Abstract
Fractures are common in patients with chronic kidney disease (CKD), but the diagnosis and treatment of bone disease in CKD are difficult due to the multiple etiologies of bone disease in these patients. Noninvasive imaging, including bone mineral density by dual energy x-ray absorptiometry, can be useful in diagnosing osteoporosis in predialysis CKD; however, consensus on the diagnosis of osteoporosis among those with advanced CKD—particularly stage 5 CKD patients on dialysis—is lacking. Treatments approved for osteoporosis in postmenopausal women may be used in patients with stage 1 to 3 CKD. Furthermore, post-hoc analyses show efficacy and safety of oral bisphosphonates, raloxifene, and denosumab in stage 4 CKD for short-term treatment. However, treatment decisions are more difficult in stage 5 CKD. Bone biopsy may be required, and most treatments, if used, would be off label. Overall, the diagnosis and treatment of bone disease in patients with CKD require further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
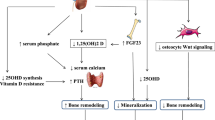
The diagnosis and treatment of bone disease in men and women with chronic kidney disease (CKD) represent a clinical challenge. Patients with CKD suffer from bone disease characterized by decreased bone quantity and quality starting early during the course of CKD. However, properly identifying the type of bone disease present in those with CKD is complicated—CKD patients may have abnormalities in mineral metabolism leading to metabolic bone disease termed chronic kidney disease mineral and bone disorder (CKD-MBD), which may also be characterized by impaired bone strength and an increased incidence of low-trauma fractures. CKD-MBD is defined in part by alterations in turnover, mineralization, and volume from bone histomorphometry. Further complicating the diagnosis of bone disease, patients with CKD may or may not have osteoporosis, defined by the National Institutes of Health Consensus Conference on Osteoporosis as a systemic skeletal disease characterized by impairment in bone strength that also increases the incidence of low-trauma fractures. Clinically, osteoporosis is defined by the occurrence of a fragility fracture or by World Health Organization criteria utilizing dual energy x-ray absorptiometry (DXA): T score of -2.5 or below.
Identifying the cause of bone loss and fractures in men and women with CKD may influence therapeutic decisions. For example, antiresorptive therapy, which suppresses bone turnover, might not be appropriate in patients with low-turnover CKD-MBD. There is consensus that in stages 1 to 3 CKD, in the absence of abnormalities of mineral metabolism such as elevated phosphorus or hyperparathyroidism, one can use therapies that have been approved for postmenopausal osteoporosis (PMO). Furthermore, post-hoc analyses of the preplanned registration studies for PMO suggest that oral bisphosphonates, raloxifene, and denosumab are safe and efficacious in stage 4 CKD. There are no scientific data showing efficacy or safety in utilizing osteoporosis therapies in patients with stage 5 CKD, including those on dialysis (stage 5D). Distinguishing CKD-MBD from osteoporosis and subsequent treatment decisions are more difficult to make in stage 5 CKD (Table 1). Bone biopsy may be required, and most of the currently available osteoporosis treatments, if used, would be off label.
This review highlights the most recent data concerning the etiology and diagnosis of bone disease in men and women with predialysis (stage 1–5) CKD, and in those with stage 5D CKD. In addition, we discuss therapeutic options and the challenges associated with treating low bone mass or fragility fractures in CKD. It is clear from our review that more research is needed in the area of noninvasive diagnosis of bone disease in CKD, as well as potential treatment regimens for low bone mass or low-trauma fractures in this population.
Diagnosis
Does My Patient Have Osteoporosis or Chronic Kidney Disease Mineral and Bone Disorder?
Fractures are common in men and women with CKD and increase in prevalence as renal function declines. Data from the Study of Osteoporotic Fractures found that compared with women with an estimated glomerular filtration rate (eGFR) of 60 mL/min or greater, hip fracture risk was increased by 1.5-fold among those with an eGFR of 45 to 50 mL/min and was doubled among women with an eGFR less than 45 mL/min [1]. Fractures are even more common among CKD patients on dialysis, of which up to 50% of patients have had a fracture [2]. The clinical challenge for physicians taking care of patients who have CKD and fractures is deciding if the fracture is due to osteoporosis or due to another metabolic bone disease associated with CKD-MBD.
Diagnosis of Bone Disease in Predialysis Chronic Kidney Disease
Current data suggest that the approach to diagnosis and treatment decisions may vary by stage of CKD. The mild metabolic derangements that occur in stages 1 to 3 CKD, such as intermittent hyperphosphatemia or mild increases in parathyroid hormone (PTH), have not been associated with alterations in bone strength or increases in rates of fractures [3]. This suggests that the primary cause of fractures in patients with stages 1 to 3 CKD, in the absence of persistent metabolic derangements, is osteoporosis and that bone mineral density (BMD) testing by DXA may be used for risk assessment in these patients. Note that this concept is supported by the Kidney Disease: Improving Global Outcomes (KDIGO) working group conclusions [4].
Further support for this concept—specifically that in early stages of CKD, the main cause of fracture is osteoporosis, and that BMD by DXA has clinical utility in these patients—comes from two sources. First are the registration trials for agents that treat osteoporosis, including teriparatide, risedronate, raloxifene, and denosumab; many of the women included in these trials had an eGFR consistent with stage 1 to 3 CKD despite a serum creatinine in the normal range (note that typically, other markers of mineral metabolism, such as PTH, phosphate, calcium, and alkaline phosphatase, were also in the normal range), and in these postmenopausal women populations, BMD did predict fracture risk [5••, 6–8].
Second are data from three cross-sectional studies measuring BMD by DXA in men and women with predialysis CKD [9, 10••, 11]. Nickolas et al. [9, 10••] published two cross-sectional studies on bone mass, microarchitecture, and fractures in patients with stage 2 to 5 (eGFR <90 mL/min) predialysis CKD. The first study included 32 individuals with fracture (mean eGFR, 33 mL/min; range, 7–68 mL/min) and 59 without fracture (mean eGFR, 30 mL/min; range, 7–76 mL/min). BMD by DXA was measured at the lumbar spine, total hip, femoral neck, ultradistal radius, and 1/3 radius. In those with fracture, BMD by DXA was lower at all sites except at the 1/3 radius. For each SD decrease in lumbar spine, total hip, femoral neck, and ultradistal radius BMD, there was a 93%, 65%, 85%, and 129% increased odds of fracture, respectively [9]. Note that in this study, those with fractures had similar calcium and phosphorus levels as those without fractures, both of which were in the normal range. In a more recent study that enrolled 82 individuals with stage 3 to 5 CKD (n = 23 with fracture; mean eGFR, 25 mL/min [range, 14–35 mL/min]; n = 59 without fracture; mean eGFR, 28 mL/min [range, 19–41 mL/min]), BMD by DXA at the lumbar spine and total hip, femoral neck, 1/3 radius, and ultradistal radius were significantly lower in those with fracture compared with those without; however, after adjusting for age, gender, and diabetes, only BMD at the ultradistal radius remained significantly lower among those with fractures compared with those without [10••]. The participants in this study had similar biochemical values as in the earlier publication, with calcium and phosphate in the normal range.
Our group assessed 211 patients with predialysis stage 3 to 5 CKD. In our cohort, there were 77 fractures in 74 individuals: 41 individuals had prevalent spine fractures, and 36 self-reported low-trauma fractures. BMD by DXA at the lumbar spine, total hip, 1/3 radius, and ultradistal radius was lower among those with fracture than those without. Furthermore, the diagnostic test characteristics for BMD to discriminate by fracture status were very good (area under the receiver operating characteristic curve [AUROC] range, 0.70–0.80) and were similar to those previously reported in a small group of postmenopausal women with and without wrist fractures [12]. Our population of stage 3 to 5 CKD had slight metabolic disturbances: individuals with fractures had a higher level of serum PTH (mean for fracture, 39.7 ± 35.7 pmol/L vs nonfracture, 22.0 ± 28.3 pmol/L [normal range, 1.6–6.9 pmol/L]; P = 0.002), and 25(OH)D was in the suboptimal range (25–75 nmol/L) for both groups. However, serum phosphate and calcium were within normal limits for both fracture and nonfracture individuals with CKD [11].
Another noninvasive method to assess fracture risk is peripheral quantitative CT (pQCT), which may have several advantages over DXA for use in fracture risk assessment in patients with CKD. In contrast to DXA, pQCT can discriminate between cortical and trabecular bone components, which allows for the determination of the differential effects of PTH on bone, as elevated PTH preferentially decreases cortical more than trabecular bone. Two studies, both of which have been discussed above, measured high-resolution (HR)pQCT in predialysis CKD in addition to BMD by DXA [9, 10••]. The first study reported that in 91 participants, those with fractures (n = 32) had lower total volumetric bone density (255 ± 63 vs 300 ± 71 mg HA/cm3 radius; 226 ± 56 vs 259 ± 63 mg HA/cm3 tibia), cortical area (45 ± 15 vs 55 ± 19 mm2 radius; 90 ± 46 vs 110 ± 38 mm2 tibia), and thickness (626 ± 179 vs 743 ± 241 μm radius; 793 ± 374 vs 979 ± 338 μm tibia) at both the radius and tibia, had lower trabecular density (122 ± 43 vs 151 ± 48 mg HA/cm3), lower trabecular number (1.7 ± 0.5 vs 2.0 ± 0.4 1/mm), and larger trabecular separation (574 ± 206 vs 477 ± 164 μm) at the radius than those without fractures (n = 59) [9]. In the second study, after adjusting for age, gender, and diabetes, those with fracture (n = 23) had a lower cortical area (40 vs 55 mm2 radius; 80 vs 113 mm2 tibia), thickness (610 vs 750 μm radius; 690 vs 1,005 μm tibia), and total density (248 vs 308 mg HA/cm3 radius; 222 vs 257 mg HA/cm3 tibia) at both the radius and tibia, lower trabecular density (114 vs 154 mg HA/cm3) and trabecular thickness (59 vs 65 μm) at the radius, and lower cortical density (718 vs 797 mg HA/cm3) at the tibia compared with those without fracture (n = 59) [10••]. However, neither of these studies [9, 10••] compared DXA and HRpQCT to determine which measure is best for fracture discrimination. This latter point is important, as to our knowledge, there are only eight HRpQCT machines in the United States and Canada, and to date no normative data comparisons, thus limiting the ability of HRpQCT to be used for fracture discrimination in clinical practice.
We also examined the ability of HRpQCT to discriminate fractures in our cohort study of stage 3 to 5 CKD patients (n = 211). We found that measures of bone microarchitecture by HRpQCT at the radius, including total volumetric BMD (255.7 ± 66.7 vs 312.8 ± 61.4 mg HA/cm3), cortical area (46.7 ± 19.3 vs 59.0 ± 14.9 mm2), cortical density (767.4 ± 88.7 vs 834.0 ± 69.3 mg HA/cm3), trabecular density (136.2 ± 44.5 vs 162.1 ± 41.8 mg HA/cm3), and cortical thickness (0.61 ± 0.2 vs 0.79 ± 0.2 mm), were lower in those with fractures (n = 74) compared with those without (n = 137), while trabecular separation (0.54 ± 0.2 vs 0.46 ± 0.2 mm) was higher in those with fractures compared with those without. Overall, cortical measures were superior to trabecular measures in discriminating fracture status. We also compared the utility of DXA and HRpQCT in discriminating fractures and found that the discriminative ability of HRpQCT was no better than that of DXA. As well, the addition of a composite HRpQCT measure (total volumetric BMD and cortical thickness) to BMD by DXA at the ultradistal radius did not improve fracture discrimination (composite HRpQCT measure added to BMD by DXA at the ultradistal radius AUROC, 0.81 [95% CI, 0.74–0.88]; BMD by DXA at ultradistal radius alone AUROC, 0.80 [95% CI, 0.74–0.87]; P = 0.37) [11].
Considered together, these data suggest that among men and women with predialysis CKD who do not have substantial derangements in markers of mineral metabolism, BMD by DXA and/or the presence of a low-trauma fracture can be used to diagnose osteoporosis, and that more detailed assessments of bone microarchitecture by HRpQCT are not necessary for fracture discrimination. Fracture risk, however, appears to be higher than in age- and BMD-matched patients without CKD such that in patients with stage 3 CKD, additional fracture risk (~2×) may need to be added to current models of predicting risk (eg, FRAX).
Diagnosis of Bone Disease in Stage 5 and 5D Chronic Kidney Disease
There is no consensus on how to diagnose osteoporosis among men and women with more advanced CKD. These patients may have profound alterations in mineral metabolism resulting in metabolic bone disease with or without decreases in BMD. The predominant diagnostic dilemma in this group is distinguishing low turnover, so-called adynamic bone disease, from other forms of metabolic bone disease—specifically osteomalacia and hyperparathyroid bone disease. Although there are no data that suggest administering an antiresorptive agent to a patient with preexisting adynamic bone disease is harmful with regard to bone strength, it seems reasonable to exclude adynamic bone disease before initiating treatment in these patients with medications that suppress bone turnover.
To date, the only way to accurately determine the type of bone disease present in men and women with advanced CKD is with double tetracycline-labeled quantitative bone histomorphometry, yet this technique is not widely utilized. Limitations of bone biopsy include cost, lack of specialized expertise to review the biopsy, and most importantly that bone histology may be fluid and thus may change with stage and duration of CKD, as bone biopsy is a view of bone from a single location at a single time point. As in patients with predialysis CKD, research has focused on noninvasive methods to assess fracture risk in patients with advanced CKD, including BMD by DXA and pQCT.
We published a meta-analysis that reported on the association between BMD by DXA and fractures in men and women with stage 5D CKD [13]. Our meta-analysis suggested that BMD is lower in those with stage 5D CKD who have fractures, particularly at cortical sites such as the radius [14, 15]. Since the publication of our meta-analysis, four studies have been published that report on the association between BMD by DXA and fracture in men and women with stage 5D CKD [16–19], three of which report on those who were actively on dialysis therapy [17–19]. Results from these recent studies are conflicting: one reported no association between fractures and lumbar spine or femoral neck BMD by DXA [17], one reported that lower BMD by DXA at the femoral neck is associated with an increased prevalence of fractures [18], while the most recent study reported that those with fracture had a reduction in BMD at the distal radius site [19].
Studies that have measured pQCT in stage 5D CKD patients are all cross-sectional and report a preferential decrease in cortical, but not trabecular density, in keeping with the hypothesis that with greater reductions in renal function, bone loss is primarily due to elevated PTH [20–23]. Only two studies have reported on the association between pQCT or HRpQCT and fracture in stage 5D CKD [20, 24]. Our group found that among 52 men and women with stage 5D CKD on hemodialysis for at least 1 year, a decrease in cortical density was associated with fractures (OR, 16.7 [95% CI, 2.9–83.3]), as was a decrease in cortical area (OR, 3.0 [95% CI, 1.3–7.3]) and a decrease in cortical thickness (OR, 3.3 [95% CI, 1.4–7.9]); however, fractures were not associated with trabecular measures [20]. The second cross-sectional study [24] measured HRpQCT at the radius and tibia in 74 stage 5D CKD patients on hemodialysis (24 with prevalent spine fracture and/or a history of previous nonspine fracture) and in 40 gender- and race-matched controls without kidney disease or fractures. There was an impairment in bone microarchitecture among those with stage 5D CKD compared with healthy controls, and among those 5D CKD patients with fracture, cortical and trabecular microarchitecture was impaired compared with those stage 5D CKD patients without fracture. Interestingly, in this study, the strongest discriminator of fracture status was trabecular density at the tibia (AUROC, 0.90 [95% CI, 0.80–0.99]) [24], different from our study in stage 5D CKD patients [20], in which we did not find an association between fractures and pQCT-measured trabecular density at the radius.
Finally, there are several novel noninvasive imaging techniques that may in the future be useful in assessing fracture risk in men and women with more advanced stages of CKD. These include micro-magnetic resolution imaging, hip structure analysis, and finite element analysis. To date, there are no published studies on the ability of these techniques to discriminate fracture status in patients with CKD.
Biochemical Markers of Bone Turnover to Diagnose Bone Disease in Chronic Kidney Disease
The clinical utility of measuring bone turnover markers in individuals with CKD is unclear [25–28], mainly because many bone turnover markers are excreted by the kidney. The markers not excreted by the kidney are bone-specific alkaline phosphatase (BSAP), N-terminal propeptide of human procollagen type I (PINP) trimer, and tartrate-resistant acid phosphatase-5b (TRAP-5b). However, there are insufficient data on the value of any marker to discriminate among diseases accompanying CKD [10••]. Data on the use of biochemical markers of bone turnover and their association with fractures in patients with CKD are limited to a small cross-sectional study (n = 82) of predialysis patients with eGFR less than 90 mL/min [10••]. Markers of bone formation (BSAP, osteocalcin, PINP) and resorption (carboxyterminal telopeptide of type I collagen [CTX], TRAP-5b) were measured in individuals with stage 3 to 5 predialysis CKD. The authors reported that compared with those without fracture (n = 23), those with fracture (n = 59) had higher osteocalcin (24.2 vs 41.8 ng/mL), PINP (54.0 vs 79.5 μL/L), CTX (0.87 vs 1.24 ng/mL), and TRAP-5b (4.41 vs. 5.33 U/L); however, BSAP was not different by fracture status [10••]. Note that while bone turnover markers were moderately able to discriminate fracture status (osteocalcin AUROC, 0.69; PINP AUROC, 0.72; TRAP-5b AUROC, 0.68), these markers did not perform better then femoral neck T score as measured by DXA (AUROC, 0.71). However, the highest tertiles of osteocalcin, PINP, and TRAP-5b were independently associated with increased odds of fracture, and combining these markers with femoral neck T score improved fracture discrimination over femoral neck T score and bone turnover markers alone (osteocalcin alone AUROC, 0.81 vs osteocalcin with femoral neck T score AUROC, 0.83; PINP alone AUROC, 0.81 vs PINP with femoral neck T score AUROC, 0.82; TRAP-5b alone AUROC, 0.79 vs TRAP-5b with femoral neck T score AUROC, 0.83) [10••].
Despite the minimal data on bone turnover markers and fractures in CKD, several studies have reported on associations between serum levels of PTH and BSAP and bone turnover (by histomorphometry) and have found that PTH and BSAP may predict bone turnover in CKD patients [28–34]. Considered together, these studies suggest that patients with high BSAP and/or very high serum PTH are unlikely to have adynamic bone disease and, after the reasons for the high BSAP/PTH have been identified and treated, may have osteoporosis. As such, KDIGO has recommended that in patients with stages 3 to 5D CKD, measurements of serum PTH or BSAP should be used to evaluate underlying bone disease [4].
Therapeutic Options
Treatment strategies to prevent fractures in patients with CKD consist of decreasing falls and fall risk, normalizing 25(OH)D serum levels, controlling PTH levels, and the consideration of treatment with pharmacologic agents that have been approved for the treatment of PMO. Falls due to myopathy, neuropathy, and postural hypotension are common in men and women with CKD and increase the risk of fractures. We have demonstrated that among patients on dialysis and among those with earlier stages of CKD, impaired performance on tests of neuromuscular function is associated with an increased risk of fracture [35, 36]. Randomized trials are needed to determine if improved neuromuscular function can decrease fracture risk.
Severe secondary hyperparathyroidism in stage 5 CKD is associated with an increased risk of fracture [37, 38], and while randomized trial data are lacking, it would seem reasonable that controlling serum PTH with adequate vitamin D replacement (25[OH]D and 1,25[OH]2D), use of calcimimetics, and parathyroidectomy should be included in the management of men and women with CKD. KDIGO recently published guidelines on managing hyperparathyroidism in CKD [4].
Nitrogen-containing oral bisphosphonates are the most commonly used medications for the treatment of postmenopausal osteoporosis. However, the US Food and Drug Administration does not recommend the use of these agents in stages 4, 5, and 5D CKD [4, 39]. There are no GFR cutoffs for denosumab, as this antiresorptive agent is not cleared by the kidney [5••, 40]. The US Food and Drug Administration recommendation has widespread health implications, as aging is associated with both osteoporosis and impairment in renal function. For example, data from the National Health and Nutrition Examination Survey indicate that 85% of women with osteoporosis have impaired renal function [41]. Fracture prevention trials for various osteoporotic treatment agents included some women with creatinine in the normal laboratory range but with decreased creatinine clearances or eGFR. Serum creatinine is generated from skeletal muscle and may be within a “normal” laboratory reference range in a person with low body mass index, yet whose eGFR is low. Post-hoc analysis of the alendronate fracture trial demonstrates an increase in total hip BMD and a decrease in clinical and vertebral fractures in the treatment compared with the placebo group (the Fracture Intervention Trial [FIT]: 581 women with eGFR by Cockcroft Gault [CG] of <45 mL/min) [42], and a pooled analysis of 9 risedronate studies (572 women with eGFR by CG of <30 mL/min) reported preserved BMD and reduced incident vertebral fractures in the treatment group [7]. None of the patients in these two post-hoc analysis trials had elevated PTH levels at baseline. Similar findings have been reported for raloxifene (Multiple Outcomes of Raloxifene Evaluation [MORE] trial: 1,480 women with creatinine clearance <45 mL/min) [8], teriparatide (the FIT using teriparatide: 731 women with eGFR 30–79 mL/min) [6], and denosumab (2,890 with eGFR 15–59 mL/min) [5••]. It is important to note that all these studies were post-hoc analyses of otherwise healthy postmenopausal women with no significant aberrations in markers of mineral metabolism and were short term in duration (at most 3 years).
How can one apply these data to clinical practice? It would seem reasonable among men and women with stages 1 to 3 CKD who are suffering from low-trauma fractures who do not have biochemical abnormalities that might suggest CKD-MBD that any agent registered for use of osteoporosis can be used without dose adjustment. Treatment decisions are more difficult in men and women with stages 4 and 5 CKD who are fracturing. Post-hoc analyses from FIT (3-year duration) [42] and MORE (3-year duration) [8] included small numbers of participants with eGFR down to 15 mL/min, and in the absence of biochemical abnormalities, fracturing patients with stage 4 CKD could be treated with these agents for no more than 3 years.
There are no published data on the safety and efficacy of any approved agent for osteoporosis among men and women with eGFR less than 15 mL/min or stage 5/5D CKD. A reasonable clinical approach would be to consider therapy only in those with fractures, not simply based on BMD results, and in those with fractures to obtain a transiliac bone biopsy. If the transiliac bone biopsy demonstrates high-turnover osteoporosis and a decision is made to treat with bisphosphonates, they should be used at half the usual dose prescribed for osteoporosis for no more than 3 years due to concerns of long-term retention of these agents in those with substantial renal impairment. Note that to date, these recommendations have not been made based on evidence, but rather clinical consensus. Further research clearly is needed.
Conclusions
Men and women with CKD suffer from bone disease and are at high risk of fractures. The diagnosis and treatment of bone disease in men and women with CKD are a clinical challenge. Noninvasive imaging techniques, including BMD by DXA and pQCT/HRpQCT, have been utilized in predialysis and stage 5D CKD patients to assess fracture status; however, the cross-sectional data are conflicting, and longitudinal studies are needed to determine the utility of these imaging techniques in predicting future fracture risk. Identifying the cause of low BMD and fractures in men and women with CKD may influence therapeutic decisions. For example, antiresorptive agents may not be efficacious in low-turnover bone disease. Treatments approved for PMO can be used in CKD stages 1 to 3 without dose modifications. In stage 4 CKD, post-hoc analyses suggest efficacy and safety for oral therapies and subcutaneous denosumab approved for PMO for no longer than 3 years’ duration. For stage 5/5D CKD, safety and efficacy of treatment with current osteoporotic drugs is unknown, and this is an important area of future research.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–9.
Jamal SA, Swan VJ, Brown JP, et al. Kidney function and rate of bone loss at the hip and spine: the Canadian Multicentre Osteoporosis Study. Am J Kidney Dis. 2010;55(2):291–9.
Miller PD. Diagnosis and treatment of osteoporosis in chronic renal disease. Semin Nephrol. 2009;29(2):144–55.
Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. 2009;76 suppl 113:s1–130.
•• Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res. 2011;26(8):1829–35. This is a post-hoc analysis examining the effects of denosumab on fracture by stage of CKD. Denosumab is of particular interest in CKD because it is not cleared by the kidney.
Miller PD, Schwartz EN, Chen P, et al. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int. 2007;18(1):59–68.
Miller PD, Roux C, Boonen S, et al. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20:2105–15.
Ishani A, Blackwell T, Jamal SA, et al. The effect of raloxifene treatment in postmenopausal women with CKD. J Am Soc Nephrol. 2008;19(7):1430–8.
Nickolas TL, Stein E, Cohen A, et al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol. 2010;21(8):1371–80.
•• Nickolas TL, Cremers S, Zhang A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. 2011;22:1560–72. This was a comprehensive cross-sectional study that examined factors associated with fracture in stages 3 to 5 CKD.
Jamal SA, Cheung AM, West SL, Lok CE. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporosis Int. 2012. doi:10.1007/s00198-012-1908-y.
Melton 3rd LJ, Riggs BL, van Lenthe GH, et al. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res. 2007;22(9):1442–8.
Jamal SA, Hayden JA, Beyene J. Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis. Am J Kidney Dis. 2007;49(5):674–81.
Duan Y, De Luca V, Seeman E. Parathyroid hormone deficiency and excess: similar effects on trabecular bone but differing effects on cortical bone. J Clin Endocrinol Metab. 1999;84:718–22.
Christiansen P, Steiniche T, Brockstedt H, et al. Primary hyperparathyroidism: iliac crest cortical thickness, structure, and remodeling evaluated by histomorphometric methods. Bone. 1993;14:755–62.
Dolgos S, Hartmann A, Bonsnes S, et al. Determinants of bone mass in end-stage renal failure patients at the time of kidney transplantation. Clin Transplant. 2008;22(4):462–8.
Ersoy FF, Passadakis SP, Tam P, et al. Bone mineral density and its correlation with clinical and laboratory factors in chronic peritoneal dialysis patients. J Bone Miner Metab. 2006;24(1):79–86.
Elder GJ, Mackun K. 25-hydroxyvitamin D deficiency and diabetes predict reduced BMD in patients with chronic kidney disease. J Bone Miner Res. 2006;21(11):1778–84.
Ambrus C, Almasi C, Berta K, et al. Vitamin D insufficiency and bone fractures in patients on maintenance hemodialysis. Int Urol Nephrol. 2011;43(2):475–82.
Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical PQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006;21:543–8.
Russo CR, Taccetti G, Caneva P, et al. Volumetric bone density and geometry assessed by peripheral quantitative computed tomography in uremic patients on maintenance hemodialysis. Osteoporos Int. 1998;8(5):443–8.
Hasegawa K, Hasegawa Y, Nagano A. Estimation of bone mineral density and architectural parameters of the distal radius in hemodialysis patients using peripheral quantitative computed tomography. J Biomech. 2004;37:751–6.
Negri AL, Barone R, Lombas C, et al. Evaluation of cortical bone by peripheral quantitative computed tomography in continuous ambulatory peritoneal dialysis patients. Hemodial Int. 2006;10(4):351–5.
Cejka D, Patsch JM, Weber M, et al. Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin J Am Soc Nephrol. 2011;6(9):2264–71.
Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8.
Vassalotti JA, Uribarri J, Chen SC, et al. Trends in mineral metabolism: Kidney Early Evaluation Program (KEEP) and the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis. 2008;51(4 Suppl 2):S56–68.
Meier C, Seibel MJ, Kraenzlin ME. Use of bone turnover markers in the real world: are we there yet? J Bone Miner Res. 2009;24(3):386–8.
Lehmann G, Ott U, Kaemmerer D, et al. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease stages 3–5. Clin Nephrol. 2008;70(4):296–305.
Hutchison AJ, Whitehouse RW, Boulton HF, et al. Correlation of bone histology with parathyroid hormone, vitamin D3, and radiology in end-stage renal disease. Kidney Int. 1993;44(5):1071–7.
Carmen Sanchez M, Auxiliadora Bajo M, Selgas R, et al.: Parathormone secretion in peritoneal dialysis patients with adynamic bone disease. Am J Kidney Dis 2000, 36:953-61.
Couttenye M, D’Haese P, VanHoof V, et al. Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transpl. 1996;11:1065–72.
Qi Q, Monier-Faugere MC, Geng Z, Malluche HH. Predictive value of serum parathyroid hormone levels for bone turnover in patients on chronic maintenance dialysis. Am J Kidney Dis. 1995;26(4):622–31.
Bervoets AR, Spasovski GB, Behets GJ, et al. Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. Am J Kidney Dis. 2003;41(5):997–1007.
Barreto FC, Barreto DV, Moyses RM, et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73(6):771–7.
Jamal SA, Leiter RE, Jassal V, et al. Impaired muscle strength is associated with fractures in hemodialysis patients. Osteoporosis Int. 2006;17(9):1390–7.
West SL, Jamal SA, Lok CE: Tests of neuromuscular function are associated with fractures in patients with chronic kidney disease. Nephrology Dialysis Transplantation 2011, E-pub ahead of print.
Danese MD, Kim J, Doan QV, et al. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47(1):149–56.
Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70(7):1358–66.
National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42 suppl 3:S1–202.
Miller PD: A review of the efficacy and safety of denosumab in postmenopausal women with osteoporosis. Therapeutic Advances in Musculoskeletal Disease 2011, E-pub ahead of print.
Klawansky S, Komaroff E, Cavanaugh PFJ, et al. Relationship between age, renal function and bone mineral density in the US population. Osteoporosis Int. 2003;14:570–6.
Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res. 2007;22(4):503–8.
Disclosure
Dr. Jamal has served on boards for Amgen, Novartis, and Warner Chilcott; has served as a consultant for Novartis, Warner Chilcott, Genzyme Corp., and Shire; has received honoraria from Amgen, Novartis, Genzyme Corp., Shire, and Warner Chilcott; has served on the speakers’ bureau for Amgen; and has had travel/accommodations expenses covered/reimbursed by Amgen, Novartis, Genzyme Corp., Shire, and Warner Chilcott.
Dr. Miller has served on boards for WCX, Merck & Co., Eli Lilly and Company, Novartis, and Amgen; has served as a consultant for WCX, Eli Lilly and Company, Amgen, and Novartis; has provided expert testimony on behalf of Novartis and Merck & Co.; has received grant support from WCX, Genentech, Eli Lilly and Company, Merck & Co., Novartis, Amgen, and Takeda Pharmaceuticals North America; and has had travel/accommodations expenses covered/reimbursed by WCX, Amgen, and Novartis.
Ms. West reported no potential conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jamal, S.A., West, S.L. & Miller, P.D. Bone and Kidney Disease: Diagnostic and Therapeutic Implications. Curr Rheumatol Rep 14, 217–223 (2012). https://doi.org/10.1007/s11926-012-0243-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-012-0243-9