiardi L, Salvarani C. Large-vessel involvement in giant cell arteritis and polymyalgia rheumatica. Clin Exp Rheumatol. 2014;32(3 Suppl 82):S106–S11. An extremely interesting and informative review article that highlights important points on the diagnosis and management of patients with large vessel involvement in the setting of GCA.
Muratore F, Kermani TA, Crowson CS, Green AB, Salvarani C, Matteson EL, et al. Large-vessel giant cell arteritis: a cohort study. Rheumatology (Oxford). 2014 Sep 5. pii: keu329. [Epub ahead of print] The clinical spectrum of patients with large vessel involvement in the setting of GCA is different from that found in patients presenting with typical cranial manifestations of GCA. Patients with large vessel involvement have less frequency of severe visual complication but they experience more relapses and have higher corticosteroid requirements than those presenting with classic cranial manifestations.
Talarico R, Boiardi L, Pipitone N, d'Ascanio A, Stagnaro C, Ferrari C, et al. Isolated aortitis versus giant cell arteritis: are they really two sides of the same coin? Clin Exp Rheumatol. 2014;32(3 Suppl 82):S55–8. An interesting study that confirms that the clinical spectrum of patients with isolated aortitis is different from that observed in those with cranial manifestations of GCA.
Salvarani C, Barozzi L, Boiardi L, Pipitone N, Bajocchi GL, Macchioni PL, et al. Lumbar interspinous bursitis in active polymyalgia rheumatica. Clin Exp Rheumatol. 2013;31:526–31. Inflammation of lumbar bursae may be responsible for the low back pain reported by patients with PMR.
Martinez-Lado L, Calviño-Díaz C, Piñeiro A, Dierssen T, Vazquez-Rodriguez TR, Miranda-Filloy JA, et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore). 2011;90:186–93.
Alba MA, García-Martínez A, Prieto-González S, Tavera-Bahillo I, Corbera-Bellalta M, Planas-Rigol E, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore). 2014;93:194–201. Relapses are common in patients with GCA. They occur predominantly within the first 2 years of treatment. PMR symptoms are common with relapses but blindness is exceptional.
Lee JH, Choi ST, Kim JS, Yoon BY, Kwok SK, Kim HS, et al. Clinical characteristics and prognostic factors for relapse in patients with polymyalgia rheumatica (PMR). Rheumatol Int. 2013;33:1475–80. As observed in GCA, relapses are also common in patients with PMR.
Yates M, Loke YK, Watts RA, MacGregor AJ. Prednisolone combined with adjunctive immunosuppression is not superior to prednisolone alone in terms of efficacy and safety in giant cell arteritis: meta-analysis. Clin Rheumatol. 2014;33:227–36. Randomized controlled trials on MTX or anti-TNF-alpha drugs versus corticosteroids do not show that the use of these adjunctive drugs may improve the outcome of GCA.
Mahr AD, Jover JA, Spiera RF, Hernández-García C, Fernández-Gutiérrez B, Lavalley MP, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–97.
Diamantopoulos AP, Hetland H, Myklebust G. Leflunomide as a corticosteroid-sparing agent in giant cell arteritis and polymyalgia rheumatica: a case series. Biomed Res Int. 2013;2013:120638. Small series of patients that showed a beneficial effect of leflunomide in GCA and PMR.
Loricera J, Blanco R, Castañeda S, Humbría A, Ortego-Centeno N, Narváez J, et al. Tocilizumab in refractory aortitis: study on 16 patients and literature review. Clin Exp Rheumatol. 2014;32(3 Suppl 82):S79. Tocilizumab may be effective in patients with inflammatory aortitis refractory to corticosteroids or to other biologic immunosuppressive drugs.
Macchioni P, Boiardi L, Catanoso M, Pulsatelli L, Pipitone N, Meliconi R, et al. Tocilizumab for polymyalgia rheumatica: report of two cases and review of the literature. Semin Arthritis Rheum. 2013;43:113–8. Tocilizumab has proved to be effective in some cases of PMR.
Gonzalez-Gay MA, Lopez-Diaz MJ, Martinez-Lado L, Peña-Sagredo JL, Lopez-Agreda H, Miranda-Filloy JA, et al. Cancer in biopsy-proven giant cell arteritis. A population-based study. Semin Arthritis Rheum. 2007;37:156–63.
Kermani TA, Schäfer VS, Crowson CS, Hunder GG, Gabriel SE, Ytterberg SR, et al. Malignancy risk in patients with giant cell arteritis: a population-based cohort study. Arthritis Care Res. 2010;62(2):149–54.
Ungprasert P, Sanguankeo A, Upala S, Knight EL. Risk of malignancy in patients with giant cell arteritis and polymyalgia rheumatica: A systematic review and meta-analysis. Semin Arthritis Rheum 2014 Jun 26. doi: 10.1016/j.semarthrit.2014.06.004. Overall, the risk of malignancy in GCA and PMR is not higher than in the general population.
Gonzalez-Gay MA, Vazquez-Rodriguez TR, Gomez-Acebo I, Pego-Reigosa R, Lopez-Diaz MJ, Vazquez-Triñanes MC, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine (Baltimore). 2009;88:227–35.
Tomasson G, Peloquin C, Mohammad A, Love TJ, Zhang Y, Choi HK, et al. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann Intern Med. 2014;160:73–80. Unlike previous studies, a recent report suggests that cardiovascular disease may be increased in GCA.
Schmidt J, Kermani TA, Muratore F, Crowson CS, Matteson EL, Warrington KJ. Statin use in giant cell arteritis: a retrospective study. J Rheumatol. 2013;40:910–5. The use of statins may reduce the risk of GCA.
Compliance with Ethics Guidelines
Conflict of Interest
Miguel A. González-Gay and Trinitario Pina declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a part of the Topical Collection on Vasculitis
Rights and permissions
About this article
Cite this article
González-Gay, M.A., Pina, T. Giant Cell Arteritis and Polymyalgia Rheumatica: an Update. Curr Rheumatol Rep 17, 6 (2015). https://doi.org/10.1007/s11926-014-0480-1
Published:
DOI: https://doi.org/10.1007/s11926-014-0480-1