Abstract
Osteoarthritis (OA) and osteoporosis (OP) are highly prevalent health problems, associated with considerable morbidity. In the past, attention was focused on a supposed inverse relationship between OA and OP, since both disorders usually affect the elderly, but were regarded to rarely coexist in a single person. However, recent studies have revealed several factors which contribute to the pathogenesis of both disorders. These insights might contribute to the development of shared new treatment options in the near future. Increased subchondral bone loss is a characteristic feature of OP and the early stage of OA, and this finding is the rationale for studies on the effect of anti-osteoporotic drugs in OA. In addition, inflammation and unfavourable body composition have been recognized as contributing factors for both disorders. Underweight is a risk factor for OP, while obesity stimulates the development of OA, by mechanical overloading of weight-bearing joints but also by supposed unfavourable effects of adipokines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
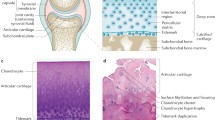
Osteoarthritis (OA) and osteoporosis (OP) are disorders of bone, which usually occur in people over the age of 50 years, and are associated with substantial morbidity and disability. Although OA and OP predominantly affect the elderly, until recently attention was focused on a supposed inverse relationship between both disorders [1], which rarely coexist in a single person [2, 3]. OP is characterized by increased bone loss and deterioration of bone microarchitecture, a process that leads to reduced bone strength and an increased risk of fragility fractures. The pathogenesis of OA has not been fully elucidated. OA is characterized by degradation of the hyaline articular cartilage, but the pathological process also involves changes in periarticular bone, the synovial membrane and other periarticular soft tissue structures such as ligaments and tendons [4]. However, although all the above-mentioned structural changes are supposed to contribute to the development of OA, the sequence and relative importance of each of these processes might be different between individuals, between joints and between different stages of the disease, and may change over time [5••].
In addition to some well-known risk factors for OA and OP (of which age is by far the most important), recent studies have elucidated that inflammation, unfavourable body composition and mechanical loading are additional shared factors influencing the development of OA and OP (Fig. 1). These more recently recognized risk factors for OA and OP are discussed.
Recent experimental and clinical studies have demonstrated that changes in subchondral bone [6–9] and a cross-talk between subchondral bone and cartilage [10] play an important role in the development of OA. Excessive bone resorption, which is a hallmark of postmenopausal OP, also occurs at the early stage in the development of OA, while increased bone formation, subchondral sclerosis, and osteophyte formation take place in both the early and late stages of OA development [5••].
The cross-talk between cartilage and subchondral bone and the subchondral bone resorption occurring at an early stage in the development of OA are the rationale for considering subchondral bone as a potential target for the treatment of (early) OA. We discuss the results of several studies, published in the last few years, on the effect of anti-osteoporotic drugs (including estrogens, selective estrogen receptor modulators, calcitonin, bisphosphonates, strontium ranelate, teriparatide, and cathepsin K inhibitors) on cartilage biomarkers, clinical signs, and progression of OA. We do realize that some of the anti-osteoporotic drugs are not often used in daily practice, but we discuss studies on these agents because they provide some insight into the relationship between OA and OP.
Role of Inflammation in OA and OP
Recent advances in the field of osteoimmunology, the cross-talk between cytokines and bone, have provided more insight into the role of inflammatory cytokines and adipokines on cartilage turnover and bone metabolism. There is substantial evidence that inflammation influences the development of both OA and OP.
The influence of inflammation on the development of OP has been recognized from the increased occurrence of OP and fractures in patients with chronic inflammatory diseases, such as rheumatoid arthritis, systemic lupus erythematosus and ankylosing spondylitis [11]. The prominent inflammation in the course of the above-mentioned diseases is associated with an increased production of a wide spectrum of cytokines that alter the balance between bone formation and bone resorption. In rheumatoid arthritis, the receptor activator of the nuclear factor κB (RANKL)/osteoprotegerin (OPG) pathway is involved in the regulation of bone resorption by stimulating the activation, differentiation, and proliferation of osteoclasts by RANKL, while OPG acts as a decoy receptor [12]. Recent studies have demonstrated that several inflammatory cytokines, such as tumor necrosis factor α, interleukin (IL)-1, IL-6 and IL-17, upregulate RANKL, which subsequently induces osteoclastogenesis [13••, 14•]. RANKL is supposed to be a major factor for joint destruction in rheumatoid arthritis, and is not only expressed by osteoblasts but also by activated T cells and B cells. In patients with rheumatoid arthritis, increased levels of RANKL and decreased OPG levels compared to healthy controls have been demonstrated [15]. These findings illustrate the occurrence of both increased bone resorption and reduced bone formation in patients with inflammatory rheumatic diseases.
However, the contribution of inflammation to the development of OP is not restricted to chronic inflammatory diseases. Schett et al. demonstrated in the Bruneck study that low-grade inflammation, as estimated by the high-sensitivity C-reactive protein level, is a significant and independent risk predictor of nontraumatic fractures in healthy individuals [16]. This finding is in line with the hypothesis of a tight link between low-grade inflammation and bone quality.
Recent epidemiological studies showing an increased risk of the development of hand OA in obese patients [17•] provided insight into the contribution of systemic inflammation to the development and progression of OA. Overweight had long been recognised as a modifiable risk factor for knee OA. Obesity induces mechanical overloading of weight-bearing joints, which leads to the development of knee OA [18]. However, animal studies of diet-induced obesity have shown that mechanical overload cannot fully explain the progression of spontaneous or post-traumatic knee OA [19]. These findings were the rationale for studies on the relationship between systemic inflammatory factors associated with obesity and the development of OA.
Among the many cytokines secreted by adipose tissue, the adipokines (adiponectin, resistin, leptin, and others) have been recognized as key mediators in the metabolic interplay between obesity and OA. Adipokines play an important role in a wide range of physiological processes, including immune function, glucose metabolism, and bone homeostasis. In a cross-sectional study, an association between increased serum adiponectin levels and erosive hand OA compared to non-erosive hand OA in females was reported [20]. Another cross-sectional study demonstrated a relationship between serum resistin levels and severe radiographic changes in hand OA, while no association was found between adiponectin levels and radiographic damage [21]. However, the Genetics, Arthrosis and Progression (GARP) study, a recent longitudinal study in 164 patients with hand OA, followed for 6 years, demonstrated that high adiponectin serum levels at baseline were associated with a decreased risk (RR 0.3, 95 % confidence interval 0.2–0.7) of progression of hand OA, a finding which suggests that adiponectin has a protective effect with respect to cartilage damage [22]. No association between baseline leptin and resistin serum levels and progression of hand OA was found in this study.
The majority of studies on the relationship between adipokines and knee OA published were relatively small, (maximum 117 patients) [23–27], had a cross-sectional design [23, 24, 26, 27] or a relatively short (maximum 2 years) followup duration [25], and showed conflicting results. Recently, the results of a large, both cross-sectional and 5-year follow-up, study on the associations between adipokines and progression of radiographic changes in 1,002 Dutch patients with early-stage symptomatic knee OA were published [28]. In this study, plasma adipokine levels were significantly but weakly associated with biochemical markers of joint metabolism and radiographic signs of OA. Leptin levels demonstrated the most statistically significant associations with biomarkers of cartilage and the presence and progression of radiographic knee OA. As expected, these associations largely disappeared after adjustment for body mass index (BMI). Resistin levels were positively associated with radiographic knee OA, largely independent of BMI. Adiponectin levels were not associated with radiographic knee OA itself, but the associations of resistin with radiographic knee OA were stronger in higher ranges of adiponectin levels. These positive associations of leptin and resistin with radiographic knee OA support the hypothesis that OA is a low-grade inflammatory disease and leptin and resistin might be proinflammatory adipokines in early-stage knee OA. The conflicting results of the above-mentioned studies illustrate that the role of adipokines in the development and progression of OA has not yet been fully elucidated. Discrepancies between study results may in part be explained by differences between study populations in size, mean age, gender, mean BMI, and the degree of OA. However, the weakness of the associations found in the largest study also point to a limited relationship between these specific adipokines investigated and early-stage knee OA. Further studies are needed to investigate the role of these and other adipokines in the development and progression of OA. In addition, intra-articularly produced adipokines might be more relevant markers for severity and progression of OA than systemic adipokine levels [28]. A recent cross-sectional study in 76 patients with knee OA demonstrated a negative association between both systemic adiponectin levels and synovial fluid adiponectin levels and the severity of OA, but the association between systemic adiponectin levels and radiographic OA disappeared after adjusting for age, gender and BMI, while the association between synovial fluid levels of adiponectin and radiographic changes persisted after the same adjustment [23].
The recognition that low-grade inflammation contributes to the development of OA and OP provides the opportunity to develop new, anti-inflammatory therapeutic interventions.
Role of Unfavourable Body Composition and Mechanical Loading in OA and OP
Unfavourable body composition contributes to the development of both OA and OP, but in different ways. Obesity stimulates the development and progression of OA by the effect of mechanical overloading of weight-bearing joints and by the supposed unfavourable effects of specific adipose tissue-derived adipokines, as discussed above. In contrast, low body weight (< 60 kg) or low BMI (< 20 kg/m2) is a well-known risk factor for the development of OP and is associated with an increased fracture risk [29]. However, a recent study in more than 800,000 postmenopausal women in Spain revealed a site-dependent association between obesity and fracture [30]. Obese women had a significantly increased risk for proximal humerus fracture than women with normal weight or underweight (RR 1.28; 95 % CI 1.04–1.58; p = 0.018), and a significantly reduced risk for hip fractures (RR 0.77; 95 % CI 0.68–0.88; p < 0.001) and pelvis fractures. Incidence rates of fractures at other sites were not different between obese and non-obese women. A study in more than 1 million postmenopausal women in the United States confirmed the reduced risk for hip fractures in obese women compared to women with normal or low BMI [31]. However, this study also demonstrated reduced wrist fracture rates and significantly increased ankle fracture rates in obese women compared to lean women. The reasons for these site-specific differences are unclear, but may be related to differences in patterns of falls, and differences in the impact of falls due to attenuation by adipose tissue. In line with the increases in both BMI and age in Western and Asian countries over the last decades, attention has recently turned to the complex problem of osteoporotic fractures in the overweight and obese. Paradoxically, many osteoporotic fractures occur in overweight or obese individuals, and obese men might be particularly at risk [32].
Effects of Anti-Osteoporotic Drugs on Osteoarthritis
Hormonal Therapies (Estrogens, Selective Estrogen Receptor Modulators)
Epidemiological studies have suggested a relationship between estrogen deprivation and the development of OA by demonstrating that age-related increases in the incidence and prevalence of OA were greater in men than in women under the age of 50 years, but were greater in women than in men older than 50 years [33]. In women, the prevalence of OA dramatically increases around 50 years of age [34], which coincides with the start of menopause. These findings suggest that estrogens have a protective effect on the development of OA.
Estrogen receptors alpha and beta have been detected in cartilage and both animal studies, and in vitro studies have shown the beneficial effects of estrogens on cartilage and subchondral bone [35–37]. A few studies investigating the influence of estrogen deprivation and the effect of hormone replacement therapy (HRT) or treatment with selective estrogen receptor modulators (SERMs) on biomarkers and development of OA in postmenopausal women have been published. These demonstrated an increase in urinary levels of the C-telopeptide of type II collagen (CTX-II) in postmenopausal women [38], and significantly lower CTX-II levels in women receiving HRT as compared to placebo-treated women [39]. In addition, a randomized controlled trial (RCT) demonstrated a significant decrease in urinary CTX-II levels in postmenopausal women treated with estrogens or SERMs as compared with placebo [40]. However, urinary CTX-II levels have long been regarded as a biomarker for cartilage damage alone, but were recently proposed to also be released from bone [4].
The effect of estrogen therapy on clinical signs and radiographic and magnetic resonance imaging (MRI) features of knee OA was investigated in a large, cross-sectional study in postmenopausal women, which demonstrated a reduction in bone marrow edema-like lesions in the subchondral bone in estrogen-treated patients. However, no association between estrogen use and knee pain and/or radiographic changes of knee OA was found [41].
Calcitonin
Several in vitro studies have demonstrated direct beneficial effects of calcitonin on articular cartilage. This protective effect of calcitonin is supposed to be mediated by inhibiting degradation and promoting formation of articular matrix components [42, 43].
The effect of oral salmon calcitonin on joint biomarker levels has been evaluated in two small RCTs in men and women with knee OA [44, 45]. These studies demonstrated significantly reduced levels of CTX-II [44, 45], and of matrix metalloproteinases 3 and 13, hyaluronan, CTX-I, and type II collagen neoepitope [45] in calcitonin-treated patients, whereas the biomarkers levels in the placebo-treated patients remained unchanged. In healthy postmenopausal women treated with oral salmon calcitonin for 3 months, similar decreases in CTX-I and CTX-II levels were found [46]. One study showed a significant functional improvement in calcitonin-treated patients [44], but no studies reporting an effect of calcitonin on pain and progression of OA have yet been published.
Bisphosphonates
Bisphosphonates are the most frequently used therapeutic agents for osteoporosis prevention and treatment, which inhibit bone resorption by their direct inhibitory effect on osteoclasts. This effect is supposed to retard subchondral bone remodelling, which might have a beneficial influence on osteophyte formation and sclerosis of subchondral bone [47]. These supposed favourable effects of bisphosphonates on subchondral bone remodelling were the rationale for studies on the effect of bisphosphonates on clinical signs and radiographic progression of OA, and on biomarkers of cartilage degradation.
Studies of animal models of OA have demonstrated that several bisphosphonates, including alendronate, risedronate, zoledronic acid, and tiludronate, may retard progression of osteoarthritis by suppressing subchondral bone resorption and possibly by reducing osteophyte formation [48–51].
In an early, cross-sectional study in postmenopausal women with knee OA, alendronate use was associated with less severe knee pain and significantly reduced subchondral bone attrition and bone marrow edema-like abnormalities as assessed by MRI, as compared with women not using bisphosphonates. However, no relationship between alendronate use and radiographic features of knee OA was found [41].
Neogi and colleagues performed a post hoc analysis of 200 patients randomly selected from the large Fracture Intervention Trial, a clinical study on the effect of alendronate in postmenopausal women with OP, and demonstrated subtle but significant reduction in radiographic features of spinal OA (both the formation of osteophytes and disc space narrowing) in alendronate-treated patients as compared to patients treated with placebo [52].
The effect of risedronate on clinical signs and radiographic progression of knee OA has been evaluated in two RCTs. Spector and colleagues reported a reduction in the Western Ontario and MacMaster Universities (WOMAC) OA index in 284 patients treated with 15 mg risedronate daily for 1 year compared to placebo, but the reduction in joint space narrowing (JSN) was not significant [53]. A much larger, 2-year RCT in 2,483 patients with knee OA demonstrated a reduction in CTX-II levels in patients treated with high dose (15 mg daily) risedronate, but not in the subgroup treated with the usually prescribed 5 mg per day. No significant effect of risedronate on WOMAC index and radiographic progression was shown [54]. In contrast to the results of these clinical studies, Buckland-Wright and colleagues demonstrated that vertical trabecular number was increased and structural integrity of subchondral bone was preserved in patients with knee OA treated with risedronate (15 mg per day or 50 mg per week) for 2 years [55]. Various explanations have been proposed for these conflicting results. First, it has been suggested that the study of Bingham and colleagues failed to demonstrate a significant effect of risedronate on clinical signs and radiographic progression of OA due to a low rate of OA progression in the placebo group [47]. Indeed, the rate of cartilage loss and JSN varies between individuals, and selecting patients with a high rate of cartilage loss might be necessary to demonstrate a significant clinical and radiographic effect of bisphosphonate therapy. Second, the timing of treatment with bisphosphonates in patients with OA may be critical, with the greatest benefit obtained early in the disease course [47]. Roux and Richette suggested the use of bone marrow lesions on MRI as inclusion criteria for clinical trials instead of the presence of JSN, since the latter might be irreversible [56]. Third, other bone antiresorptive agents with a different mechanism of action on subchondral bone and cartilage might be more effective. Recently, a significant reduction in knee pain and bone marrow lesions on MRI 6 months after a single infusion of zoledronic acid compared to placebo treatment was demonstrated [57].
Strontium Ranelate
Strontium ranelate is an anti-osteoporotic drug with a supposed dual mode of action, since it decreases bone resorption and increases bone formation simultaneously [58]. Results of post hoc analyses of two large intervention studies in patients with osteoporosis were the rationale for studies on the effect of strontium ranelate in OA. In the first study, CTX-II levels were measured in a subgroup of 2,617 women who had participated in the TROPOS trial, a placebo-controlled study on the effect of strontium ranelate on the occurrence of non-vertebral osteoporotic fractures. The post hoc analysis demonstrated a significant reduction in CTX-II levels in strontium ranelate-treated patients as compared to placebo [59]. The second study was performed in the same study population, but investigated changes in CTX-II levels in subgroups of patients with and without OA at baseline. Patients with OA treated with strontium ranelate had a significant decrease in urinary CTX-II levels as compared to placebo-treated women [60]. Interestingly, urinary CTX-II levels not only decreased in patients with OA but also in patients without OA. Therefore, it has been suggested that CTX-II is not a marker of cartilage degradation alone but may also be released from bone [61].
A post hoc analysis of pooled data from two clinical trials performed in 1,105 women with OP and concomitantly radiological spinal OA suggested that strontium ranelate might reduce the progression of spinal OA after 3 years of therapy [62]. Based on these findings, the strontium ranelate efficacy in knee osteoarthritis trial (SEKOIA) was designed: a large, double-blind, placebo-controlled RCT on the effect of strontium ranelate 1 g/day, 2 g/day or placebo in 1,371 patients with knee OA (Kellgren and Lawrence grade 2 or 3, and joint space width 2.5–5 mm) [63•]. Both strontium ranelate-treated subgroups demonstrated significantly less radiological progression of JSN after 3 years of treatment (1 g/day: −0.23 (SD 0.56) mm, 2 g/day: −0.27 (SD 0.63) mm, placebo: −0.37 (SD 0.59) mm) compared to placebo (p < 0.001 for 1 g/day and p = 0.018 for 2 g/day). In addition, therapy with strontium ranelate 2 g daily was associated with significant greater reductions in (total) WOMAC score, and pain subscore.
The exact mechanisms of action by which strontium ranelate exerts beneficial effects on subchondral bone and cartilage are not fully understood and subject of debate [4]. In vitro experiments demonstrated that strontium ranelate has inhibitory effects on human osteoarthritic subchondral bone osteoblasts, which leads to reduced production of key factors associated with subchondral bone resorption [64]. In addition, other in vitro experiments have suggested that strontium ranelate also acts directly on cartilage. Strontium ranelate strongly increased cartilage matrix formation by human chondrocytes in vitro [65].
The results of the SEKOIA study, demonstrating efficacy of strontium ranelate in the treatment of moderate knee OA, might be an important step forwards in the development of new treatment strategies for OA. However, although the SEKOIA study is a high quality study, which convincingly demonstrated reduction in radiological progression, the clinical relevance of these differences in radiological progression and the small decreases in pain subscores can be debated. Therefore, further studies are necessary to determine for which patients and in what stage of OA this drug may be most effective [4]. Another issue of importance might be compliance to therapy in OA patients, which might be negatively influenced by the need for daily oral intake and the expected long duration of therapy.
Teriparatide
The well-known stimulatory effects of parathyroid hormone (PTH) on bone formation have led to the development of recombinant human PTH 1-34 (teriparatide), a bone anabolic drug for the treatment of osteoporosis. PTH induces matrix synthesis and suppresses maturation of chondrocytes. OA is associated with inappropriate maturation of articular chondrocytes [66]. In addition, the PTH receptor is up-regulated in articular chondrocytes during human OA and in an animal model of injury-induced knee OA [67]. Based on these findings, teriparatide might have a benefit in OA. A recent study demonstrated positive effects of teriparatide in an animal model of OA [66]. However, studies in human OA have not yet been published.
Cathepsin K Inhibitors
Another group of anti-osteoporotic drugs suggested as potential therapeutic agents in OA are cathepsin K inhibitors. The selective cathepsin K inhibitor odanacatib has been demonstrated to increase bone mineral density and decrease bone resorption markers in postmenopausal women with OP [68]. A recent study in two animal models of OA showed beneficial effects of a cathepsin K inhibitor on protection of subchondral bone loss and against cartilage degradation, and suggested reduced osteophyte formation [69]. Results of further clinical studies on cathepsin K inhibitors in human OA and OP are awaited.
Conclusions
What is the overlap between OA and OP?
OP and OA are both disorders of bone, which usually occur in elderly people and are associated with considerable morbidity and disability. Both diseases are huge health problems because of their high prevalence rates and rising incidence rates due to the ageing population. Apart from that, in patients with inflammatory rheumatic disorders, both OA and (secondary) OP may occur at younger ages.
Recent advances in the field of osteoimmunology have elucidated that increased bone loss occurs not only in OP but also in the early stage of OA, a finding which is the rationale for studies on the effect of anti-osteoporotic drugs on the development and progression of OA. Post hoc analyses of RCTs on the effect of alendronate and strontium ranelate, and a prospective intervention study on the effect of strontium ranelate, have demonstrated subtle but significant effects on clinical and radiographic signs of OA. However, none of the currently available anti-osteoporotic drugs is ready for use as a disease-modifying OA drug in daily clinical practice, because studies have failed to provide sufficient evidence of both clinical and structural improvement as defined by the guidelines of regulatory authorities [70].
Inflammation has been recognized as a risk factor for both OP and OA. In addition, unfavourable body composition contributes to the development of both disorders, but while low BMI is associated with an increased risk for OP, obesity stimulates the development of OA by the effect of increased mechanical loading of weight-bearing joints as well as by supposed unfavourable effects of specific adipose tissue-derived adipokines.
Hopefully, these insights will contribute to the development of new pharmacological and non-pharmacological treatment options for both disorders in the near future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res. 2003;15:426–39.
Hart DJ, Mootoosamy I, Doyle DV, et al. The relationship between osteoarthritis and osteoporosis in the general population: the Chingford Study. Ann Rheum Dis. 1994;53:158–62.
Healey JH, Vigorita VJ, Lane JM. The coexistence and characteristics of osteoarthritis and osteoporosis. J Bone Joint Surg Am. 1985;67:586–92.
Lafeber FPJG, Van Laar JM. Strontium ranelate: ready for clinical use as disease-modifying osteoarthritis drug? Ann Rheum Dis. 2013;72:157–61.
•• Bijlsma JWJ, Berenbaum E, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–26. This is an outstanding state-of-the-art article on the epidemiology, etiology, clinical characteristics, diagnostic measures, and therapeutic options for osteoarthritis.
Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6.
Berry PA, Maciewicz RA, Cicuttini FM, et al. Markers of bone formation and resorption identify subgroups of patients with clinical knee osteoarthritis who have reduced rates of cartilage loss. J Rheumatol. 2010;37:1252–9.
Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7.
Zhang ZM, Li ZC, Jiang LS, et al. Micro-CT and mechanical evaluation of subchondral trabecular bone structure between postmenopausal women with osteoarthritis and osteoporosis. Osteoporos Int. 2010;21:1383–90.
Funck-Brentano T, Cohen-Solal M. Crosstalk between cartilage and bone: when bone cytokines matter. Cytokine Growth Factor Rev. 2011;22:91–7.
Bultink IEM, Vis M, Van der Horst-Bruinsma IE, Lems WF. Inflammatory rheumatic disorders and bone. Curr Rheumatol Rep. 2012;14:224–30.
Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76.
•• Schett G, Saag KG, Bijlsma JWJ. From bone biology to clinical outcome: state of the art and future perspectives. Ann Rheum Dis. 2010;69:1415–9. This is an excellent state-of-the-art article on bone biology.
• Geusens PP, Lems WF. Osteoimmunology and osteoporosis. Arthritis Res Ther. 2011;13:242. Epub. This article is a large overview on osteoimmunology and osteoporosis.
Xu S, Wang Y, Lu J, Xu J. Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arhritis-induced osteoporosis. Rheumatol Int. 2012;32:3397–403.
Schett G, Kiechl S, Weger S, et al. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch Intern Med. 2006;166:2495–501.
• Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761–5. This is an excellent systematic review on the association between obesity and hand osteoarthritis.
Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332:639–42.
Berenbaum F, Wymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25:114–8.
Filková M, Lišková M, Hulejová H, et al. Increased serum adiponectin levels in female patients with erosive compared with non-erosive osteoarthritis. Ann Rheum Dis. 2009;68:295–6.
Choe JY, Bae J, Jung HY, et al. Serum resistin level is associated with radiographic changes in hand osteoarthritis: cross-sectional study. Joint Bone Spine. 2012;79:160–5.
Yusuf E, Ioan-Facsinay A, Bijsterbosch J, et al. Association between leptin, adiponectin and resistin and long-term progression of hand osteoarthritis. Ann Rheum Dis. 2011;70:282–4.
Honsawek H, Chayanupatkul M. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res. 2010;41:593–8.
Koskinen A, Juslin S, Nieminen R, et al. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther. 2011;13:R184.
Berry PA, Jones SW, Cicuttini FM, Wluka AE, Maciewicz RA. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum. 2011;63:700–7.
Iwamoto J, Takeda T, Sato Y, Matsumoto H. Serum leptin concentration positively correlates with body weight and total fat mass in postmenopausal Japanese women with osteoarthritis of the knee. Arthritis. 2011;2011:580632.
Anandacoomarasamy A, Giuffre BM, Leibman S, et al. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage: clinical associations in obese adults. J Rheumatol. 2009;36:1056–62.
Van Spil WE, Welsing PMJ, Kloppenburg M, et al. Cross-sectional and predictive associations between plasma adipokines and radiographic signs of early-stage knee osteoarthritis: data from CHECK. Osteoarthr Cartil. 2012;20:1278–85.
De Laet C, Kanis JA, Odén A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–8.
Prieto-Alhambra D, Premaor MO, Fina Avilés F, et al. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27:294–300.
Armstrong ME, Cairns BJ, Banks E, et al. Different effects of age, adiposity and physical activity on the risk of ankle, wrist and hip fractures in postmenopausal women. Bone. 2012;50:1394–400.
Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture risk in men and women: an epidemiologic perspective. J Bone Miner Res. 2012;27:1–10.
Richette P, Corvol M, Bardin T. Estrogens, cartilage, and osteoarthritis. Joint Bone Spine. 2003;70:257–62.
Wilson MG, Michet Jr CJ, Ilstrup DM, et al. Idiopathic symptomatic osteoarthritis of the hip and knee: a population-based incidence study. Mayo Clin Proc. 1990;65:1214–21.
Sniekers YH, Weinans H, Bierma-Zeinstra SM, et al. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment – a systematic approach. Osteoarthr Cartil. 2008;16:533–41.
Sniekers YH, Weinans H, van Osch GJ, et al. Oestrogen is important for maintenance of cartilage and subchondral bone in a murine model of knee osteoarthritis. Arthritis Res Ther. 2010;12:R182.
Richette P, Dumontier MF, Tahiri K, et al. Oestrogens inhibit interleukin 1beta-mediated nitric oxide synthase expression in articular chondrocytes through nuclear factor-kappa B impairment. Ann Rheum Dis. 2007;66:345–50.
Bay-Jensen AC, Tabassi NC, Sondergaard LV, et al. The response to estrogen deprivation of the cartilage collagen degradation marker, CTX-II, is unique compared with other markers of collagen turnover. Arthritis Res Ther. 2009;11:R9.
Mouritzen U, Christgau S, Lehmann HJ, et al. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62:332–6.
Christgau S, Tanko LB, Cloos PA, et al. Suppression of elevated cartilage turnover in postmenopausal women and in ovariectomized rats by estrogen and a selective estrogen-receptor modulator (SERM). Menopause. 2004;11:508–18.
Carbone LD, Nevitt MC, Wildy K, et al. The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum. 2004;11:3516–25.
Karsdal MA, Sondergaard BC, Arnold M, et al. Calcitonin affects both bone and cartilage: a dual action treatment for osteoarthritis? Ann N Y Acad Sci. 2007;1117:181–95.
Sondergaard BC, Madsen SH, Segovia-Silvestre T, et al. Investigation of the direct effects of salmon calcitonin on human osteoarthritic chondrocytes. BMC Musculoskelet Disord. 2010;11:62.
Manicourt DH, Azria M, Mindeholm L, et al. Oral salmon calcitonin reduces Lequesne’s algofunctional index scores and decreases urinary and serum levels of biomarkers of joint metabolism in knee osteoarthritis. Arthritis Rheum. 2006;54:3205–11.
Karsdal MA, Byrjalsen I, Henriksen K, et al. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritis patients: a 14-day randomized study. Osteoarthr Cartil. 2010;18:150–9.
Bagger YZ, Tanko LB, Alexandersen P, et al. Oral salmon calcitonin induced suppression of urinary collagen type II degradation in postmenopausal women: a new potential treatment of osteoarthritis. Bone. 2005;37:425–30.
Saag KG. Bisphosphonates for osteoarthritis prevention: “Holy Grail” or not? Ann Rheum Dis. 2008;67:1358–9.
Podworny NV, Kandel RA, Renlund RC, et al. Partial chondroprotective effect of zoledronate in a rabbit model of inflammatory arthritis. J Rheumatol. 1999;26:1972–82.
Hayami T, Pickarski M, Wesolowski GA, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–206.
Kadri A, Funck-Brentano T, Lin H, et al. Inhibition of bone resorption blunts osteoarthritis in mice with high bone remodeling. Ann Rheum Dis. 2010;69:1533–8.
Moreau M, Rialland P, Pelletier JP, et al. Tiludronate treatment improves structural changes and symptoms of osteoarthritis in the canine anterior cruciate ligament model. Arthritis Res Ther. 2011;13:R98.
Neogi T, Nevitt MC, Ensrud KE, et al. The effect of alendronate on progression of spinal osteophytes and disc space narrowing. Ann Rheum Dis. 2008;67:1427–30.
Spector TD, Conaghan PG, Buckland-Wright JC, et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial. Arthritis Res Ther. 2005;7:R625–33.
Bingham CO, Buckland-Wright JC, Garnero P, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symtpoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee. Arthritis Rheum. 2006;54:3493–507.
Buckland-Wright JC, Messent EA, Bingham CO, et al. A 2 year longitudinal radiographic study examining the effect of a bisphosphonate (risedronate) upon subchondral bone loss in osteoarthritis knee patients. Rheumatology. 2007;46:257–64.
Roux C, Richette P. Impact of treatments for osteoporosis on osteoarthritis progression. Osteoporos Int. 2012;23:S881–3.
Laslett LL, Doré DA, Quinn SJ, et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis. 2012;71:1322–8.
Marie PJ. Strontium ranelate: new insights into its dual mode of action. Bone. 2007;40:S5–8.
Alexandersen P, Karsdal MA, Qvist P, et al. Strontium ranelate reduces the urinary level of cartilage degradation biomarker CTX-II in postmenopausal women. Bone. 2007;40:218–22.
Alexandersen P, Karsdal MA, Byrjalsen I, et al. Strontium ranelate effect in postmenopausal women with different clinical levels of osteoarthritis. Climacteric. 2011;14:236–43.
Van Spil WE, Drossaers-Bakker KW, Lafeber FPJG. Associations of CTX-II with biochemical markers of bone turnover rise questions on its tissue origin: data from CHECK, a cohort study of early osteoarthritis. Ann Rheum Dis. 2013;72:29–36.
Bruyere O, Delferriere D, Roux C, et al. Effects of strontium ranelate on spinal osteoarthritis progression. Ann Rheum Dis. 2008;67:335–9.
• Reginster J-Y, Badurski J, Bellamy N, et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis. 2013;72:179–86. In this article, the results of the first prospective, placebo-controlled, clinical study on the effect of strontium ranelate in the treatment of osteoarthritis (of the knee) are presented.
Tat SK, Pelletier J-P, Mineau F, et al. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone. 2011;49:559–67.
Henrotin Y, Labasse A, Zheng SX, et al. Strontium ranelate increases cartilage matrix formation. J Bone Miner Res. 2001;16:299–308.
Simpson ER, Hilton MJ, Tian Y, et al. Teriparatide, a chondro-regenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra93.
Terkeltaub R, Lotz M, Johnson K, et al. Parathyroid hormone-related proteins is abundant in osteoarthritic cartilage, and the parathyroid hormone-related protein 1-173 isoform is selectively induced by transforming growth factor beta in articular chondrocytes and suppresses generation of extracellular inorganic pyrophosphate. Arthritis Rheum. 1998;41:2152–64.
Bone HG, McClung MR, Roux C, et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone mineral density. J Bone Miner Res. 2010;25:937–47.
Hayami T, Zhuo Y, Wesolowski GA, et al. Inhibition of cathepsin K reduces cartilage degeneration in the anterior cruciate ligament transection rabbit and murine models of osteoarthritis. Bone. 2012;50:1250–9.
FDA – Clinical development programs for drugs, devices and biological products intended for the treatment of OA. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances (7/99).
Conflict of Interest
Irene E.M. Bultink has received honoraria from Merck & Co. and Servier Laboratories.
Willem F. Lems has received speakers fees from Merck, Sharp & Dohme, Eli Lilly and Company, Servier Laboratories, and Amgen, and has received payment for development of educational presentations from Servier Laboratories.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Osteoporosis and Metabolic Bone Disease
Rights and permissions
About this article
Cite this article
Bultink, I.E.M., Lems, W.F. Osteoarthritis and Osteoporosis: What Is the Overlap?. Curr Rheumatol Rep 15, 328 (2013). https://doi.org/10.1007/s11926-013-0328-0
Published:
DOI: https://doi.org/10.1007/s11926-013-0328-0